

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50095997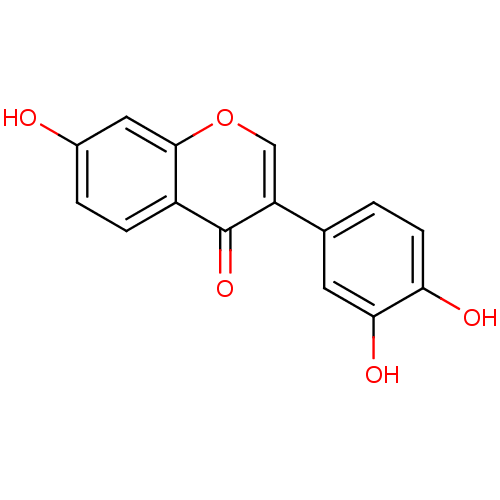 (3',4',7-trihydroxyisoflavone | CHEMBL13486) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096004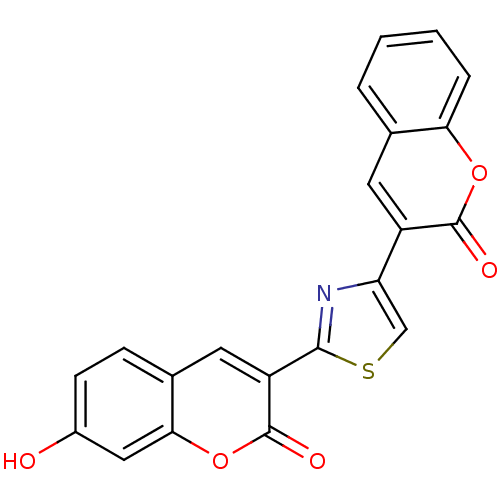 (7-Hydroxy-3-[4-(2-oxo-2H-chromen-3-yl)-thiazol-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096003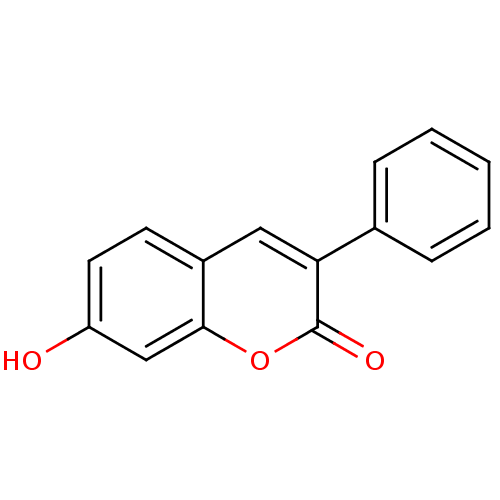 (7-Hydroxy-3-phenyl-chromen-2-one | 7-hydroxy-3-phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096001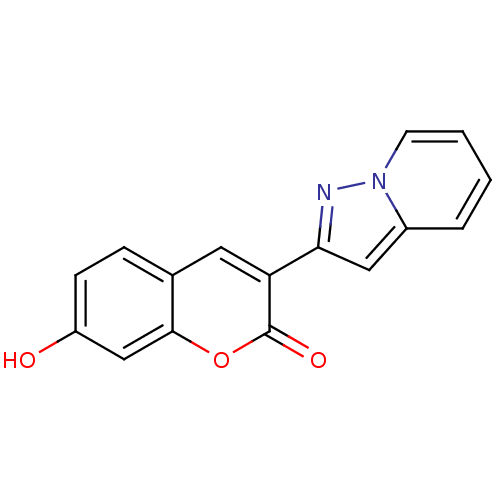 (7-Hydroxy-3-pyrazolo[1,5-a]pyridin-2-yl-chromen-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50095993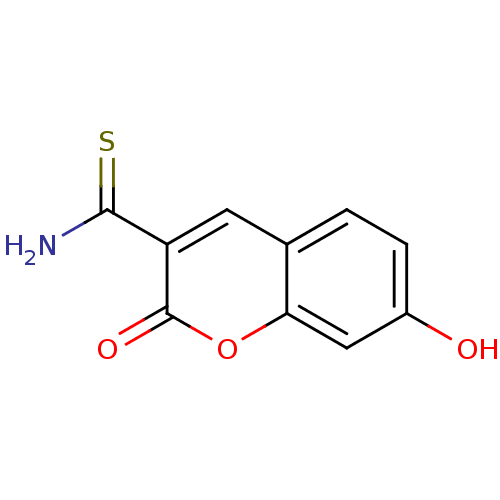 (7-Hydroxy-2-oxo-2H-chromene-3-carbothioic acid ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096002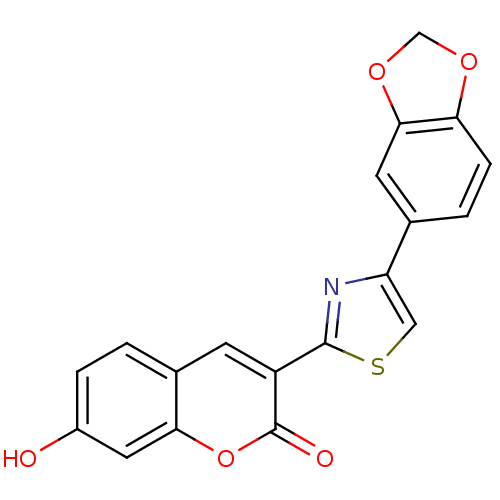 (3-(4-Benzo[1,3]dioxol-5-yl-thiazol-2-yl)-7-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096007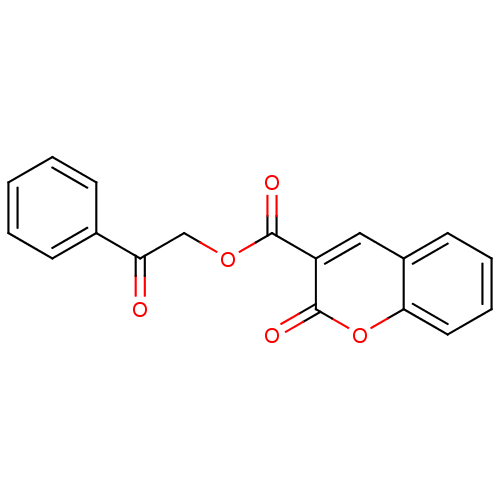 (2-Oxo-2H-chromene-3-carboxylic acid 2-oxo-2-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096006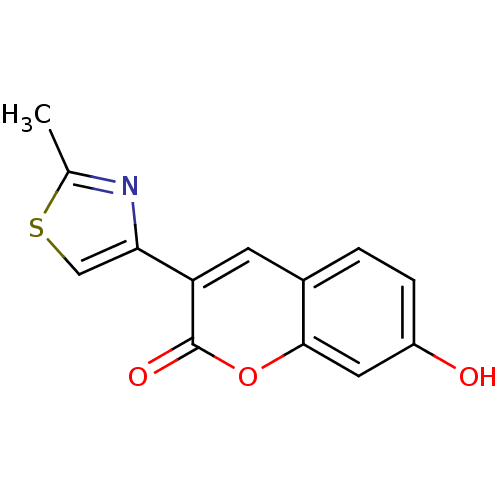 (7-Hydroxy-3-(2-methyl-thiazol-4-yl)-chromen-2-one ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096000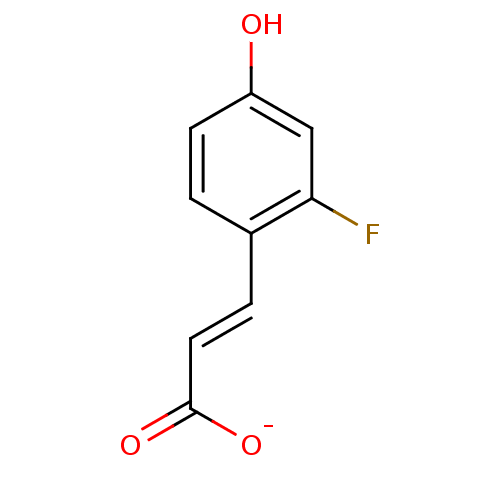 (3-(2-Fluoro-4-hydroxy-phenyl)-acrylic acid anion) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50095994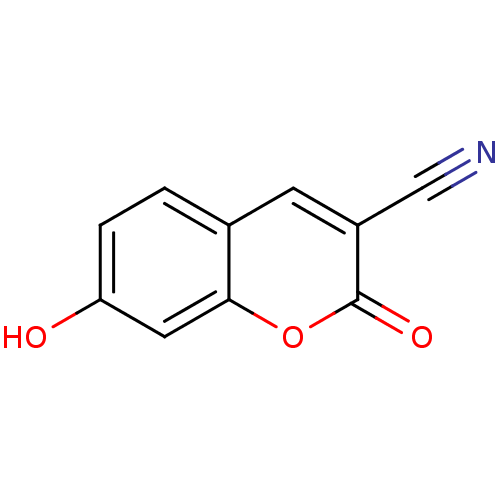 (3-Cyano-7-hydroxycoumarin (2) | 7-Hydroxy-2-oxo-2H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096005 (7-Hydroxy-3-(4-methyl-thiazol-2-yl)-chromen-2-one ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096008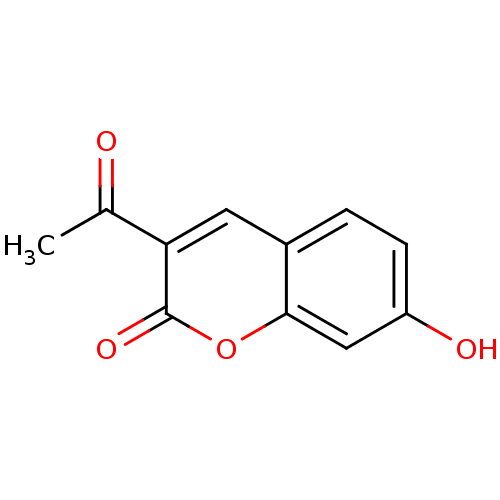 (3-Acetyl-7-hydroxy-chromen-2-one | 3-acetyl-7-hydr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50095996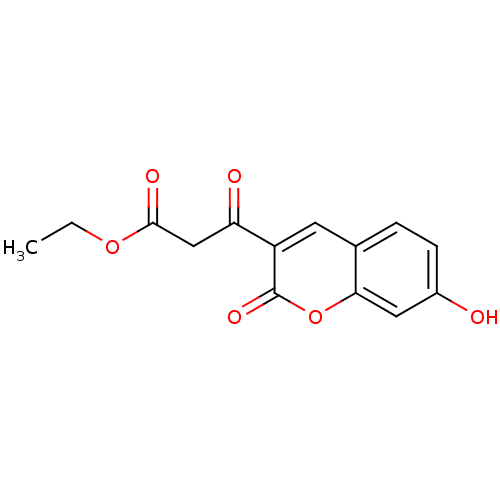 (3-(7-Hydroxy-2-oxo-2H-chromen-3-yl)-3-oxo-propioni...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50096009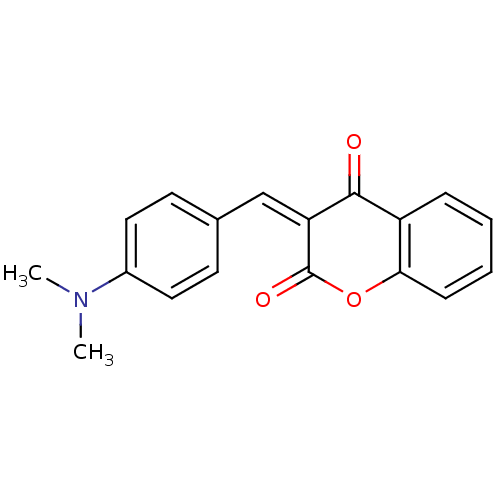 (3-(4-Dimethylamino-benzylidene)-chroman-2,4-dione ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50095995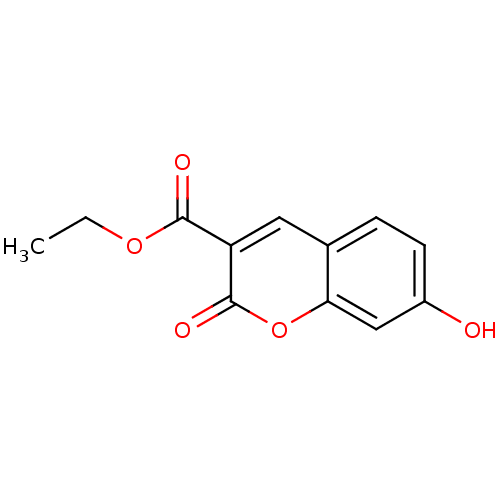 (7-HYDROXY-2-OXO-CHROMENE-3-CARBOXYLIC ACID ETHYL E...