

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (Homo sapiens (Human)) | BDBM50318924 (2-(3,5-bis(trifluoromethyl)phenyl)-N-(4-(2-chlorop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 3405-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.008 BindingDB Entry DOI: 10.7270/Q2S75GHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50318925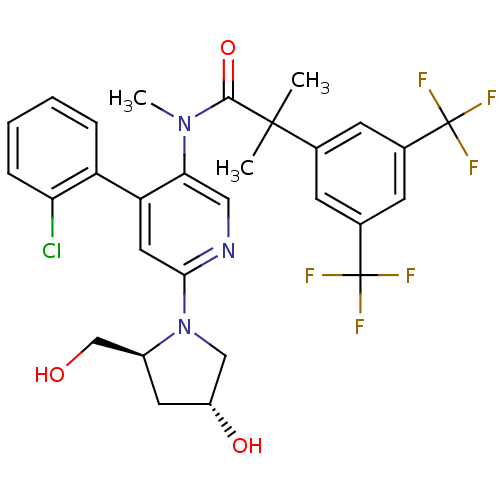 (2-(3,5-bis(trifluoromethyl)phenyl)-N-(4-(2-chlorop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 3405-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.008 BindingDB Entry DOI: 10.7270/Q2S75GHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50318922 (2-(3,5-bis(trifluoromethyl)phenyl)-N-(4-(2-chlorop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]SR142801 from human NK3 receptor expressed in HEK-293-EBNA cells | Bioorg Med Chem Lett 20: 3405-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.008 BindingDB Entry DOI: 10.7270/Q2S75GHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50318930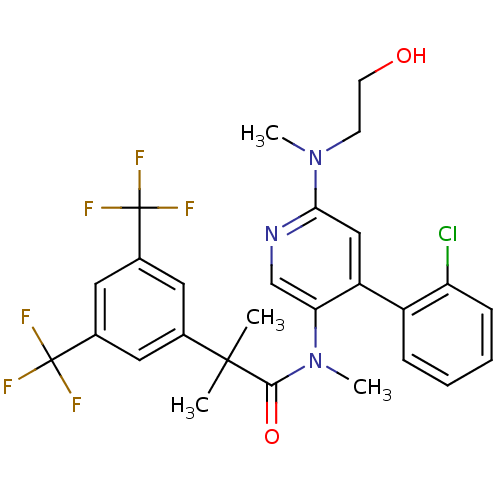 (2-(3,5-bis(trifluoromethyl)phenyl)-N-(4-(2-chlorop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 3405-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.008 BindingDB Entry DOI: 10.7270/Q2S75GHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50318919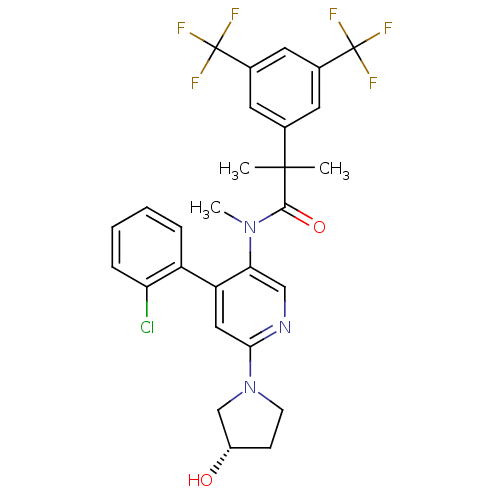 (2-(3,5-bis(trifluoromethyl)phenyl)-N-(4-(2-chlorop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 3405-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.008 BindingDB Entry DOI: 10.7270/Q2S75GHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50318926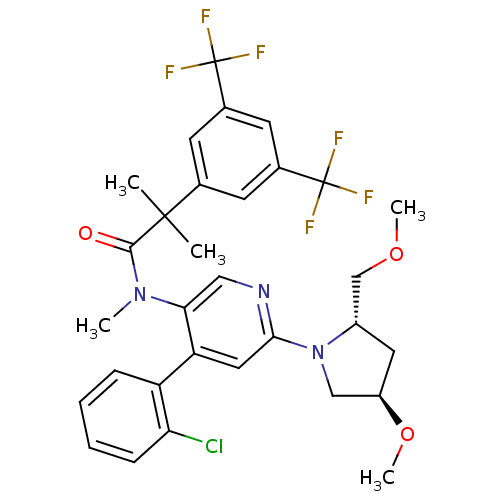 (2-(3,5-bis(trifluoromethyl)phenyl)-N-(4-(2-chlorop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 