 Found 330 hits with Last Name = 'swartz' and Initial = 's'
Found 330 hits with Last Name = 'swartz' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50199865
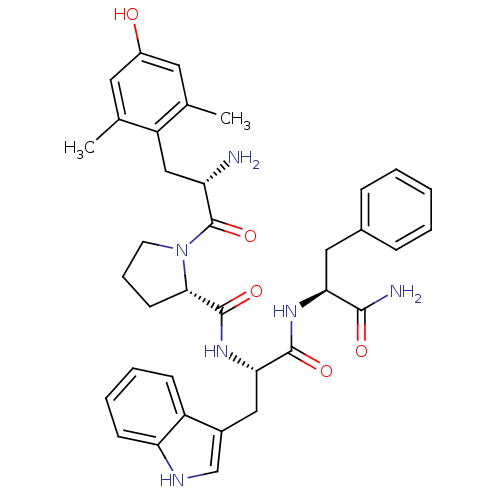
((S)-N-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-y...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C36H42N6O5/c1-21-15-25(43)16-22(2)27(21)19-28(37)36(47)42-14-8-13-32(42)35(46)41-31(18-24-20-39-29-12-7-6-11-26(24)29)34(45)40-30(33(38)44)17-23-9-4-3-5-10-23/h3-7,9-12,15-16,20,28,30-32,39,43H,8,13-14,17-19,37H2,1-2H3,(H2,38,44)(H,40,45)(H,41,46)/t28-,30-,31-,32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain P2 synaptosome |
Bioorg Med Chem 15: 1237-51 (2007)
Article DOI: 10.1016/j.bmc.2006.11.019
BindingDB Entry DOI: 10.7270/Q2348K1F |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50199863
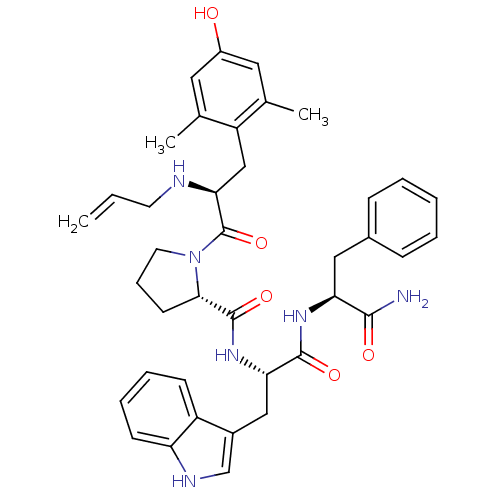
((S)-1-((S)-2-(allylamino)-3-(4-hydroxy-2,6-dimethy...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](NCC=C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C39H46N6O5/c1-4-16-41-34(22-30-24(2)18-28(46)19-25(30)3)39(50)45-17-10-15-35(45)38(49)44-33(21-27-23-42-31-14-9-8-13-29(27)31)37(48)43-32(36(40)47)20-26-11-6-5-7-12-26/h4-9,11-14,18-19,23,32-35,41-42,46H,1,10,15-17,20-22H2,2-3H3,(H2,40,47)(H,43,48)(H,44,49)/t32-,33-,34-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain P2 synaptosome |
Bioorg Med Chem 15: 1237-51 (2007)
Article DOI: 10.1016/j.bmc.2006.11.019
BindingDB Entry DOI: 10.7270/Q2348K1F |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50199861
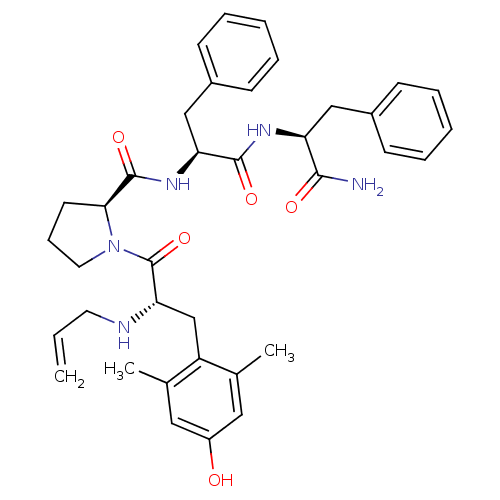
((S)-1-((S)-2-(allylamino)-3-(4-hydroxy-2,6-dimethy...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](NCC=C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C37H45N5O5/c1-4-17-39-32(23-29-24(2)19-28(43)20-25(29)3)37(47)42-18-11-16-33(42)36(46)41-31(22-27-14-9-6-10-15-27)35(45)40-30(34(38)44)21-26-12-7-5-8-13-26/h4-10,12-15,19-20,30-33,39,43H,1,11,16-18,21-23H2,2-3H3,(H2,38,44)(H,40,45)(H,41,46)/t30-,31-,32-,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain P2 synaptosome |
Bioorg Med Chem 15: 1237-51 (2007)
Article DOI: 10.1016/j.bmc.2006.11.019
BindingDB Entry DOI: 10.7270/Q2348K1F |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50095155

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C34H38N6O5/c35-26(17-22-12-14-24(41)15-13-22)34(45)40-16-6-11-30(40)33(44)39-29(19-23-20-37-27-10-5-4-9-25(23)27)32(43)38-28(31(36)42)18-21-7-2-1-3-8-21/h1-5,7-10,12-15,20,26,28-30,37,41H,6,11,16-19,35H2,(H2,36,42)(H,38,43)(H,39,44)/t26-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain P2 synaptosome |
Bioorg Med Chem 15: 1237-51 (2007)
Article DOI: 10.1016/j.bmc.2006.11.019
BindingDB Entry DOI: 10.7270/Q2348K1F |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50139013