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) | J Med Chem 44: 540-7 (2001) BindingDB Entry DOI: 10.7270/Q2W66MGR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50608178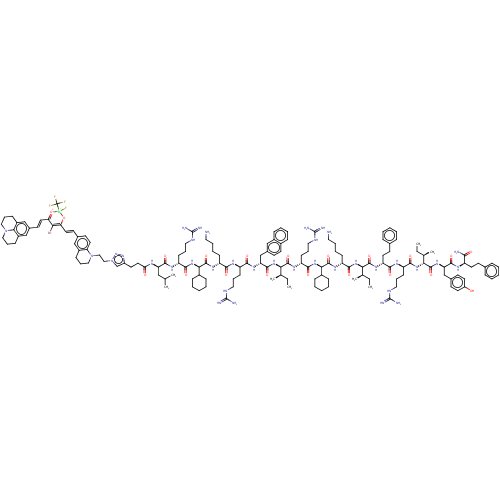 (CHEMBL5268044) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50608179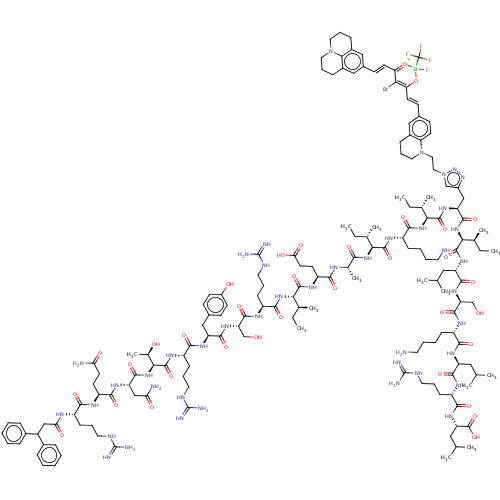 (CHEMBL5285634) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50539387 (CHEMBL4648474) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of myostatin (unknown origin) expressed in HEK293 cells incubated for 4 hrs by dual luciferase reporter gene assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126892 BindingDB Entry DOI: 10.7270/Q2G73J8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50525413 (CHEMBL4448117) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of myostatin (unknown origin) expressed in HEK293 cells incubated for 4 hrs by dual luciferase reporter gene assay | ACS Med Chem Lett 10: 985-990 (2019) Article DOI: 10.1021/acsmedchemlett.9b00174 BindingDB Entry DOI: 10.7270/Q2V1287X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Death-associated protein kinase 3 (Homo sapiens (Human)) | BDBM50314672 (2-(3-bromophenyl)-4-(pyridin-3-ylmethylene)oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmaDesign, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant DAPK3 | Bioorg Med Chem 18: 2728-34 (2010) Article DOI: 10.1016/j.bmc.2010.02.018 BindingDB Entry DOI: 10.7270/Q2RB74R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Death-associated protein kinase 3 (Homo sapiens (Human)) | BDBM50314673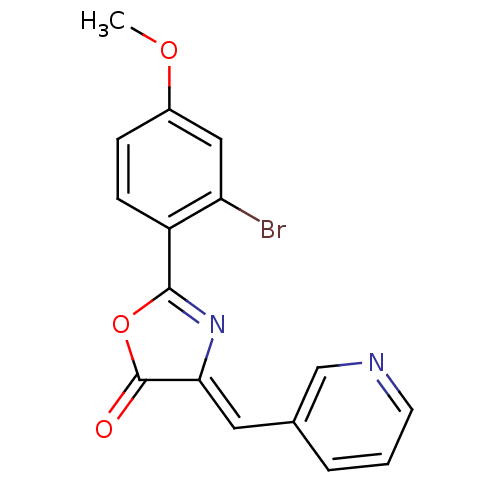 (2-(2-bromo-4-methoxyphenyl)-4-(pyridin-3-ylmethyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 157 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmaDesign, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant DAPK3 | Bioorg Med Chem 18: 2728-34 (2010) Article DOI: 