

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119489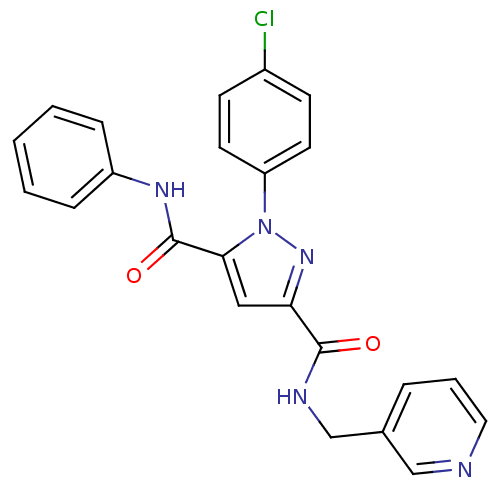 (1-(4-Chloro-phenyl)-1H-pyrazole-3,5-dicarboxylic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119488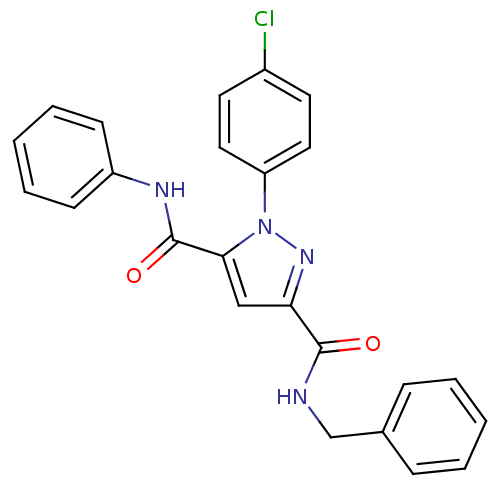 (CHEMBL143628 | X1-(4-Chloro-phenyl)-1H-pyrazole-3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119481 (1-(4-Chloro-phenyl)-1H-pyrazole-3,5-dicarboxylic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119483 (1-(4-Chloro-phenyl)-1H-pyrazole-3,5-dicarboxylic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119485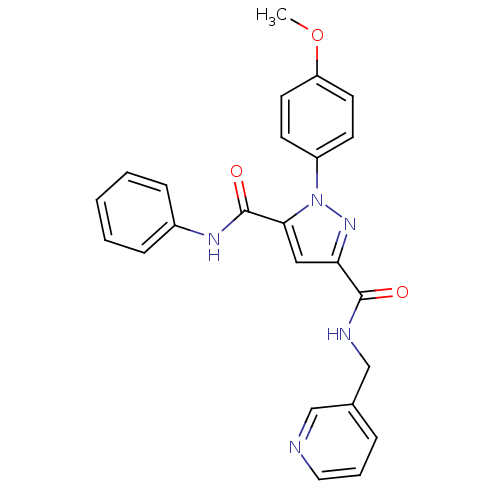 (1-(4-Methoxy-phenyl)-1H-pyrazole-3,5-dicarboxylic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119490 (1-(4-Methoxy-phenyl)-1H-pyrazole-3,5-dicarboxylic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119492 (1-(4-Chloro-phenyl)-1H-pyrazole-3,5-dicarboxylic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119487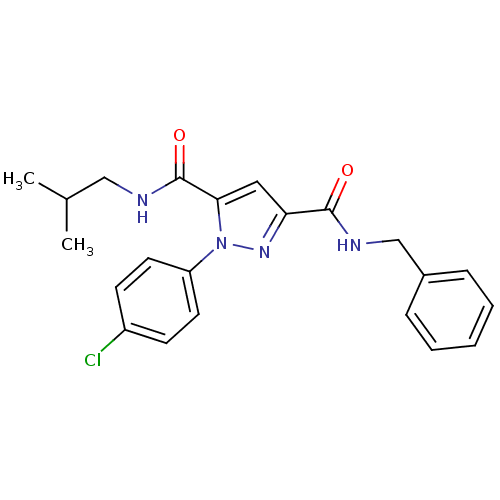 (1-(4-Chloro-phenyl)-1H-pyrazole-3,5-dicarboxylic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119491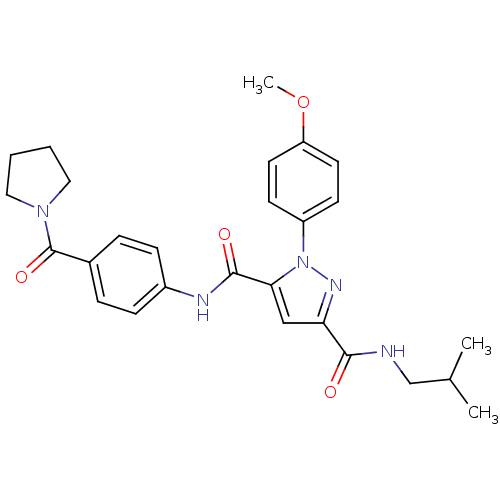 (1-(4-Methoxy-phenyl)-1H-pyrazole-3,5-dicarboxylic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119484 (1-(4-Methoxy-phenyl)-1H-pyrazole-3,5-dicarboxylic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 331 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119482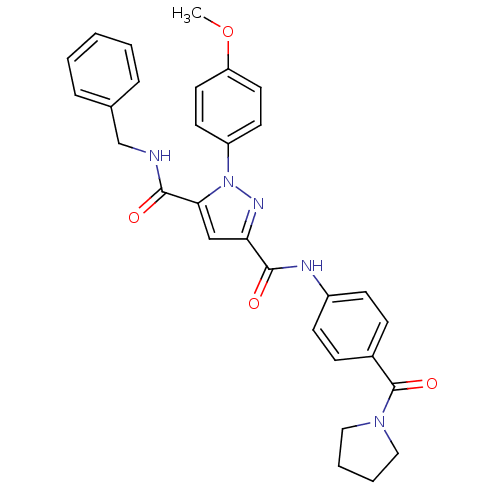 (1-(4-Methoxy-phenyl)-1H-pyrazole-3,5-dicarboxylic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119486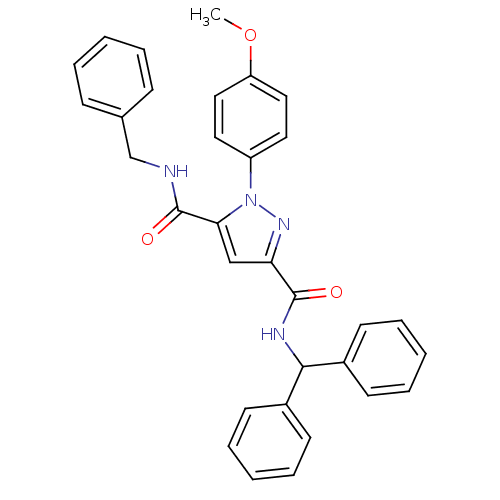 (1-(4-Methoxy-phenyl)-1H-pyrazole-3,5-dicarboxylic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119485 (1-(4-Methoxy-phenyl)-1H-pyrazole-3,5-dicarboxylic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase (DHODase) | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119484 (1-(4-Methoxy-phenyl)-1H-pyrazole-3,5-dicarboxylic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase (DHODase) | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119486 (1-(4-Methoxy-phenyl)-1H-pyrazole-3,5-dicarboxylic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase (DHODase) | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119491 (1-(4-Methoxy-phenyl)-1H-pyrazole-3,5-dicarboxylic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase (DHODase) | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119487 (1-(4-Chloro-phenyl)-1H-pyrazole-3,5-dicarboxylic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase (DHODase) | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119490 (1-(4-Methoxy-phenyl)-1H-pyrazole-3,5-dicarboxylic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase (DHODase) | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119489 (1-(4-Chloro-phenyl)-1H-pyrazole-3,5-dicarboxylic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase (DHODase) | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119488 (CHEMBL143628 | X1-(4-Chloro-phenyl)-1H-pyrazole-3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase (DHODase) | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119483 (1-(4-Chloro-phenyl)-1H-pyrazole-3,5-dicarboxylic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase (DHODase) | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119492 (1-(4-Chloro-phenyl)-1H-pyrazole-3,5-dicarboxylic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase (DHODase) | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119481 (1-(4-Chloro-phenyl)-1H-pyrazole-3,5-dicarboxylic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase (DHODase) | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50119482 (1-(4-Methoxy-phenyl)-1H-pyrazole-3,5-dicarboxylic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human dihydroorotate dehydrogenase (DHODase) | J Med Chem 45: 4669-78 (2002) BindingDB Entry DOI: 10.7270/Q2C24VSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50400690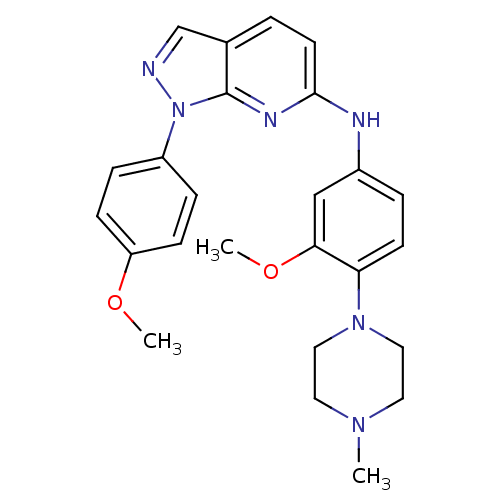 (CHEMBL2203524) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant STK33 using myelin basic protein as substrate incubated for 15 mins before initiat... | ACS Med Chem Lett 3: 1034-1038 (2012) Article DOI: 10.1021/ml300246r BindingDB Entry DOI: 10.7270/Q2PK0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50400689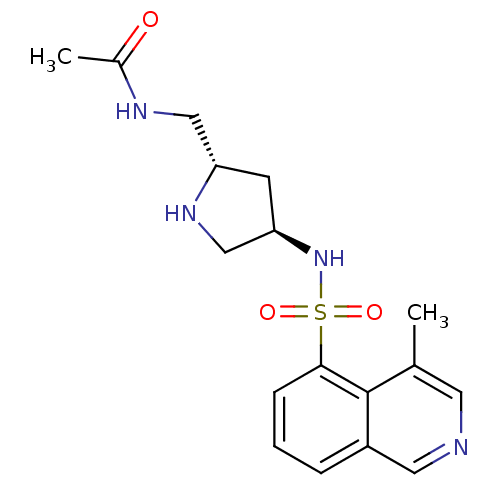 (CHEMBL2203525) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant STK33 using myelin basic protein as substrate incubated for 15 mins before initiat... | ACS Med Chem Lett 3: 1034-1038 (2012) Article DOI: 10.1021/ml300246r BindingDB Entry DOI: 10.7270/Q2PK0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50400664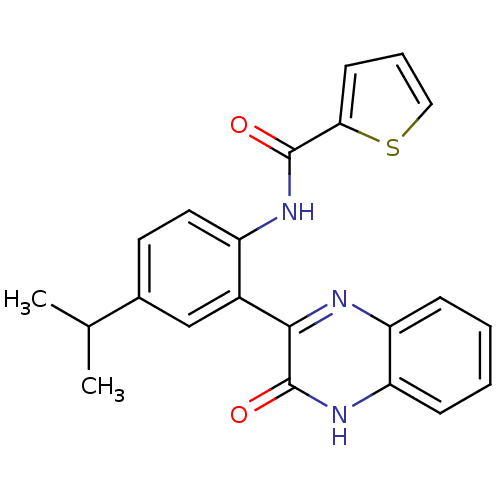 (CHEMBL2204239) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant STK33 using myelin basic protein as substrate incubated for 15 mins before initiat... | ACS Med Chem Lett 3: 1034-1038 (2012) Article DOI: 10.1021/ml300246r BindingDB Entry DOI: 