3405-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.008 BindingDB Entry DOI: 10.7270/Q2S75GHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50318915 (CHEMBL1082737 | N-(6-(bis(2-hydroxyethyl)amino)-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]SR142801 from human NK3 receptor expressed in HEK-293-EBNA cells | Bioorg Med Chem Lett 20: 3405-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.008 BindingDB Entry DOI: 10.7270/Q2S75GHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50318927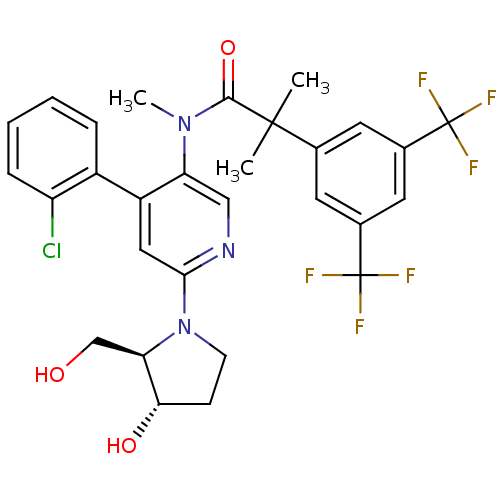 (2-(3,5-bis(trifluoromethyl)phenyl)-N-(4-(2-chlorop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]SR142801 from human NK3 receptor expressed in HEK-293-EBNA cells | Bioorg Med Chem Lett 20: 3405-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.008 BindingDB Entry DOI: 10.7270/Q2S75GHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50318916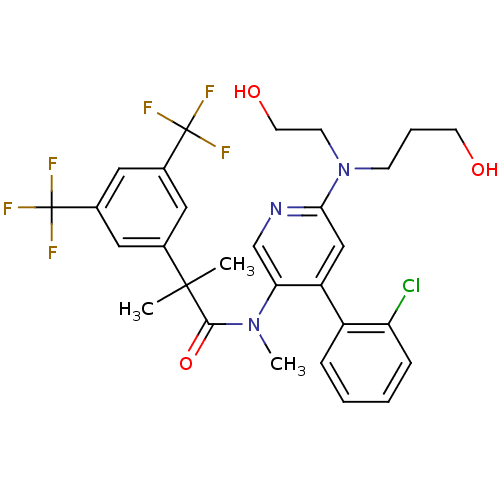 (2-(3,5-bis(trifluoromethyl)phenyl)-N-(4-(2-chlorop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]SR142801 from human NK3 receptor expressed in HEK-293-EBNA cells | Bioorg Med Chem Lett 20: 3405-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.008 BindingDB Entry DOI: 10.7270/Q2S75GHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50318922 (2-(3,5-bis(trifluoromethyl)phenyl)-N-(4-(2-chlorop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 3405-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.008 BindingDB Entry DOI: 10.7270/Q2S75GHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50371462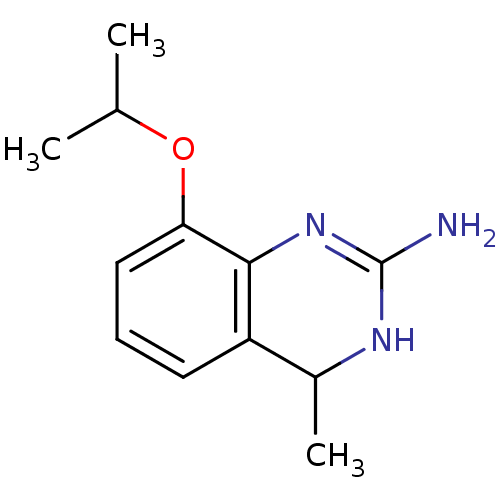 (CHEMBL258075) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human recombinant 5HT7 receptor expressed in