10.1016/j.bmc.2010.02.018 BindingDB Entry DOI: 10.7270/Q2RB74R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50585028 (CHEMBL5078185) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of myostatin (unknown origin) expressed in HEK293 cells transfected with Smad2/3 responsive reporter plasmid incubated for 4 hrs by dual l... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00705 BindingDB Entry DOI: 10.7270/Q28S4TT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50585028 (CHEMBL5078185) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Death-associated protein kinase 3 (Homo sapiens (Human)) | BDBM50314664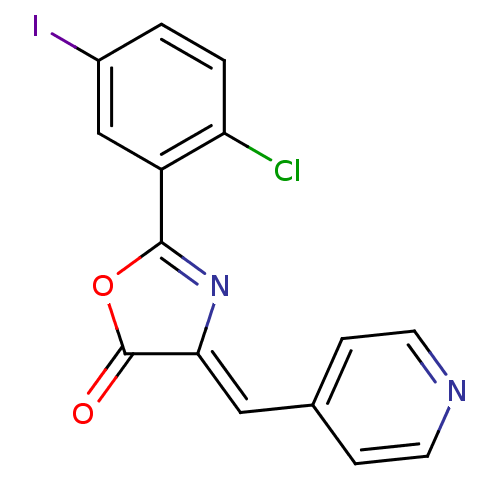 (2-(2-chloro-5-iodophenyl)-4-(pyridin-4-ylmethylene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 227 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmaDesign, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant DAPK3 | Bioorg Med Chem 18: 2728-34 (2010) Article DOI: 10.1016/j.bmc.2010.02.018 BindingDB Entry DOI: 10.7270/Q2RB74R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50525413 (CHEMBL4448117) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of myostatin (unknown origin) expressed in HEK293 cells transfected with Smad2/3 responsive reporter plasmid incubated for 4 hrs by dual l... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00705 BindingDB Entry DOI: 10.7270/Q28S4TT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50537170 (CHEMBL4516740) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of myostatin (unknown origin) expressed in HEK293 cells after 4 hrs by dual luciferase reporter gene assay | Bioorg Med Chem 27: 1437-1443 (2019) Article DOI: 10.1016/j.bmc.2019.02.019 BindingDB Entry DOI: 10.7270/Q2W099FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Death-associated protein kinase 3 (Homo sapiens (Human)) | BDBM50314665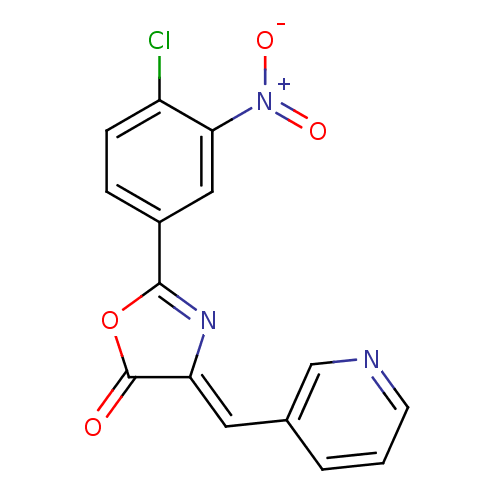 (2-(4-chloro-3-nitrophenyl)-4-(pyridin-3-ylmethylen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 279 