10.7270/Q2PK0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50400684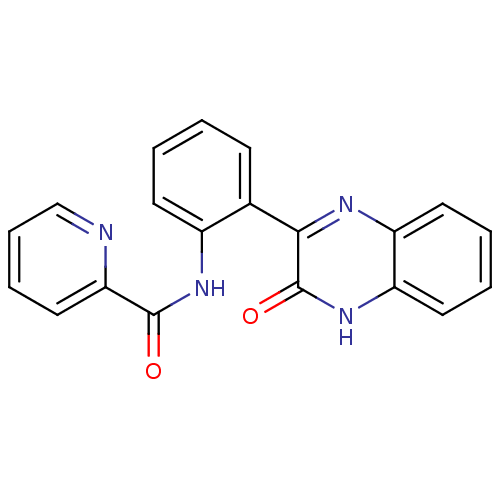 (CHEMBL2203528) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant STK33 using myelin basic protein as substrate incubated for 15 mins before initiat... | ACS Med Chem Lett 3: 1034-1038 (2012) Article DOI: 10.1021/ml300246r BindingDB Entry DOI: 10.7270/Q2PK0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50400662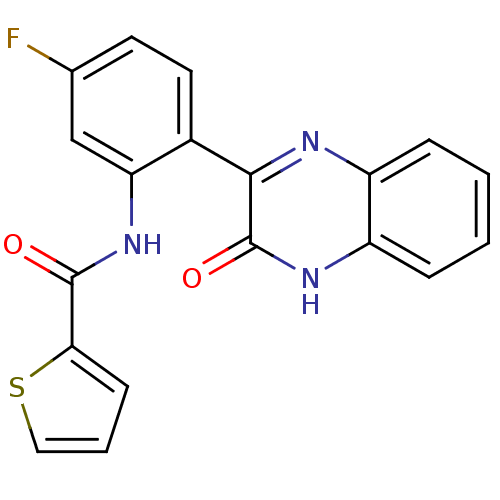 (CHEMBL2204241) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant STK33 using myelin basic protein as substrate incubated for 15 mins before initiat... | ACS Med Chem Lett 3: 1034-1038 (2012) Article DOI: 10.1021/ml300246r BindingDB Entry DOI: 10.7270/Q2PK0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50400665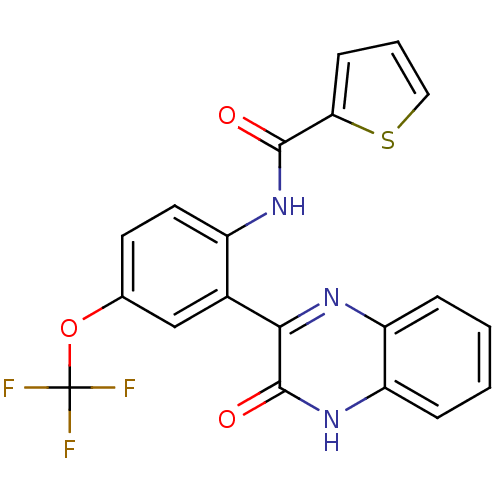 (CHEMBL2204238) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant STK33 using myelin basic protein as substrate incubated for 15 mins before initiat... | ACS Med Chem Lett 3: 1034-1038 (2012) Article DOI: 10.1021/ml300246r BindingDB Entry DOI: 10.7270/Q2PK0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50400682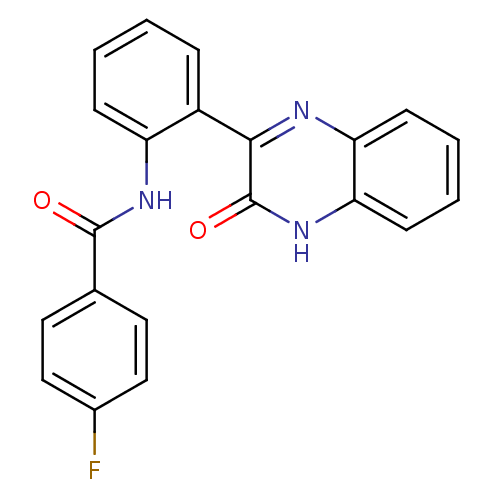 (CHEMBL2203530) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant STK33 using myelin basic protein as substrate incubated for 15 mins before initiat... | ACS Med Chem Lett 3: 1034-1038 (2012) Article DOI: 10.1021/ml300246r BindingDB Entry DOI: 10.7270/Q2PK0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50400668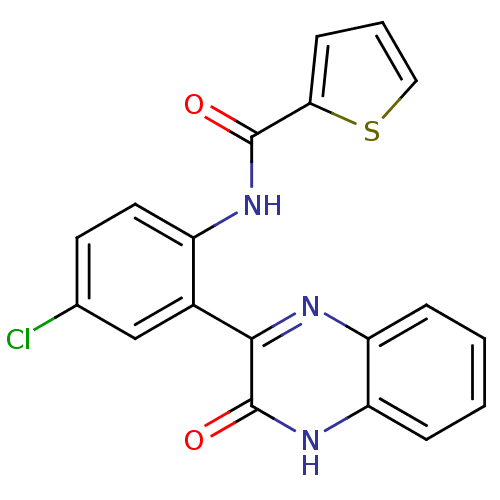 (CHEMBL2204235) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant STK33 using myelin basic protein