HEK293-EBNA cells | Bioorg Med Chem Lett 18: 256-61 (2008) Article DOI: 10.1016/j.bmcl.2007.10.080 BindingDB Entry DOI: 10.7270/Q2KD1ZQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50318928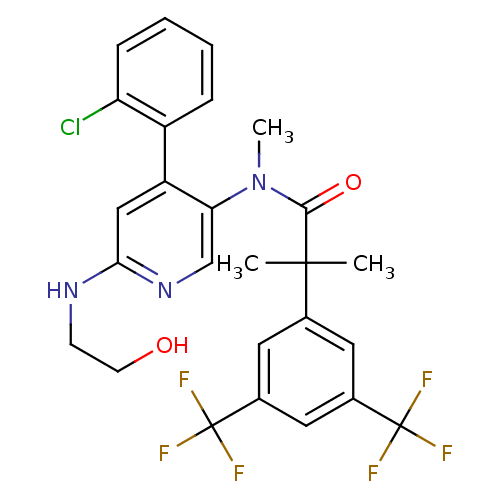 (2-(3,5-bis(trifluoromethyl)phenyl)-N-(4-(2-chlorop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 3405-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.008 BindingDB Entry DOI: 10.7270/Q2S75GHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50318925 (2-(3,5-bis(trifluoromethyl)phenyl)-N-(4-(2-chlorop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]SR142801 from human NK3 receptor expressed in HEK-293-EBNA cells | Bioorg Med Chem Lett 20: 3405-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.008 BindingDB Entry DOI: 10.7270/Q2S75GHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50318915 (CHEMBL1082737 | N-(6-(bis(2-hydroxyethyl)amino)-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 3405-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.008 BindingDB Entry DOI: 10.7270/Q2S75GHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (RAT) | BDBM50371998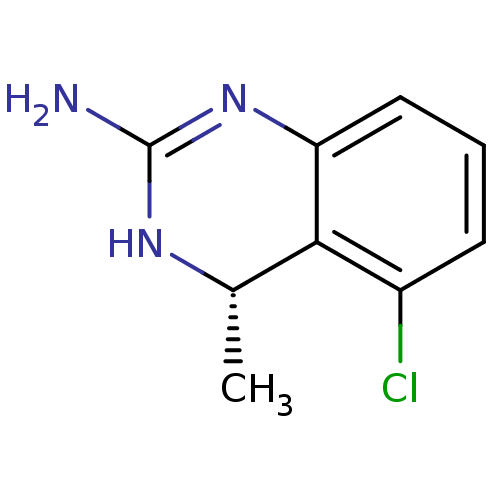 (CHEMBL404372) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to rat 5HT5A receptor | Bioorg Med Chem Lett 18: 262-6 (2008) Article DOI: 10.1016/j.bmcl.2007.10.078 BindingDB Entry DOI: 10.7270/Q2FJ2HNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50318916 (2-(3,5-bis(trifluoromethyl)phenyl)-N-(4-(2-chlorop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 3405-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.008 BindingDB Entry DOI: 10.7270/Q2S75GHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM180718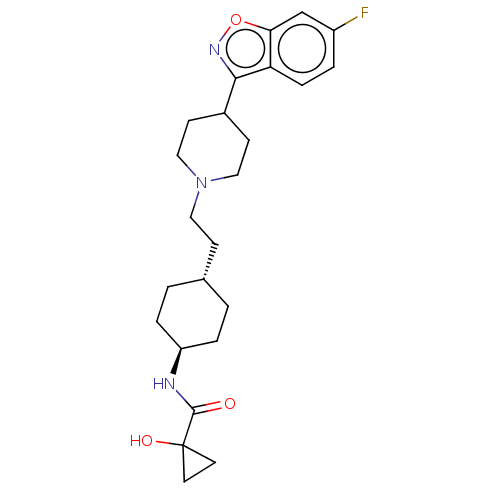 (US8829029, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... | US Patent US8829029 (2014) BindingDB Entry DOI: 10.7270/Q22B8WSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50318924 (2-(3,5-bis(trifluoromethyl)phenyl)-N-(4-(2-chlorop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]SR142801 from human NK3 receptor expressed in HEK-293-EBNA cells | Bioorg Med Chem Lett 20: 3405-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.008 BindingDB Entry DOI: 10.7270/Q2S75GHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50318923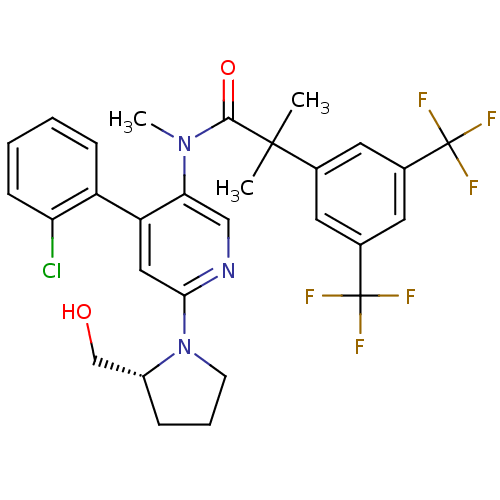 (2-(3,5-bis(trifluoromethyl)phenyl)-N-(4-(2-chlorop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 3405-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.008 BindingDB Entry DOI: 10.7270/Q2S75GHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50318927 (2-(3,5-bis(trifluoromethyl)phenyl)-N-(4-(2-chlorop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 3405-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.008 BindingDB Entry DOI: 10.7270/Q2S75GHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50371998 (CHEMBL404372) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to human 5HT5A receptor | Bioorg Med Chem Lett 18: 262-6 (2008) Article DOI: 10.1016/j.bmcl.2007.10.078 BindingDB Entry DOI: 10.7270/Q2FJ2HNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM180713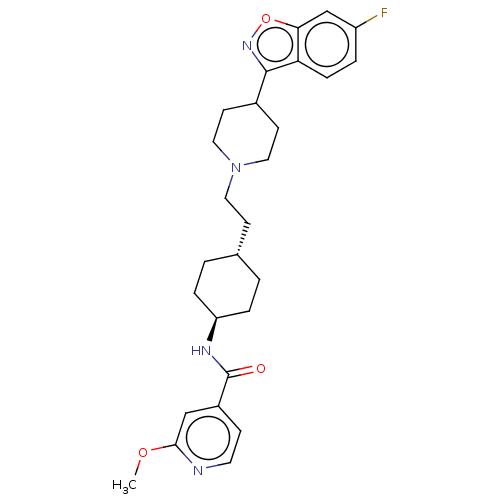 (US8829029, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... | US Patent US8829029 (2014) BindingDB Entry DOI: 10.7270/Q22B8WSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM180752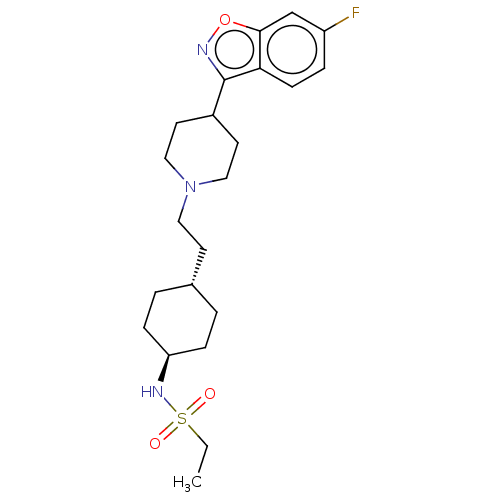 (US8829029, 47) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... | US Patent US8829029 (2014) BindingDB