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmaDesign, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant DAPK3 | Bioorg Med Chem 18: 2728-34 (2010) Article DOI: 10.1016/j.bmc.2010.02.018 BindingDB Entry DOI: 10.7270/Q2RB74R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Death-associated protein kinase 3 (Homo sapiens (Human)) | BDBM50314670 (2-(4-bromo-3-methylphenyl)-4-(pyridin-3-ylmethylen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 279 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmaDesign, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant DAPK3 | Bioorg Med Chem 18: 2728-34 (2010) Article DOI: 10.1016/j.bmc.2010.02.018 BindingDB Entry DOI: 10.7270/Q2RB74R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50254771 (CHEMBL4061416) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of myostatin (unknown origin) expressed in HEK293 cells incubated for 4 hrs by dual luciferase reporter gene assay | ACS Med Chem Lett 10: 985-990 (2019) Article DOI: 10.1021/acsmedchemlett.9b00174 BindingDB Entry DOI: 10.7270/Q2V1287X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Death-associated protein kinase 3 (Homo sapiens (Human)) | BDBM50314671 (2-(4-nitrophenyl)-4-(pyridin-3-ylmethylene)oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 291 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmaDesign, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant DAPK3 | Bioorg Med Chem 18: 2728-34 (2010) Article DOI: 10.1016/j.bmc.2010.02.018 BindingDB Entry DOI: 10.7270/Q2RB74R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50585029 (CHEMBL5092917) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of myostatin (unknown origin) expressed in HEK293 cells transfected with Smad2/3 responsive reporter plasmid incubated for 4 hrs by dual l... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00705 BindingDB Entry DOI: 10.7270/Q28S4TT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50254771 (CHEMBL4061416) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Department of Drug Delivery and Molecular Biopharmaceutics, Tokyo University of Pharmacy