as substrate incubated for 15 mins before initiat... | ACS Med Chem Lett 3: 1034-1038 (2012) Article DOI: 10.1021/ml300246r BindingDB Entry DOI: 10.7270/Q2PK0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50400687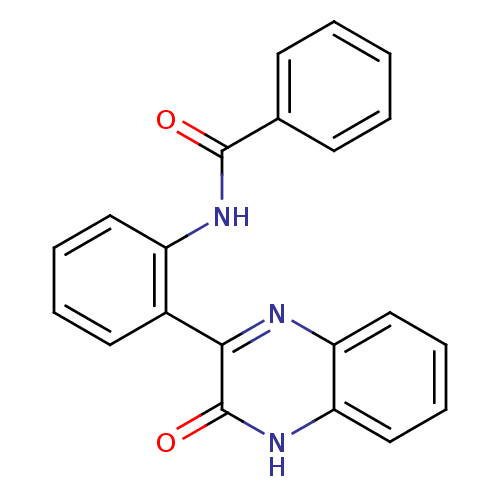 (CHEMBL1992679) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant STK33 using myelin basic protein as substrate incubated for 15 mins before initiat... | ACS Med Chem Lett 3: 1034-1038 (2012) Article DOI: 10.1021/ml300246r BindingDB Entry DOI: 10.7270/Q2PK0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50400666 (CHEMBL2204237) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant STK33 using myelin basic protein as substrate incubated for 15 mins before initiat... | ACS Med Chem Lett 3: 1034-1038 (2012) Article DOI: 10.1021/ml300246r BindingDB Entry DOI: 10.7270/Q2PK0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50400663 (CHEMBL2204240) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant STK33 using myelin basic protein as substrate incubated for 15 mins before initiat... | ACS Med Chem Lett 3: 1034-1038 (2012) Article DOI: 10.1021/ml300246r BindingDB Entry DOI: 10.7270/Q2PK0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM67675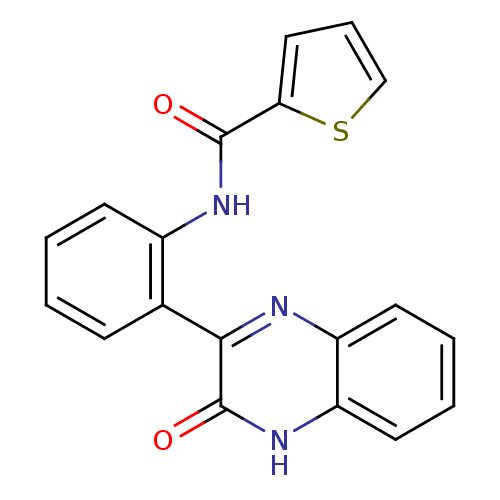 (CHEMBL1543343 | MLS001221673 | N-[2-(3-keto-4H-qui...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant STK33 using myelin basic protein as substrate incubated for 15 mins before initiat... | ACS Med Chem Lett 3: 1034-1038 (2012) Article DOI: 10.1021/ml300246r BindingDB Entry DOI: 10.7270/Q2PK0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50400681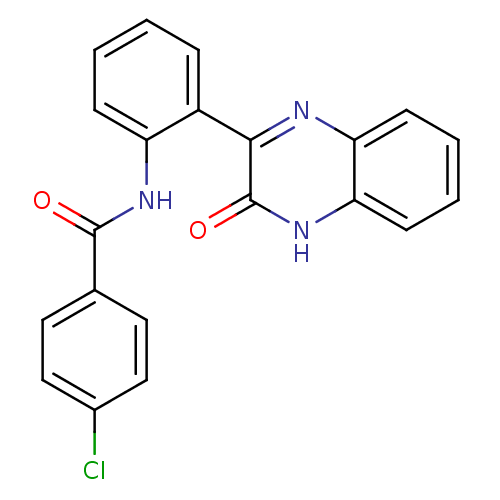 (CHEMBL2203531) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant STK33 using myelin basic protein as substrate incubated for 15 mins before initiat... | ACS Med Chem Lett 3: 1034-1038 (2012) Article DOI: 10.1021/ml300246r BindingDB Entry DOI: 10.7270/Q2PK0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50400686 (CHEMBL2203526) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant STK33 using myelin basic protein as substrate incubated for 15 mins before initiat... | ACS Med Chem Lett 3: 1034-1038 (2012) Article DOI: 10.1021/ml300246r BindingDB Entry DOI: 10.7270/Q2PK0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50400667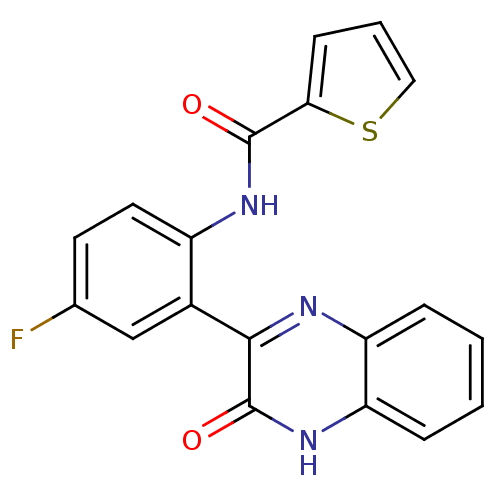 (CHEMBL2204236) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant STK33 using myelin basic protein as substrate incubated for 15 mins before initiat... | ACS Med Chem Lett 3: 1034-1038 (2012) Article DOI: 10.1021/ml300246r BindingDB Entry DOI: 10.7270/Q2PK0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50400685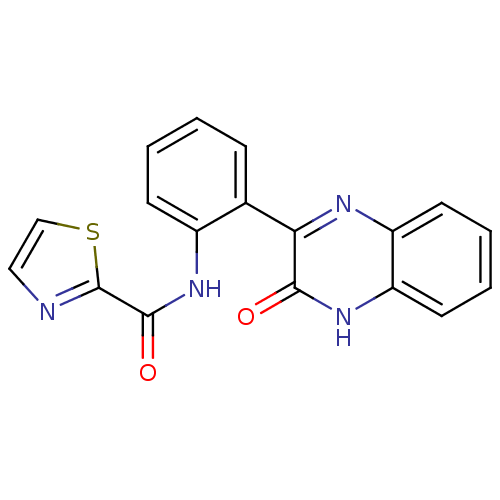 (CHEMBL2203527) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant STK33 using myelin basic protein as substrate incubated for 15 mins before initiat... | ACS Med Chem Lett 3: 1034-1038 (2012) Article DOI: 10.1021/ml300246r BindingDB Entry DOI: 10.7270/Q2PK0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50400683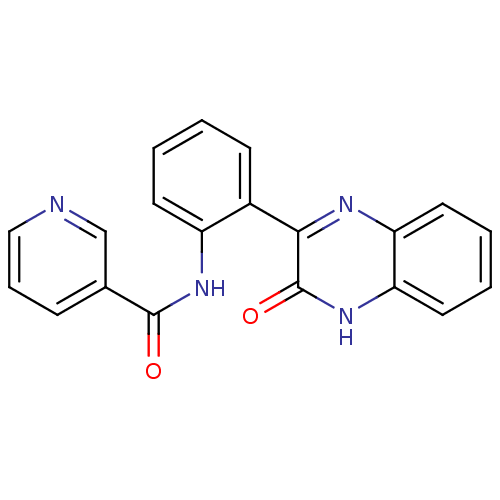 (CHEMBL2203529) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant STK33 using myelin basic protein as substrate incubated for 15 mins before initiat... | ACS Med Chem Lett 3: 1034-1038 (2012) Article DOI: 10.1021/ml300246r