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C32H37N5O5/c33-25(18-23-13-15-24(38)16-14-23)32(42)37-17-7-12-28(37)31(41)36-27(20-22-10-5-2-6-11-22)30(40)35-26(29(34)39)19-21-8-3-1-4-9-21/h1-6,8-11,13-16,25-28,38H,7,12,17-20,33H2,(H2,34,39)(H,35,40)(H,36,41)/t25-,26-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain P2 synaptosome |
Bioorg Med Chem 15: 1237-51 (2007)
Article DOI: 10.1016/j.bmc.2006.11.019
BindingDB Entry DOI: 10.7270/Q2348K1F |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50199859
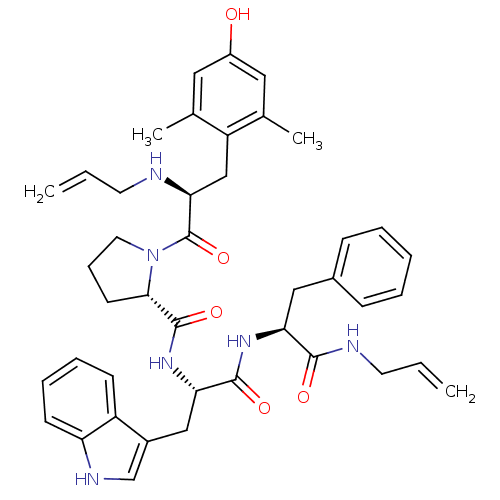
((S)-N-((S)-1-((S)-1-(allylamino)-1-oxo-3-phenylpro...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](NCC=C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC=C Show InChI InChI=1S/C42H50N6O5/c1-5-18-43-37(25-33-27(3)21-31(49)22-28(33)4)42(53)48-20-12-17-38(48)41(52)47-36(24-30-26-45-34-16-11-10-15-32(30)34)40(51)46-35(39(50)44-19-6-2)23-29-13-8-7-9-14-29/h5-11,13-16,21-22,26,35-38,43,45,49H,1-2,12,17-20,23-25H2,3-4H3,(H,44,50)(H,46,51)(H,47,52)/t35-,36-,37-,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain P2 synaptosome |
Bioorg Med Chem 15: 1237-51 (2007)
Article DOI: 10.1016/j.bmc.2006.11.019
BindingDB Entry DOI: 10.7270/Q2348K1F |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50199865

((S)-N-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-y...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C36H42N6O5/c1-21-15-25(43)16-22(2)27(21)19-28(37)36(47)42-14-8-13-32(42)35(46)41-31(18-24-20-39-29-12-7-6-11-26(24)29)34(45)40-30(33(38)44)17-23-9-4-3-5-10-23/h3-7,9-12,15-16,20,28,30-32,39,43H,8,13-14,17-19,37H2,1-2H3,(H2,38,44)(H,40,45)(H,41,46)/t28-,30-,31-,32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Displacement of [3H]deltorphin 2 from delta opioid receptor in rat brain P2 synaptosome |
Bioorg Med Chem 15: 1237-51 (2007)
Article DOI: 10.1016/j.bmc.2006.11.019
BindingDB Entry DOI: 10.7270/Q2348K1F |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50371684
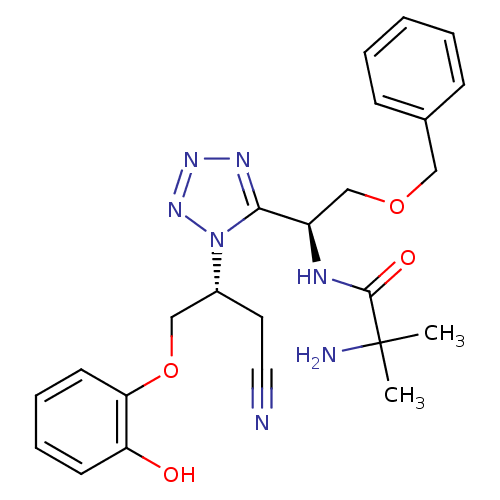
(CHEMBL270666)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccccc1)c1nnnn1[C@@H](COc1ccccc1O)CC#N Show InChI InChI=1S/C24H29N7O4/c1-24(2,26)23(33)27-19(16-34-14-17-8-4-3-5-9-17)22-28-29-30-31(22)18(12-13-25)15-35-21-11-7-6-10-20(21)32/h3-11,18-19,32H,12,14-16,26H2,1-2H3,(H,27,33)/t18-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at human GHS receptor expressed in H4 glioma cells |
Bioorg Med Chem Lett 18: 2067-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.100
BindingDB Entry DOI: 10.7270/Q21G0N4D |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50199867
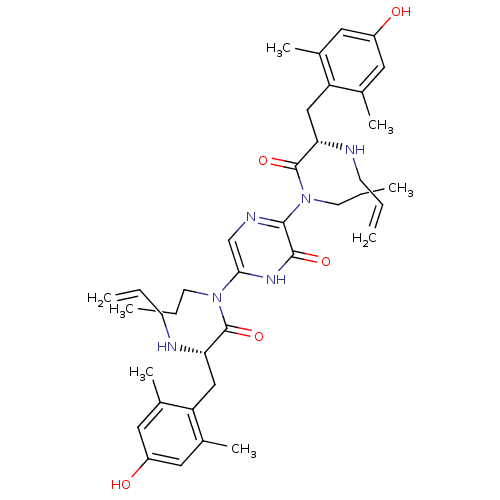
((S)-2-allylamino-N-(5-{[(S)-2-allylamino-3-(4-hydr...)Show SMILES CCCN(C(=O)[C@H](Cc1c(C)cc(O)cc1C)NCC=C)c1cnc(N(CCC)C(=O)[C@H](Cc2c(C)cc(O)cc2C)NCC=C)c(=O)[nH]1 Show InChI InChI=1S/C38H52N6O5/c1-9-13-39-32(21-30-24(5)17-28(45)18-25(30)6)37(48)43(15-11-3)34-23-41-35(36(47)42-34)44(16-12-4)38(49)33(40-14-10-2)22-31-26(7)19-29(46)20-27(31)8/h9-10,17-20,23,32-33,39-40,45-46H,1-2,11-16,21-22H2,3-8H3,(H,42,47)/t32-,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain P2 synaptosome |
Bioorg Med Chem 15: 1237-51 (2007)
Article DOI: 10.1016/j.bmc.2006.11.019
BindingDB Entry DOI: 10.7270/Q2348K1F |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50371688

(CHEMBL404545)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccccc1)c1nnnn1[C@@H](COc1ccccc1)CC#N Show InChI InChI=1S/C24H29N7O3/c1-24(2,26)23(32)27-21(17-33-15-18-9-5-3-6-10-18)22-28-29-30-31(22)19(13-14-25)16-34-20-11-7-4-8-12-20/h3-12,19,21H,13,15-17,26H2,1-2H3,(H,27,32)/t19-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at human GHS receptor expressed in H4 glioma cells |
Bioorg Med Chem Lett 18: 2067-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.100
BindingDB Entry DOI: 10.7270/Q21G0N4D |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50199863