and Life Sciences, Hachioji, Tokyo 192-0392, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant myostatin (unknown origin) expressed in HEK293 cells after 4 hrs by dual-luciferase reporter gene assay | ACS Med Chem Lett 8: 751-756 (2017) Article DOI: 10.1021/acsmedchemlett.7b00168 BindingDB Entry DOI: 10.7270/Q2QC05ZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Death-associated protein kinase 3 (Homo sapiens (Human)) | BDBM50314668 (2-(3,4-difluorophenyl)-4-(pyridin-3-ylmethylene)ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 346 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmaDesign, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant DAPK3 | Bioorg Med Chem 18: 2728-34 (2010) Article DOI: 10.1016/j.bmc.2010.02.018 BindingDB Entry DOI: 10.7270/Q2RB74R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Death-associated protein kinase 3 (Homo sapiens (Human)) | BDBM50314669 (2-(3-chlorophenyl)-4-(pyridin-3-ylmethylene)oxazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmaDesign, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant DAPK3 | Bioorg Med Chem 18: 2728-34 (2010) Article DOI: 10.1016/j.bmc.2010.02.018 BindingDB Entry DOI: 10.7270/Q2RB74R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50254771 (CHEMBL4061416) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of myostatin (unknown origin) expressed in HEK293 cells after 4 hrs by dual luciferase reporter gene assay | Bioorg Med Chem 27: 1437-1443 (2019) Article DOI: 10.1016/j.bmc.2019.02.019 BindingDB Entry DOI: 10.7270/Q2W099FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Death-associated protein kinase 3 (Homo sapiens (Human)) | BDBM50314674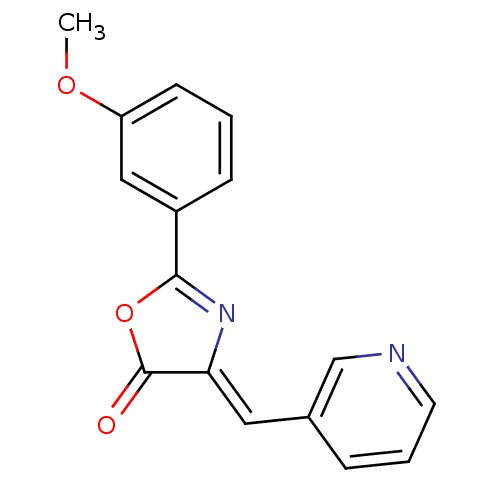 (2-(3-methoxyphenyl)-4-(pyridin-3-ylmethylene)oxazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 395 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmaDesign, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant DAPK3 | Bioorg Med Chem 18: 2728-34 (2010) Article DOI: 10.1016/j.bmc.2010.02.018 BindingDB Entry DOI: 