BindingDB Entry DOI: 10.7270/Q2PK0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50400680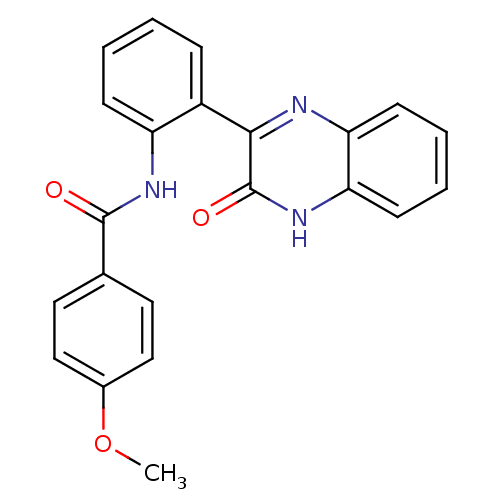 (CHEMBL2203532) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant STK33 using myelin basic protein as substrate incubated for 15 mins before initiat... | ACS Med Chem Lett 3: 1034-1038 (2012) Article DOI: 10.1021/ml300246r BindingDB Entry DOI: 10.7270/Q2PK0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50400669 (CHEMBL2204234) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant STK33 using myelin basic protein as substrate incubated for 15 mins before initiat... | ACS Med Chem Lett 3: 1034-1038 (2012) Article DOI: 10.1021/ml300246r BindingDB Entry DOI: 10.7270/Q2PK0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50400670 (CHEMBL2204233) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant STK33 using myelin basic protein as substrate incubated for 15 mins before initiat... | ACS Med Chem Lett 3: 1034-1038 (2012) Article DOI: 10.1021/ml300246r BindingDB Entry DOI: 10.7270/Q2PK0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50400676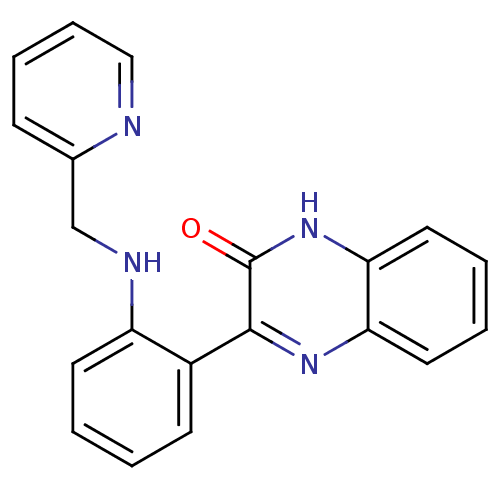 (CHEMBL2203536) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant STK33 using myelin basic protein as substrate incubated for 15 mins before initiat... | ACS Med Chem Lett 3: 1034-1038 (2012) Article DOI: 10.1021/ml300246r BindingDB Entry DOI: 10.7270/Q2PK0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50400671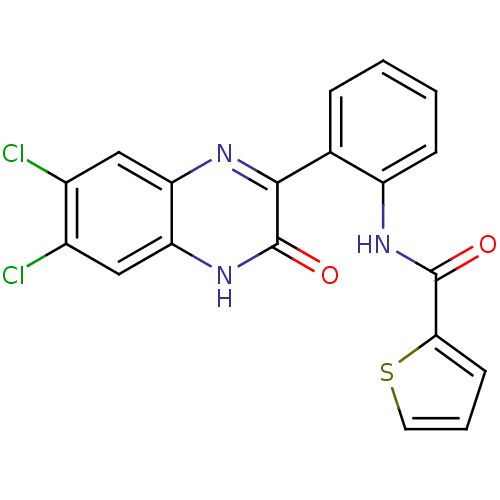 (CHEMBL2204232) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant STK33 using myelin basic protein as substrate incubated for 15 mins before