((S)-1-((S)-2-(allylamino)-3-(4-hydroxy-2,6-dimethy...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](NCC=C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C39H46N6O5/c1-4-16-41-34(22-30-24(2)18-28(46)19-25(30)3)39(50)45-17-10-15-35(45)38(49)44-33(21-27-23-42-31-14-9-8-13-29(27)31)37(48)43-32(36(40)47)20-26-11-6-5-7-12-26/h4-9,11-14,18-19,23,32-35,41-42,46H,1,10,15-17,20-22H2,2-3H3,(H2,40,47)(H,43,48)(H,44,49)/t32-,33-,34-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Displacement of [3H]deltorphin 2 from delta opioid receptor in rat brain P2 synaptosome |
Bioorg Med Chem 15: 1237-51 (2007)
Article DOI: 10.1016/j.bmc.2006.11.019
BindingDB Entry DOI: 10.7270/Q2348K1F |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50199862

((S)-2-allylamino-N-{6-[(S)-2-allylamino-3-(4-hydro...)Show SMILES Oc1ccc(C[C@H](NCC=C)C(=O)NCCCCCCNC(=O)[C@H](Cc2ccc(O)cc2)NCC=C)cc1 Show InChI InChI=1S/C30H42N4O4/c1-3-17-31-27(21-23-9-13-25(35)14-10-23)29(37)33-19-7-5-6-8-20-34-30(38)28(32-18-4-2)22-24-11-15-26(36)16-12-24/h3-4,9-16,27-28,31-32,35-36H,1-2,5-8,17-22H2,(H,33,37)(H,34,38)/t27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain P2 synaptosome |
Bioorg Med Chem 15: 1237-51 (2007)
Article DOI: 10.1016/j.bmc.2006.11.019
BindingDB Entry DOI: 10.7270/Q2348K1F |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50199864
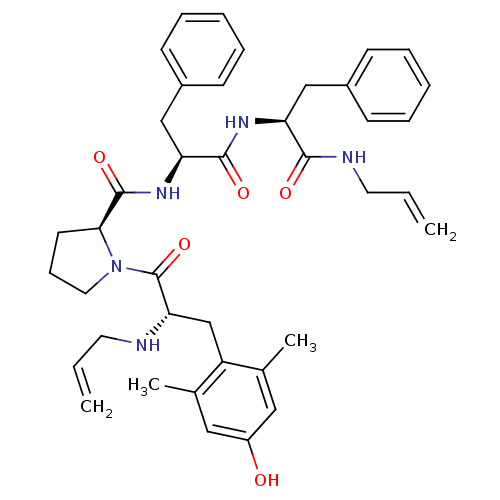
((S)-N-((S)-1-((S)-1-(allylamino)-1-oxo-3-phenylpro...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](NCC=C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC=C Show InChI InChI=1S/C40H49N5O5/c1-5-19-41-35(26-32-27(3)22-31(46)23-28(32)4)40(50)45-21-13-18-36(45)39(49)44-34(25-30-16-11-8-12-17-30)38(48)43-33(37(47)42-20-6-2)24-29-14-9-7-10-15-29/h5-12,14-17,22-23,33-36,41,46H,1-2,13,18-21,24-26H2,3-4H3,(H,42,47)(H,43,48)(H,44,49)/t33-,34-,35-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain P2 synaptosome |
Bioorg Med Chem 15: 1237-51 (2007)
Article DOI: 10.1016/j.bmc.2006.11.019
BindingDB Entry DOI: 10.7270/Q2348K1F |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50371686
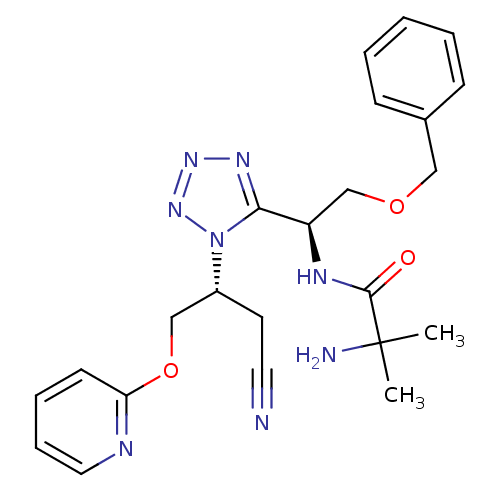
(CHEMBL271876)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccccc1)c1nnnn1[C@@H](COc1ccccn1)CC#N Show InChI InChI=1S/C23H28N8O3/c1-23(2,25)22(32)27-19(16-33-14-17-8-4-3-5-9-17)21-28-29-30-31(21)18(11-12-24)15-34-20-10-6-7-13-26-20/h3-10,13,18-19H,11,14-16,25H2,1-2H3,(H,27,32)/t18-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at human GHS receptor expressed in H4 glioma cells |
Bioorg Med Chem Lett 18: 2067-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.100
BindingDB Entry DOI: 10.7270/Q21G0N4D |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50199862

((S)-2-allylamino-N-{6-[(S)-2-allylamino-3-(4-hydro...)Show SMILES Oc1ccc(C[C@H](NCC=C)C(=O)NCCCCCCNC(=O)[C@H](Cc2ccc(O)cc2)NCC=C)cc1 Show InChI InChI=1S/C30H42N4O4/c1-3-17-31-27(21-23-9-13-25(35)14-10-23)29(37)33-19-7-5-6-8-20-34-30(38)28(32-18-4-2)22-24-11-15-26(36)16-12-24/h3-4,9-16,27-28,31-32,35-36H,1-2,5-8,17-22H2,(H,33,37)(H,34,38)/t27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 51.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Displacement of [3H]deltorphin 2 from delta opioid receptor in rat brain P2 synaptosome |
Bioorg Med Chem 15: 1237-51 (2007)
Article DOI: 10.1016/j.bmc.2006.11.019
BindingDB Entry DOI: 10.7270/Q2348K1F |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50199867