10.7270/Q2RB74R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Death-associated protein kinase 3 (Homo sapiens (Human)) | BDBM50314667 (2-(4-chlorophenyl)-4-(pyridin-3-ylmethylene)oxazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmaDesign, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant DAPK3 | Bioorg Med Chem 18: 2728-34 (2010) Article DOI: 10.1016/j.bmc.2010.02.018 BindingDB Entry DOI: 10.7270/Q2RB74R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Death-associated protein kinase 3 (Homo sapiens (Human)) | BDBM50314675 (2-(4-methyl-3-nitrophenyl)-4-(pyridin-3-ylmethylen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 534 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmaDesign, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant DAPK3 | Bioorg Med Chem 18: 2728-34 (2010) Article DOI: 10.1016/j.bmc.2010.02.018 BindingDB Entry DOI: 10.7270/Q2RB74R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Death-associated protein kinase 3 (Homo sapiens (Human)) | BDBM50314663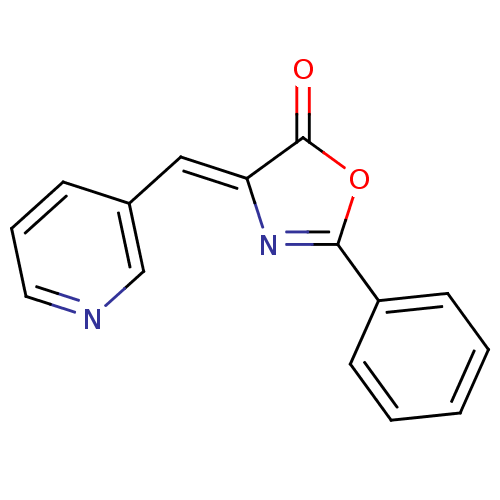 (2-phenyl-4-(pyridin-3-ylmethylene)oxazol-5(4H)-one...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 583 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmaDesign, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant DAPK3 | Bioorg Med Chem 18: 2728-34 (2010) Article DOI: 10.1016/j.bmc.2010.02.018 BindingDB Entry DOI: 10.7270/Q2RB74R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Death-associated protein kinase 3 (Homo sapiens (Human)) | BDBM50314666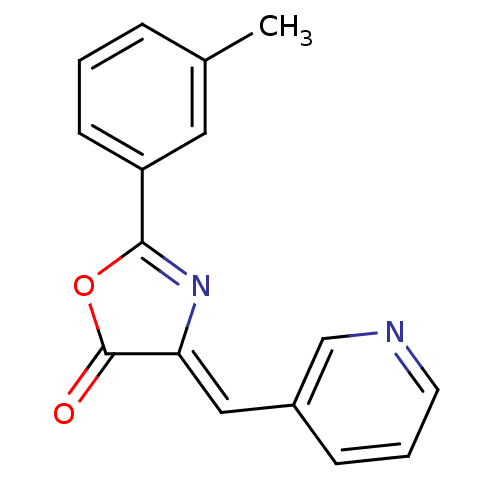 (4-(pyridin-3-ylmethylene)-2-m-tolyloxazol-5(4H)-on...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 714 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmaDesign, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant DAPK3 | Bioorg Med Chem 18: 2728-34 (2010) Article DOI: 10.1016/j.bmc.2010.02.018 BindingDB Entry DOI: 10.7270/Q2RB74R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50206029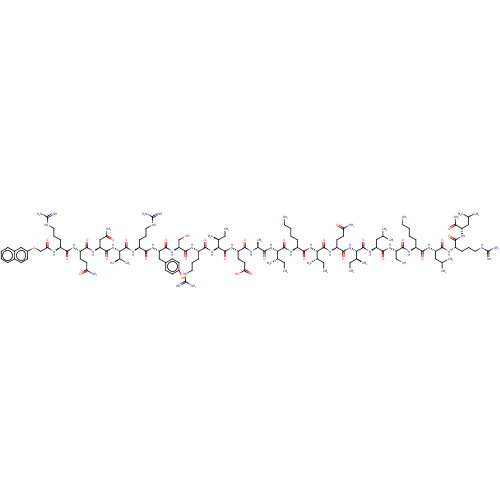 (CHEMBL3892004) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Department