Entry DOI: 10.7270/Q22B8WSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM180723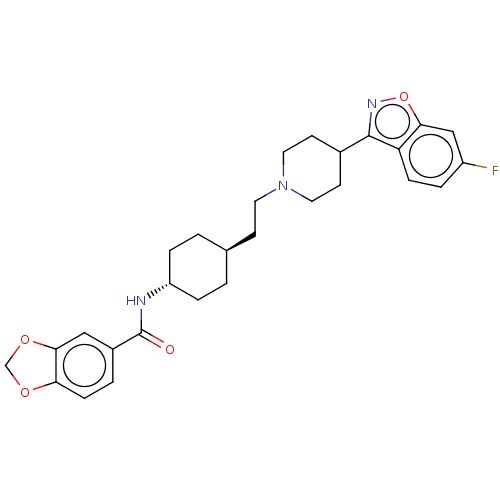 (US8829029, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... | US Patent US8829029 (2014) BindingDB Entry DOI: 10.7270/Q22B8WSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50318917 (2-(3,5-bis(trifluoromethyl)phenyl)-N-(4-(2-chlorop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 3405-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.008 BindingDB Entry DOI: 10.7270/Q2S75GHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM180709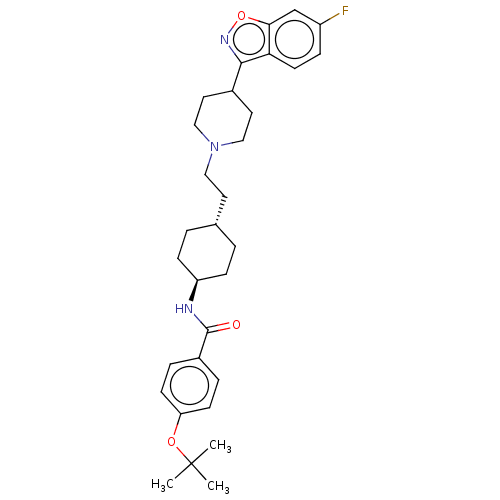 (US8829029, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... | US Patent US8829029 (2014) BindingDB Entry DOI: 10.7270/Q22B8WSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM180698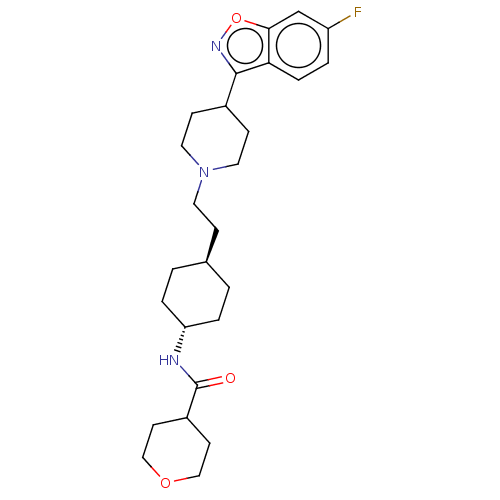 (US8829029, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... | US Patent US8829029 (2014) BindingDB Entry DOI: 10.7270/Q22B8WSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50371981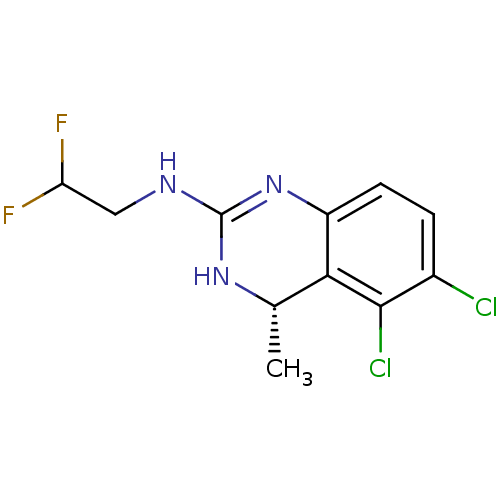 (CHEMBL256694) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to human 5HT5A receptor | Bioorg Med Chem Lett 18: 262-6 (2008) Article DOI: 10.1016/j.bmcl.2007.10.078 BindingDB Entry DOI: 10.7270/Q2FJ2HNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM180699 (US8829029, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... | US Patent US8829029 (2014) BindingDB Entry DOI: 10.7270/Q22B8WSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM180755 (US8829029, 50) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... | US Patent US8829029 (2014) BindingDB Entry DOI: 10.7270/Q22B8WSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50371464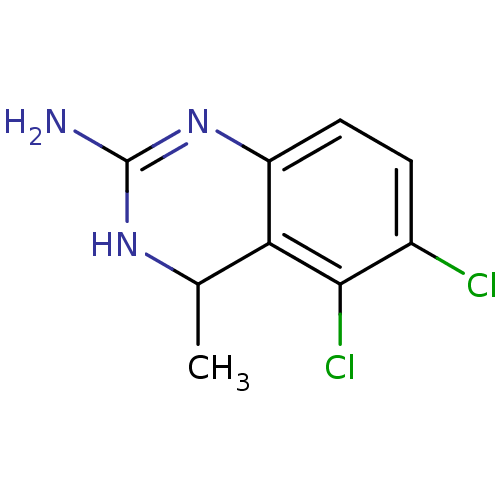 (CHEMBL273170) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human recombinant 5HT5A receptor expressed in HEK293-EBNA cells | Bioorg Med Chem Lett 18: 256-61 (2008) Article DOI: 10.1016/j.bmcl.2007.10.080 BindingDB Entry DOI: 10.7270/Q2KD1ZQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM180707 (US8829029, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... | US Patent US8829029 (2014) BindingDB Entry DOI: 10.7270/Q22B8WSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM180697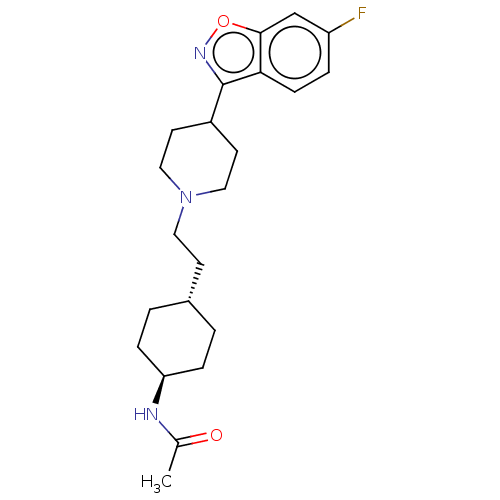 (US8829029, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... | US Patent US8829029 (2014) BindingDB Entry DOI: 10.7270/Q22B8WSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50318932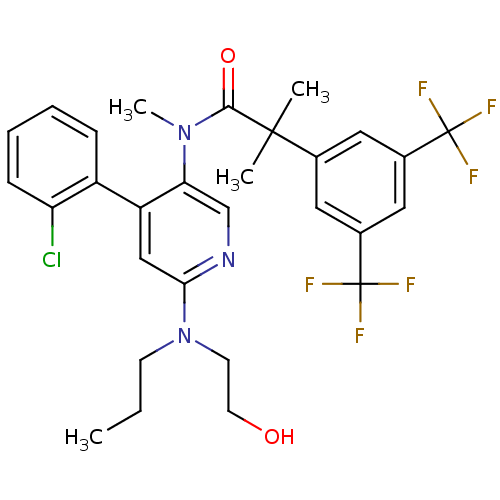 (2-(3,5-bis(trifluoromethyl)phenyl)-N-(4-(2-chlorop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 3405-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.008 BindingDB Entry DOI: 10.7270/Q2S75GHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM180719 (US8829029, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... | US Patent US8829029 (2014) BindingDB Entry DOI: 10.7270/Q22B8WSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50318921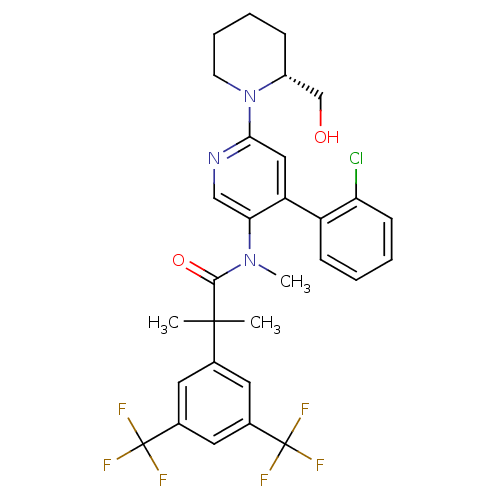 (2-(3,5-bis(trifluoromethyl)phenyl)-N-(4-(2-chlorop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 3405-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.008 BindingDB Entry DOI: 10.7270/Q2S75GHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50371461 (CHEMBL402179) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human recombinant 5HT7 receptor expressed in HEK293-EBNA cells | Bioorg Med Chem Lett 18: 256-61 (2008) Article DOI: 10.1016/j.bmcl.2007.10.080 BindingDB Entry DOI: 10.7270/Q2KD1ZQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50371459 (CHEMBL272781) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human recombinant 5HT7 receptor expressed in HEK293-EBNA cells | Bioorg Med Chem Lett 18: 256-61 (2008) Article DOI: 10.1016/j.bmcl.2007.10.080 BindingDB Entry DOI: 10.7270/Q2KD1ZQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM180724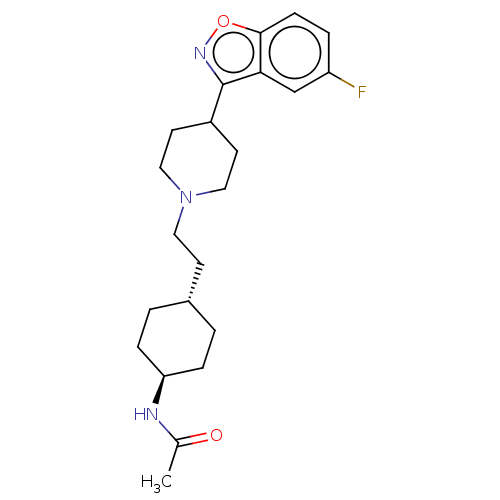 (US8829029, 24) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... | US Patent US8829029 (2014) BindingDB Entry DOI: 10.7270/Q22B8WSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM180717 (US8829029, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... | US Patent US8829029 (2014) BindingDB Entry DOI: 10.7270/Q22B8WSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM180704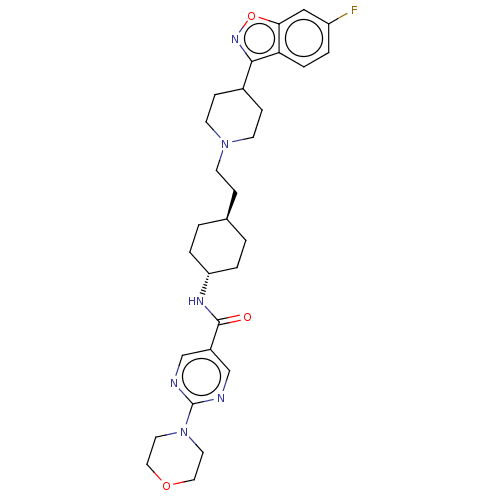 (US8829029, 4D) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... | US Patent US8829029 (2014) BindingDB Entry DOI: 10.7270/Q22B8WSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM180712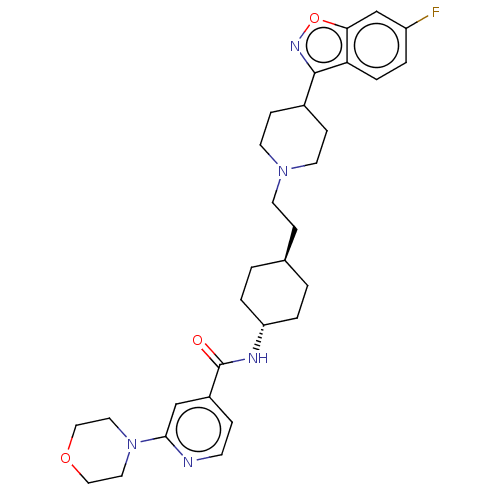 (US8829029, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... | US Patent US8829029 (2014) BindingDB Entry DOI: 10.7270/Q22B8WSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM180755 (US8829029, 50) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... | US Patent US8829029 (2014) BindingDB Entry DOI: 10.7270/Q22B8WSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM180711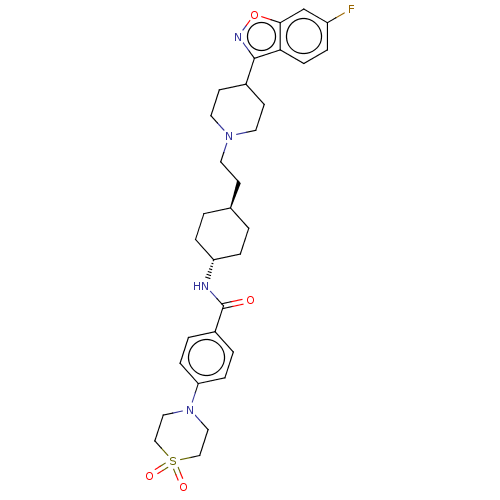 (US8829029, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... | US Patent US8829029 (2014) BindingDB Entry DOI: 