((S)-2-allylamino-N-(5-{[(S)-2-allylamino-3-(4-hydr...)Show SMILES CCCN(C(=O)[C@H](Cc1c(C)cc(O)cc1C)NCC=C)c1cnc(N(CCC)C(=O)[C@H](Cc2c(C)cc(O)cc2C)NCC=C)c(=O)[nH]1 Show InChI InChI=1S/C38H52N6O5/c1-9-13-39-32(21-30-24(5)17-28(45)18-25(30)6)37(48)43(15-11-3)34-23-41-35(36(47)42-34)44(16-12-4)38(49)33(40-14-10-2)22-31-26(7)19-29(46)20-27(31)8/h9-10,17-20,23,32-33,39-40,45-46H,1-2,11-16,21-22H2,3-8H3,(H,42,47)/t32-,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 77.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Displacement of [3H]deltorphin 2 from delta opioid receptor in rat brain P2 synaptosome |
Bioorg Med Chem 15: 1237-51 (2007)
Article DOI: 10.1016/j.bmc.2006.11.019
BindingDB Entry DOI: 10.7270/Q2348K1F |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50199860
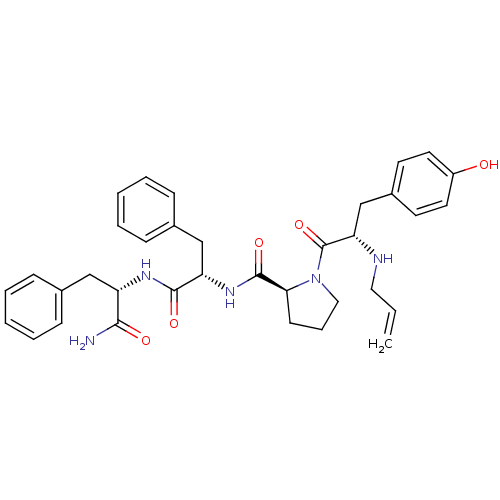
((S)-1-((S)-2-(allylamino)-3-(4-hydroxyphenyl)propa...)Show SMILES NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(O)cc1)NCC=C Show InChI InChI=1S/C35H41N5O5/c1-2-19-37-30(23-26-15-17-27(41)18-16-26)35(45)40-20-9-14-31(40)34(44)39-29(22-25-12-7-4-8-13-25)33(43)38-28(32(36)42)21-24-10-5-3-6-11-24/h2-8,10-13,15-18,28-31,37,41H,1,9,14,19-23H2,(H2,36,42)(H,38,43)(H,39,44)/t28-,29-,30-,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain P2 synaptosome |
Bioorg Med Chem 15: 1237-51 (2007)
Article DOI: 10.1016/j.bmc.2006.11.019
BindingDB Entry DOI: 10.7270/Q2348K1F |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50222142
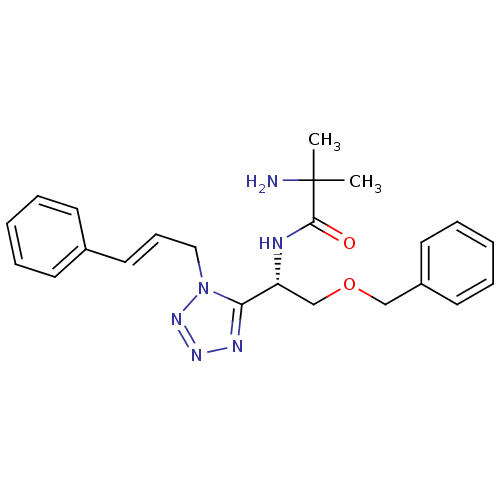
((S,E)-2-amino-N-(2-(benzyloxy)-1-(1-cinnamyl-1H-te...)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccccc1)c1nnnn1C\C=C\c1ccccc1 Show InChI InChI=1S/C23H28N6O2/c1-23(2,24)22(30)25-20(17-31-16-19-12-7-4-8-13-19)21-26-27-28-29(21)15-9-14-18-10-5-3-6-11-18/h3-14,20H,15-17,24H2,1-2H3,(H,25,30)/b14-9+/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHSR1a after 1 hr |
Bioorg Med Chem Lett 17: 5928-33 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.099
BindingDB Entry DOI: 10.7270/Q28P607D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50371687

(CHEMBL272085)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccccc1)c1nnnn1[C@H](COc1ccccc1)CC#N Show InChI InChI=1S/C24H29N7O3/c1-24(2,26)23(32)27-21(17-33-15-18-9-5-3-6-10-18)22-28-29-30-31(22)19(13-14-25)16-34-20-11-7-4-8-12-20/h3-12,19,21H,13,15-17,26H2,1-2H3,(H,27,32)/t19-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 197 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at human GHS receptor expressed in H4 glioma cells |
Bioorg Med Chem Lett 18: 2067-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.100
BindingDB Entry DOI: 10.7270/Q21G0N4D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50222164
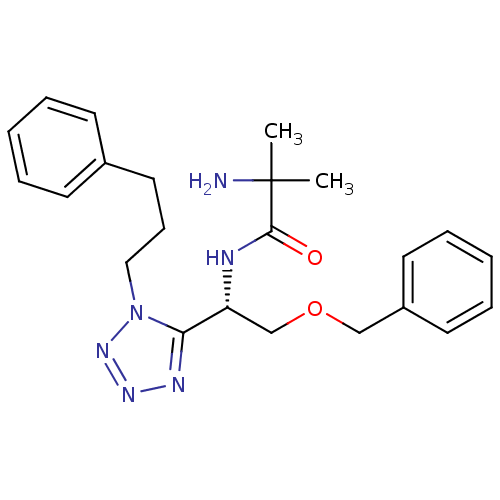
((S)-2-amino-N-(2-(benzyloxy)-1-(1-(3-phenylpropyl)...)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccccc1)c1nnnn1CCCc1ccccc1 Show InChI InChI=1S/C23H30N6O2/c1-23(2,24)22(30)25-20(17-31-16-19-12-7-4-8-13-19)21-26-27-28-29(21)15-9-14-18-10-5-3-6-11-18/h3-8,10-13,20H,9,14-17,24H2,1-2H3,(H,25,30)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at human GHS receptor expressed in H4 glioma cells |
Bioorg Med Chem Lett 18: 2067-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.100
BindingDB Entry DOI: 10.7270/Q21G0N4D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50222164

((S)-2-amino-N-(2-(benzyloxy)-1-(1-(3-phenylpropyl)...)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccccc1)c1nnnn1CCCc1ccccc1 Show InChI InChI=1S/C23H30N6O2/c1-23(2,24)22(30)25-20(17-31-16-19-12-7-4-8-13-19)21-26-27-28-29(21)15-9-14-18-10-5-3-6-11-18/h3-8,10-13,20H,9,14-17,24H2,1-2H3,(H,25,30)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHSR1a after 1 hr |
Bioorg Med Chem Lett 17: 5928-33 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.099
BindingDB Entry DOI: 10.7270/Q28P607D |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50199859