of Drug Delivery and Molecular Biopharmaceutics, Tokyo University of Pharmacy and Life Sciences, Hachioji, Tokyo 192-0392, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant myostatin (unknown origin) expressed in HEK293 cells after 4 hrs by dual-luciferase reporter gene assay | ACS Med Chem Lett 8: 751-756 (2017) Article DOI: 10.1021/acsmedchemlett.7b00168 BindingDB Entry DOI: 10.7270/Q2QC05ZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50206029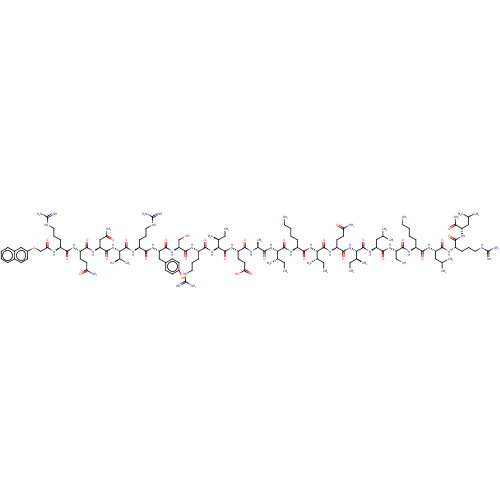 (CHEMBL3892004) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant myostatin expressed in HEK293 cells after 4 hrs by SBE4 based luciferase reporter gene assay | ACS Med Chem Lett 8: 113-117 (2017) Article DOI: 10.1021/acsmedchemlett.6b00420 BindingDB Entry DOI: 10.7270/Q2MG7RHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50030766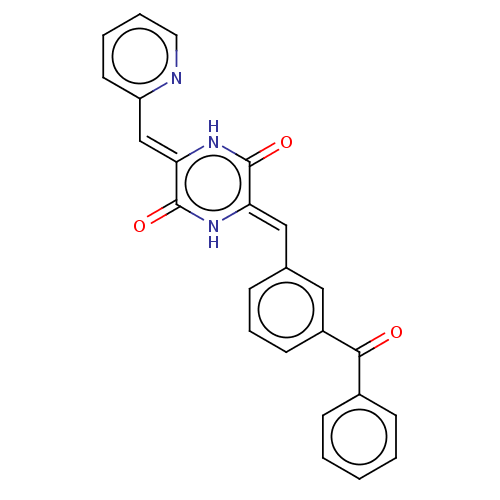 (CHEMBL3342339) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of porcine tubulin polymerization by spectrophotometry | ACS Med Chem Lett 5: 1094-8 (2014) Article DOI: 10.1021/ml5001883 BindingDB Entry DOI: 10.7270/Q2R49SCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transforming growth factor beta-1 proprotein (Homo sapiens (Human)) | BDBM50585028 (CHEMBL5078185) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TGF beta 1 (unknown origin) expressed in HEK293 cells transfected with Smad2/3 responsive reporter plasmid incubated for 4 hrs by dual ... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00705 BindingDB Entry DOI: 10.7270/Q28S4TT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50030765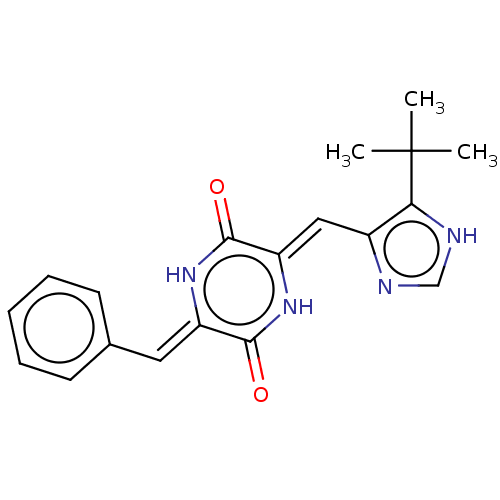 (NPI-2358 | Plinabulin) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of porcine tubulin polymerization by spectrophotometry | ACS Med Chem Lett 5: 1094-8 (2014) Article DOI: 10.1021/ml5001883 BindingDB Entry DOI: 10.7270/Q2R49SCP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50525413 (CHEMBL4448117) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of myostatin (unknown origin) expressed in HEK293 cells incubated for 4 hrs by dual luciferase reporter gene assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126892 BindingDB Entry DOI: 10.7270/Q2G73J8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50071380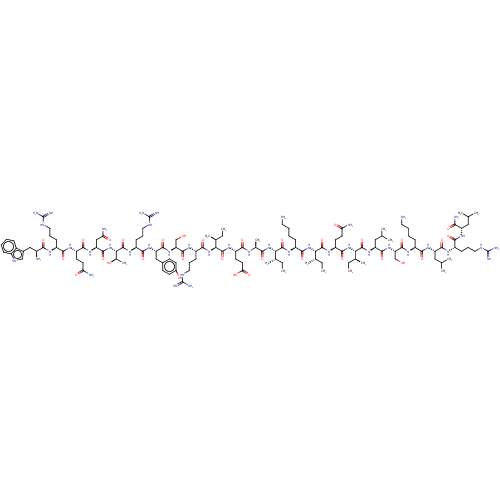 (CHEMBL3410232) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Department of Drug Delivery and Molecular Biopharmaceutics, Tokyo University of Pharmacy and Life Sciences, Hachioji, Tokyo 192-0392, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant myostatin (unknown origin) expressed in HEK293 cells after 4 hrs by dual-luciferase reporter gene assay | ACS Med Chem Lett 8: 751-756 (2017) Article DOI: 10.1021/acsmedchemlett.7b00168 BindingDB Entry DOI: 10.7270/Q2QC05ZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50071379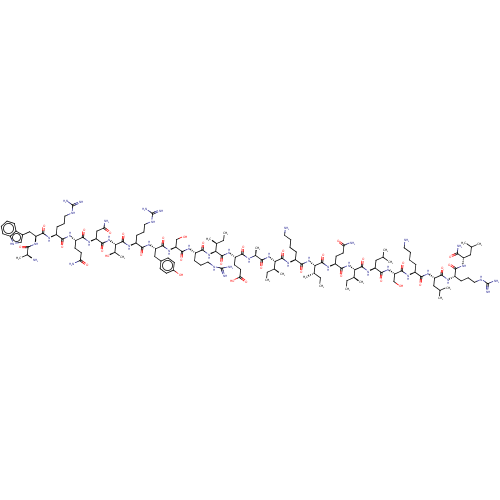 (CHEMBL3410227) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant myostatin pre-incubated with compound for 1 hr before addition to human HepG2 cells assessed as reduction in myostati... | J Med Chem 58: 1544-9 (2015) Article DOI: 10.1021/jm501170d BindingDB Entry DOI: 10.7270/Q2DJ5HBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50539386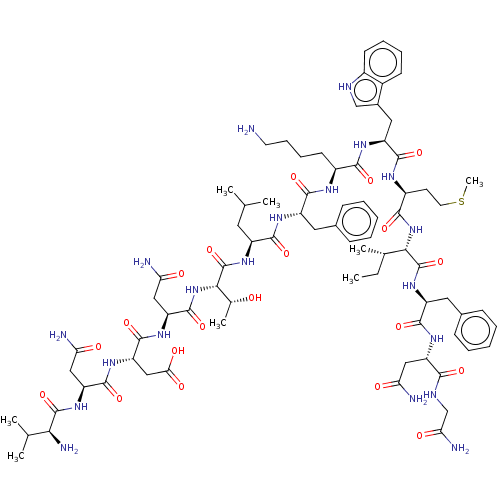 (CHEMBL4646947) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of myostatin (unknown origin) expressed in HEK293 cells incubated for 4 hrs by dual luciferase reporter gene assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126892 BindingDB Entry DOI: 10.7270/Q2G73J8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transforming growth factor beta-1 proprotein (Homo sapiens (Human)) | BDBM50525413 (CHEMBL4448117) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TGF beta 1 (unknown origin) expressed in HEK293 cells transfected with Smad2/3 responsive reporter plasmid incubated for 4 hrs by dual ... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00705 BindingDB Entry DOI: 10.7270/Q28S4TT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 88 total ) | Next | Last >> |