10.7270/Q22B8WSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50371460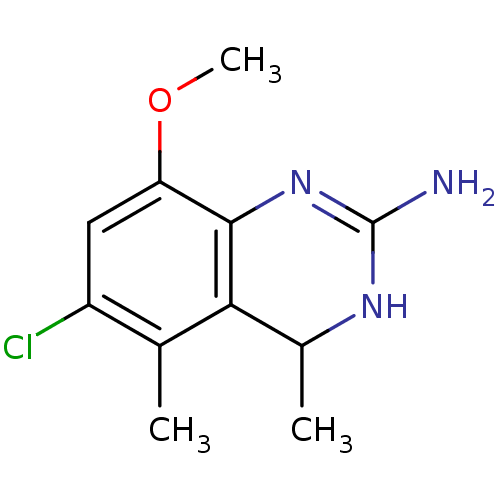 (CHEMBL401745) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human recombinant 5HT7 receptor expressed in HEK293-EBNA cells | Bioorg Med Chem Lett 18: 256-61 (2008) Article DOI: 10.1016/j.bmcl.2007.10.080 BindingDB Entry DOI: 10.7270/Q2KD1ZQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM180715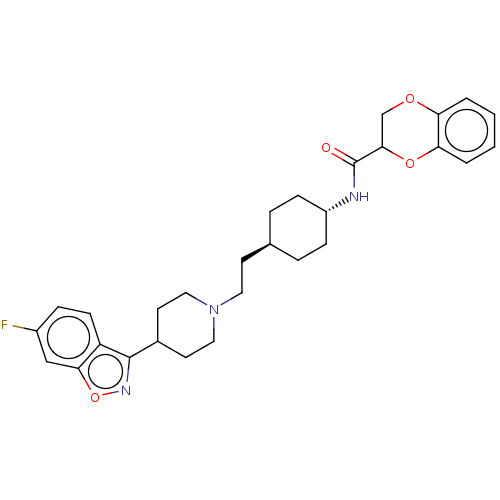 (US8829029, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... | US Patent US8829029 (2014) BindingDB Entry DOI: 10.7270/Q22B8WSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM180714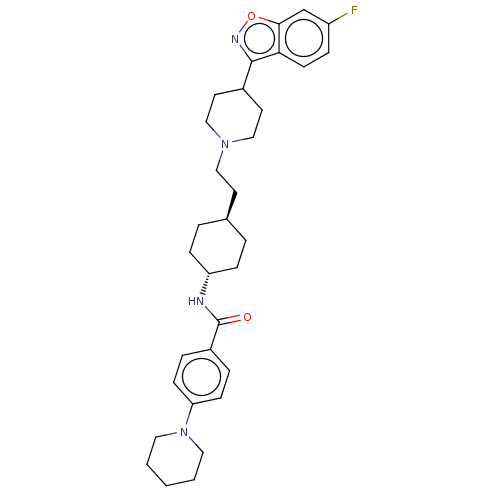 (US8829029, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... | US Patent US8829029 (2014) BindingDB Entry DOI: 10.7270/Q22B8WSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50371981 (CHEMBL256694) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to histamine H1 receptor | Bioorg Med Chem Lett 18: 262-6 (2008) Article DOI: 10.1016/j.bmcl.2007.10.078 BindingDB Entry DOI: 10.7270/Q2FJ2HNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM180717 (US8829029, 17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Aliquots of membrane preparations were thawed at RT, resuspended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KC... | US Patent US8829029 (2014) BindingDB Entry DOI: 10.7270/Q22B8WSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50318918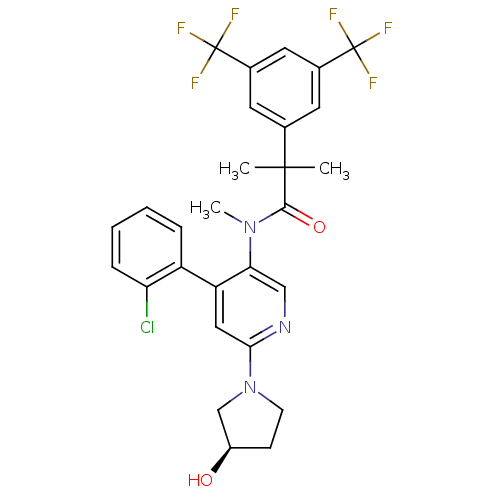 (2-(3,5-bis(trifluoromethyl)phenyl)-N-(4-(2-chlorop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 3405-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.008 BindingDB Entry DOI: 10.7270/Q2S75GHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 424 total ) | Next | Last >> |