((S)-N-((S)-1-((S)-1-(allylamino)-1-oxo-3-phenylpro...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](NCC=C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC=C Show InChI InChI=1S/C42H50N6O5/c1-5-18-43-37(25-33-27(3)21-31(49)22-28(33)4)42(53)48-20-12-17-38(48)41(52)47-36(24-30-26-45-34-16-11-10-15-32(30)34)40(51)46-35(39(50)44-19-6-2)23-29-13-8-7-9-14-29/h5-11,13-16,21-22,26,35-38,43,45,49H,1-2,12,17-20,23-25H2,3-4H3,(H,44,50)(H,46,51)(H,47,52)/t35-,36-,37-,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 217 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Displacement of [3H]deltorphin 2 from delta opioid receptor in rat brain P2 synaptosome |
Bioorg Med Chem 15: 1237-51 (2007)
Article DOI: 10.1016/j.bmc.2006.11.019
BindingDB Entry DOI: 10.7270/Q2348K1F |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50371683

(CHEMBL410060)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccccc1)c1nnnn1[C@H](COc1ccccc1O)CC#N Show InChI InChI=1S/C24H29N7O4/c1-24(2,26)23(33)27-19(16-34-14-17-8-4-3-5-9-17)22-28-29-30-31(22)18(12-13-25)15-35-21-11-7-6-10-20(21)32/h3-11,18-19,32H,12,14-16,26H2,1-2H3,(H,27,33)/t18-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at human GHS receptor expressed in H4 glioma cells |
Bioorg Med Chem Lett 18: 2067-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.100
BindingDB Entry DOI: 10.7270/Q21G0N4D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50371693

(CHEMBL402086)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccccc1)c1nnnn1CC(Cc1ccccc1)C#N |w:23.24| Show InChI InChI=1S/C24H29N7O2/c1-24(2,26)23(32)27-21(17-33-16-19-11-7-4-8-12-19)22-28-29-30-31(22)15-20(14-25)13-18-9-5-3-6-10-18/h3-12,20-21H,13,15-17,26H2,1-2H3,(H,27,32)/t20?,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 298 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at human GHS receptor expressed in H4 glioma cells |
Bioorg Med Chem Lett 18: 2067-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.100
BindingDB Entry DOI: 10.7270/Q21G0N4D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50222147

((S)-benzyl 5-(5-(1-(2-amino-2-methylpropanamido)-2...)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccccc1)c1nnnn1CCCCCNC(=O)OCc1ccccc1 Show InChI InChI=1S/C27H37N7O4/c1-27(2,28)25(35)30-23(20-37-18-21-12-6-3-7-13-21)24-31-32-33-34(24)17-11-5-10-16-29-26(36)38-19-22-14-8-4-9-15-22/h3-4,6-9,12-15,23H,5,10-11,16-20,28H2,1-2H3,(H,29,36)(H,30,35)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHSR1a after 1 hr |
Bioorg Med Chem Lett 17: 5928-33 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.099
BindingDB Entry DOI: 10.7270/Q28P607D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50222136
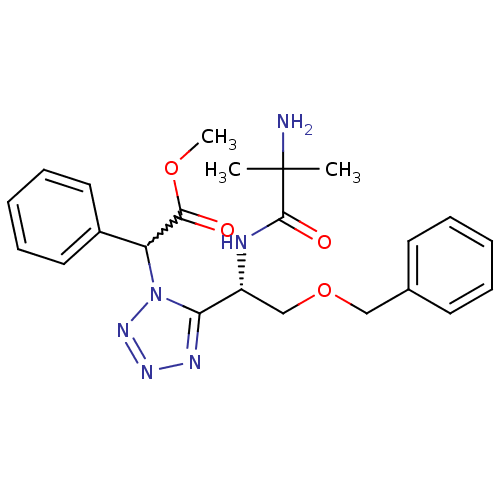
(CHEMBL235793 | methyl 2-(5-((S)-1-(2-amino-2-methy...)Show SMILES COC(=O)C(c1ccccc1)n1nnnc1[C@@H](COCc1ccccc1)NC(=O)C(C)(C)N |w:4.3| Show InChI InChI=1S/C23H28N6O4/c1-23(2,24)22(31)25-18(15-33-14-16-10-6-4-7-11-16)20-26-27-28-29(20)19(21(30)32-3)17-12-8-5-9-13-17/h4-13,18-19H,14-15,24H2,1-3H3,(H,25,31)/t18-,19?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHSR1a after 1 hr |
Bioorg Med Chem Lett 17: 5928-33 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.099
BindingDB Entry DOI: 10.7270/Q28P607D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50371685

(CHEMBL270876)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccccc1)c1nnnn1[C@H](COc1ccccn1)CC#N Show InChI InChI=1S/C23H28N8O3/c1-23(2,25)22(32)27-19(16-33-14-17-8-4-3-5-9-17)21-28-29-30-31(21)18(11-12-24)15-34-20-10-6-7-13-26-20/h3-10,13,18-19H,11,14-16,25H2,1-2H3,(H,27,32)/t18-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 503 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at human GHS receptor expressed in H4 glioma cells |
Bioorg Med Chem Lett 18: 2067-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.100
BindingDB Entry DOI: 10.7270/Q21G0N4D |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50199861

((S)-1-((S)-2-(allylamino)-3-(4-hydroxy-2,6-dimethy...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](NCC=C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C37H45N5O5/c1-4-17-39-32(23-29-24(2)19-28(43)20-25(29)3)37(47)42-18-11-16-33(42)36(46)41-31(22-27-14-9-6-10-15-27)35(45)40-30(34(38)44)21-26-12-7-5-8-13-26/h4-10,12-15,19-20,30-33,39,43H,1,11,16-18,21-23H2,2-3H3,(H2,38,44)(H,40,45)(H,41,46)/t30-,31-,32-,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Displacement of [3H]deltorphin 2 from delta opioid receptor in rat brain P2 synaptosome |
Bioorg Med Chem 15: 1237-51 (2007)
Article DOI: 10.1016/j.bmc.2006.11.019
BindingDB Entry DOI: 10.7270/Q2348K1F |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50371691