initiat... | ACS Med Chem Lett 3: 1034-1038 (2012) Article DOI: 10.1021/ml300246r BindingDB Entry DOI: 10.7270/Q2PK0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50400672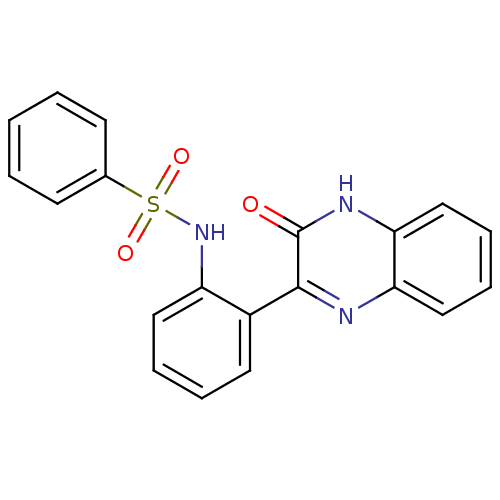 (CHEMBL2204231) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant STK33 using myelin basic protein as substrate incubated for 15 mins before initiat... | ACS Med Chem Lett 3: 1034-1038 (2012) Article DOI: 10.1021/ml300246r BindingDB Entry DOI: 10.7270/Q2PK0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50400679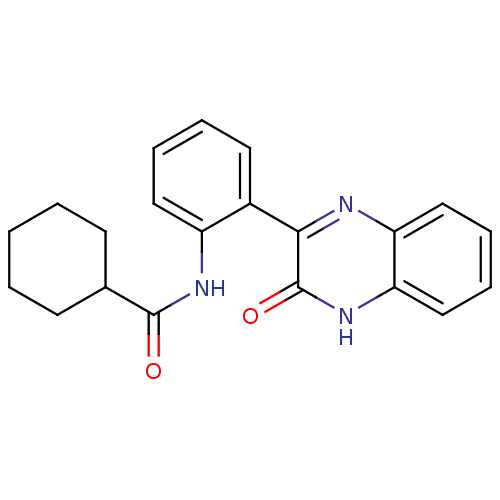 (CHEMBL2203533) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant STK33 using myelin basic protein as substrate incubated for 15 mins before initiat... | ACS Med Chem Lett 3: 1034-1038 (2012) Article DOI: 10.1021/ml300246r BindingDB Entry DOI: 10.7270/Q2PK0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50400678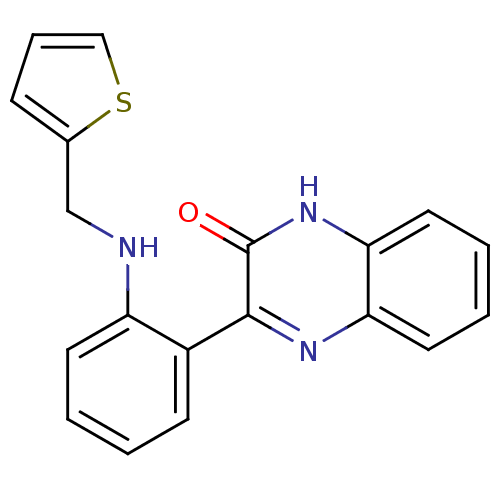 (CHEMBL2203534) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant STK33 using myelin basic protein as substrate incubated for 15 mins before initiat... | ACS Med Chem Lett 3: 1034-1038 (2012) Article DOI: 10.1021/ml300246r BindingDB Entry DOI: 10.7270/Q2PK0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50400675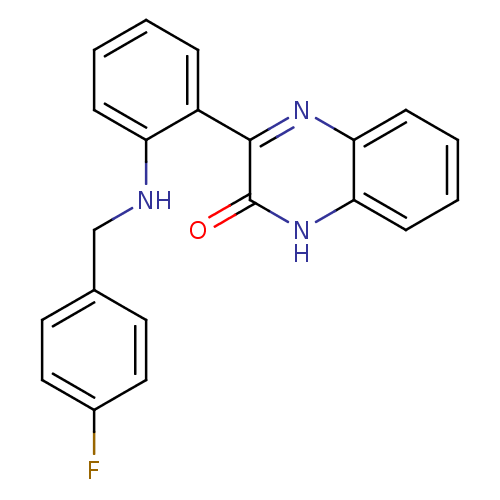 (CHEMBL2204228) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant STK33 using myelin basic protein as substrate incubated for 15 mins before initiat... | ACS Med Chem Lett 3: 1034-1038 (2012) Article DOI: 10.1021/ml300246r BindingDB Entry DOI: 10.7270/Q2PK0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 54 total ) | Next | Last >> |