(CHEMBL270851)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccccc1)c1nnnn1CCC(C#N)c1ccccc1 |w:24.25| Show InChI InChI=1S/C24H29N7O2/c1-24(2,26)23(32)27-21(17-33-16-18-9-5-3-6-10-18)22-28-29-30-31(22)14-13-20(15-25)19-11-7-4-8-12-19/h3-12,20-21H,13-14,16-17,26H2,1-2H3,(H,27,32)/t20?,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 611 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at human GHS receptor expressed in H4 glioma cells |
Bioorg Med Chem Lett 18: 2067-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.100
BindingDB Entry DOI: 10.7270/Q21G0N4D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50371689
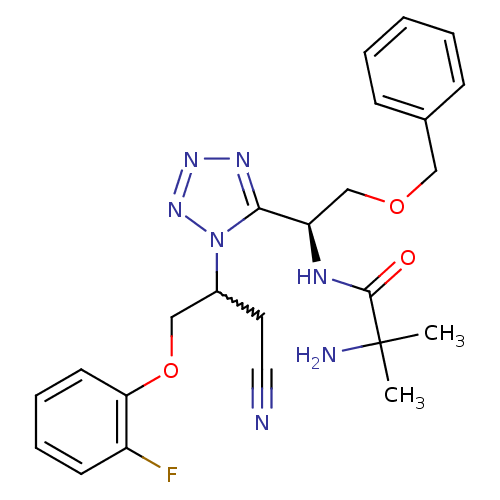
(CHEMBL256235)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccccc1)c1nnnn1C(COc1ccccc1F)CC#N |w:22.34| Show InChI InChI=1S/C24H28FN7O3/c1-24(2,27)23(33)28-20(16-34-14-17-8-4-3-5-9-17)22-29-30-31-32(22)18(12-13-26)15-35-21-11-7-6-10-19(21)25/h3-11,18,20H,12,14-16,27H2,1-2H3,(H,28,33)/t18?,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 696 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at human GHS receptor expressed in H4 glioma cells |
Bioorg Med Chem Lett 18: 2067-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.100
BindingDB Entry DOI: 10.7270/Q21G0N4D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50371689

(CHEMBL256235)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccccc1)c1nnnn1C(COc1ccccc1F)CC#N |w:22.34| Show InChI InChI=1S/C24H28FN7O3/c1-24(2,27)23(33)28-20(16-34-14-17-8-4-3-5-9-17)22-29-30-31-32(22)18(12-13-26)15-35-21-11-7-6-10-19(21)25/h3-11,18,20H,12,14-16,27H2,1-2H3,(H,28,33)/t18?,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 696 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at human GHS receptor expressed in H4 glioma cells |
Bioorg Med Chem Lett 18: 2067-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.100
BindingDB Entry DOI: 10.7270/Q21G0N4D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50371697

(CHEMBL404336)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccccc1)c1nnnn1C(CC#N)\C=C\c1ccccc1 |w:22.24| Show InChI InChI=1S/C25H29N7O2/c1-25(2,27)24(33)28-22(18-34-17-20-11-7-4-8-12-20)23-29-30-31-32(23)21(15-16-26)14-13-19-9-5-3-6-10-19/h3-14,21-22H,15,17-18,27H2,1-2H3,(H,28,33)/b14-13+/t21?,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 737 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at human GHS receptor expressed in H4 glioma cells |
Bioorg Med Chem Lett 18: 2067-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.100
BindingDB Entry DOI: 10.7270/Q21G0N4D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50371697

(CHEMBL404336)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccccc1)c1nnnn1C(CC#N)\C=C\c1ccccc1 |w:22.24| Show InChI InChI=1S/C25H29N7O2/c1-25(2,27)24(33)28-22(18-34-17-20-11-7-4-8-12-20)23-29-30-31-32(23)21(15-16-26)14-13-19-9-5-3-6-10-19/h3-14,21-22H,15,17-18,27H2,1-2H3,(H,28,33)/b14-13+/t21?,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 737 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at human GHS receptor expressed in H4 glioma cells |
Bioorg Med Chem Lett 18: 2067-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.100
BindingDB Entry DOI: 10.7270/Q21G0N4D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50222157
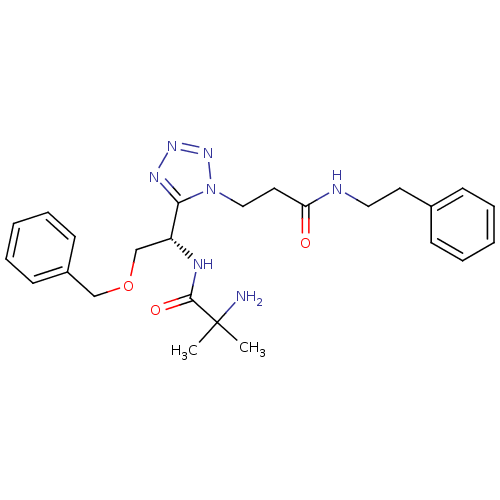
(2-amino-N-{(S)-2-benzyloxy-1-[1-(2-phenethylcarbam...)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccccc1)c1nnnn1CCC(=O)NCCc1ccccc1 Show InChI InChI=1S/C25H33N7O3/c1-25(2,26)24(34)28-21(18-35-17-20-11-7-4-8-12-20)23-29-30-31-32(23)16-14-22(33)27-15-13-19-9-5-3-6-10-19/h3-12,21H,13-18,26H2,1-2H3,(H,27,33)(H,28,34)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHSR1a after 1 hr |
Bioorg Med Chem Lett 17: 5928-33 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.099
BindingDB Entry DOI: 10.7270/Q28P607D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50222166

(2-amino-N-((S)-2-benzyloxy-1-{1-[2-(3-phenyl-propy...)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccccc1)c1nnnn1CCC(=O)NCCCc1ccccc1 Show InChI InChI=1S/C26H35N7O3/c1-26(2,27)25(35)29-22(19-36-18-21-12-7-4-8-13-21)24-30-31-32-33(24)17-15-23(34)28-16-9-14-20-10-5-3-6-11-20/h3-8,10-13,22H,9,14-19,27H2,1-2H3,(H,28,34)(H,29,35)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHSR1a after 1 hr |
Bioorg Med Chem Lett 17: 5928-33 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.099
BindingDB Entry DOI: 10.7270/Q28P607D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50222162

((S)-2-amino-N-(2-(benzyloxy)-1-(1-(2-(1-(methylsul...)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccccc1)c1nnnn1CCc1cc2ccccc2n1S(C)(=O)=O Show InChI InChI=1S/C25H31N7O4S/c1-25(2,26)24(33)27-21(17-36-16-18-9-5-4-6-10-18)23-28-29-30-31(23)14-13-20-15-19-11-7-8-12-22(19)32(20)37(3,34)35/h4-12,15,21H,13-14,16-17,26H2,1-3H3,(H,27,33)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHSR1a after 1 hr |
Bioorg Med Chem Lett 17: 5928-33 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.099
BindingDB Entry DOI: 10.7270/Q28P607D |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50199866
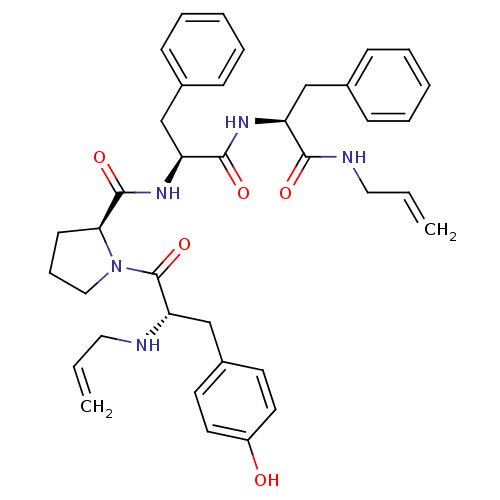
((S)-N-((S)-1-((S)-1-(allylamino)-1-oxo-3-phenylpro...)Show SMILES Oc1ccc(C[C@H](NCC=C)C(=O)N2CCC[C@H]2C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)NCC=C)cc1 Show InChI InChI=1S/C38H45N5O5/c1-3-21-39-33(26-29-17-19-30(44)20-18-29)38(48)43-23-11-16-34(43)37(47)42-32(25-28-14-9-6-10-15-28)36(46)41-31(35(45)40-22-4-2)24-27-12-7-5-8-13-27/h3-10,12-15,17-20,31-34,39,44H,1-2,11,16,21-26H2,(H,40,45)(H,41,46)(H,42,47)/t31-,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain P2 synaptosome |
Bioorg Med Chem 15: 1237-51 (2007)
Article DOI: 10.1016/j.bmc.2006.11.019
BindingDB Entry DOI: 10.7270/Q2348K1F |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50222167

(2-amino-N-{(S)-1-[1-(2-benzylcarbamoyl-ethyl)-1H-t...)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccccc1)c1nnnn1CCC(=O)NCc1ccccc1 Show InChI InChI=1S/C24H31N7O3/c1-24(2,25)23(33)27-20(17-34-16-19-11-7-4-8-12-19)22-28-29-30-31(22)14-13-21(32)26-15-18-9-5-3-6-10-18/h3-12,20H,13-17,25H2,1-2H3,(H,26,32)(H,27,33)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHSR1a after 1 hr |
Bioorg Med Chem Lett 17: 5928-33 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.099
BindingDB Entry DOI: 10.7270/Q28P607D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50222173

((S)-2-amino-N-(2-(benzyloxy)-1-(2-(2-(1-(methylsul...)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccccc1)c1nnn(CCc2cc3ccccc3n2S(C)(=O)=O)n1 Show InChI InChI=1S/C25H31N7O4S/c1-25(2,26)24(33)27-21(17-36-16-18-9-5-4-6-10-18)23-28-30-31(29-23)14-13-20-15-19-11-7-8-12-22(19)32(20)37(3,34)35/h4-12,15,21H,13-14,16-17,26H2,1-3H3,(H,27,33)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHSR1a after 1 hr |
Bioorg Med Chem Lett 17: 5928-33 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.099
BindingDB Entry DOI: 10.7270/Q28P607D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50222150
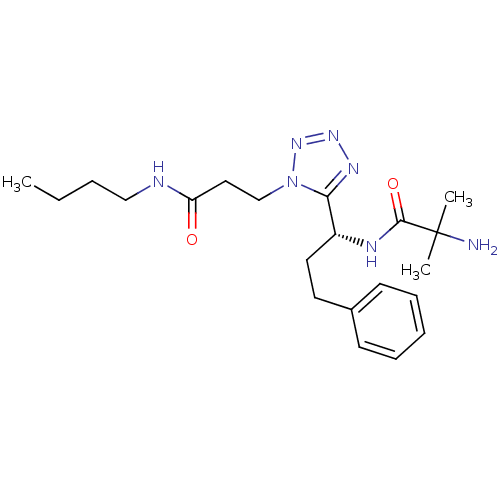
(2-amino-N-{(R)-1-[1-(2-butylcarbamoyl-ethyl)-1H-te...)Show SMILES CCCCNC(=O)CCn1nnnc1[C@@H](CCc1ccccc1)NC(=O)C(C)(C)N Show InChI InChI=1S/C21H33N7O2/c1-4-5-14-23-18(29)13-15-28-19(25-26-27-28)17(24-20(30)21(2,3)22)12-11-16-9-7-6-8-10-16/h6-10,17H,4-5,11-15,22H2,1-3H3,(H,23,29)(H,24,30)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHSR1a after 1 hr |
Bioorg Med Chem Lett 17: 5928-33 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.099
BindingDB Entry DOI: 10.7270/Q28P607D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50371695

(CHEMBL403180)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccccc1)c1nnnn1C(CCc1ccccc1)CC#N |w:22.33| Show InChI InChI=1S/C25H31N7O2/c1-25(2,27)24(33)28-22(18-34-17-20-11-7-4-8-12-20)23-29-30-31-32(23)21(15-16-26)14-13-19-9-5-3-6-10-19/h3-12,21-22H,13-15,17-18,27H2,1-2H3,(H,28,33)/t21?,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at human GHS receptor expressed in H4 glioma cells |
Bioorg Med Chem Lett 18: 2067-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.100
BindingDB Entry DOI: 10.7270/Q21G0N4D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50371695

(CHEMBL403180)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccccc1)c1nnnn1C(CCc1ccccc1)CC#N |w:22.33| Show InChI InChI=1S/C25H31N7O2/c1-25(2,27)24(33)28-22(18-34-17-20-11-7-4-8-12-20)23-29-30-31-32(23)21(15-16-26)14-13-19-9-5-3-6-10-19/h3-12,21-22H,13-15,17-18,27H2,1-2H3,(H,28,33)/t21?,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at human GHS receptor expressed in H4 glioma cells |
Bioorg Med Chem Lett 18: 2067-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.100
BindingDB Entry DOI: 10.7270/Q21G0N4D |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50199864

((S)-N-((S)-1-((S)-1-(allylamino)-1-oxo-3-phenylpro...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](NCC=C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC=C Show InChI InChI=1S/C40H49N5O5/c1-5-19-41-35(26-32-27(3)22-31(46)23-28(32)4)40(50)45-21-13-18-36(45)39(49)44-34(25-30-16-11-8-12-17-30)38(48)43-33(37(47)42-20-6-2)24-29-14-9-7-10-15-29/h5-12,14-17,22-23,33-36,41,46H,1-2,13,18-21,24-26H2,3-4H3,(H,42,47)(H,43,48)(H,44,49)/t33-,34-,35-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Displacement of [3H]deltorphin 2 from delta opioid receptor in rat brain P2 synaptosome |
Bioorg Med Chem 15: 1237-51 (2007)
Article DOI: 10.1016/j.bmc.2006.11.019
BindingDB Entry DOI: 10.7270/Q2348K1F |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50222151

(2-amino-N-((S)-2-(benzyloxy)-1-(1-((1-(methylsulfo...)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccccc1)c1nnnn1CC1CN(c2ccccc12)S(C)(=O)=O |w:23.24| Show InChI InChI=1S/C24H31N7O4S/c1-24(2,25)23(32)26-20(16-35-15-17-9-5-4-6-10-17)22-27-28-29-30(22)13-18-14-31(36(3,33)34)21-12-8-7-11-19(18)21/h4-12,18,20H,13-16,25H2,1-3H3,(H,26,32)/t18?,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHSR1a after 1 hr |
Bioorg Med Chem Lett 17: 5928-33 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.099
BindingDB Entry DOI: 10.7270/Q28P607D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50222161

((S)-2-amino-N-(2-(benzyloxy)-1-(1-(3-(2-(N-methylm...)Show SMILES CN(c1ccccc1CCCn1nnnc1[C@@H](COCc1ccccc1)NC(=O)C(C)(C)N)S(C)(=O)=O Show InChI InChI=1S/C25H35N7O4S/c1-25(2,26)24(33)27-21(18-36-17-19-11-6-5-7-12-19)23-28-29-30-32(23)16-10-14-20-13-8-9-15-22(20)31(3)37(4,34)35/h5-9,11-13,15,21H,10,14,16-18,26H2,1-4H3,(H,27,33)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHSR1a after 1 hr |
Bioorg Med Chem Lett 17: 5928-33 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.099
BindingDB Entry DOI: 10.7270/Q28P607D |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50095155

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C34H38N6O5/c35-26(17-22-12-14-24(41)15-13-22)34(45)40-16-6-11-30(40)33(44)39-29(19-23-20-37-27-10-5-4-9-25(23)27)32(43)38-28(31(36)42)18-21-7-2-1-3-8-21/h1-5,7-10,12-15,20,26,28-30,37,41H,6,11,16-19,35H2,(H2,36,42)(H,38,43)(H,39,44)/t26-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Displacement of [3H]deltorphin 2 from delta opioid receptor in rat brain P2 synaptosome |
Bioorg Med Chem 15: 1237-51 (2007)
Article DOI: 10.1016/j.bmc.2006.11.019
BindingDB Entry DOI: 10.7270/Q2348K1F |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50222146

((S)-4-(5-(1-(2-amino-2-methylpropanamido)-2-(benzy...)Show SMILES CCCCNC(=O)CCCn1nnnc1[C@@H](COCc1ccccc1)NC(=O)C(C)(C)N Show InChI InChI=1S/C22H35N7O3/c1-4-5-13-24-19(30)12-9-14-29-20(26-27-28-29)18(25-21(31)22(2,3)23)16-32-15-17-10-7-6-8-11-17/h6-8,10-11,18H,4-5,9,12-16,23H2,1-3H3,(H,24,30)(H,25,31)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHSR1a after 1 hr |
Bioorg Med Chem Lett 17: 5928-33 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.099
BindingDB Entry DOI: 10.7270/Q28P607D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50222153

((S)-2-amino-N-(1-(1-benzyl-1H-tetrazol-5-yl)-2-(be...)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccccc1)c1nnnn1Cc1ccccc1 Show InChI InChI=1S/C21H26N6O2/c1-21(2,22)20(28)23-18(15-29-14-17-11-7-4-8-12-17)19-24-25-26-27(19)13-16-9-5-3-6-10-16/h3-12,18H,13-15,22H2,1-2H3,(H,23,28)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHSR1a after 1 hr |
Bioorg Med Chem Lett 17: 5928-33 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.099
BindingDB Entry DOI: 10.7270/Q28P607D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50222165

(2-amino-N-{(S)-2-benzyloxy-1-[1-(2-butylcarbamoyl-...)Show SMILES CCCCNC(=O)CCn1nnnc1[C@@H](COCc1ccccc1)NC(=O)C(C)(C)N Show InChI InChI=1S/C21H33N7O3/c1-4-5-12-23-18(29)11-13-28-19(25-26-27-28)17(24-20(30)21(2,3)22)15-31-14-16-9-7-6-8-10-16/h6-10,17H,4-5,11-15,22H2,1-3H3,(H,23,29)(H,24,30)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHSR1a after 1 hr |
Bioorg Med Chem Lett 17: 5928-33 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.099
BindingDB Entry DOI: 10.7270/Q28P607D |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50222143
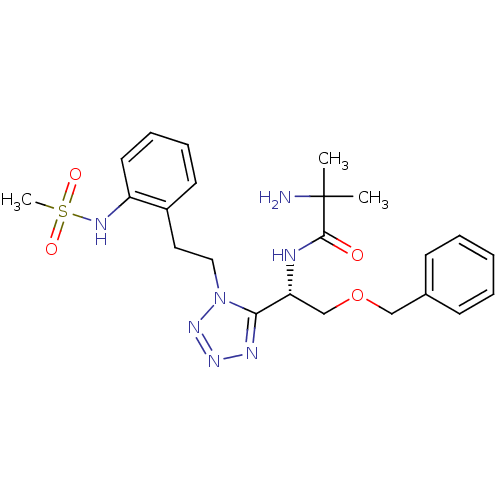
((S)-N-(1-(1-(2-(methylsulfonamido)phenethyl)-1H-te...)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccccc1)c1nnnn1CCc1ccccc1NS(C)(=O)=O Show InChI InChI=1S/C23H31N7O4S/c1-23(2,24)22(31)25-20(16-34-15-17-9-5-4-6-10-17)21-26-28-29-30(21)14-13-18-11-7-8-12-19(18)27-35(3,32)33/h4-12,20,27H,13-16,24H2,1-3H3,(H,25,31)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHSR1a after 1 hr |
Bioorg Med Chem Lett 17: 5928-33 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.099
BindingDB Entry DOI: 10.7270/Q28P607D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data