

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448761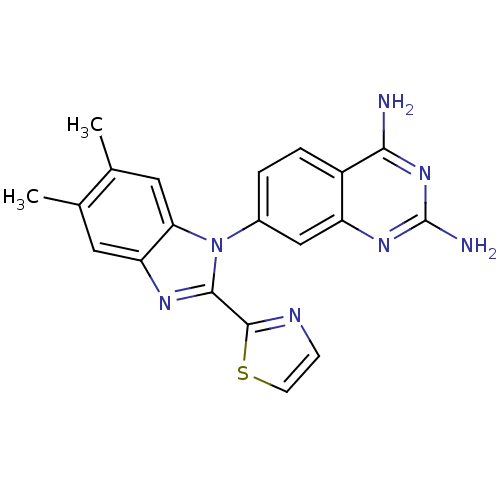 (CHEMBL3128021 | US8835445, 22) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448757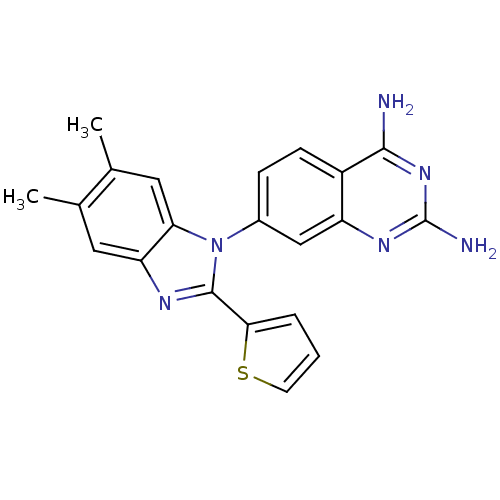 (CHEMBL3128025 | US8835445, 18) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131853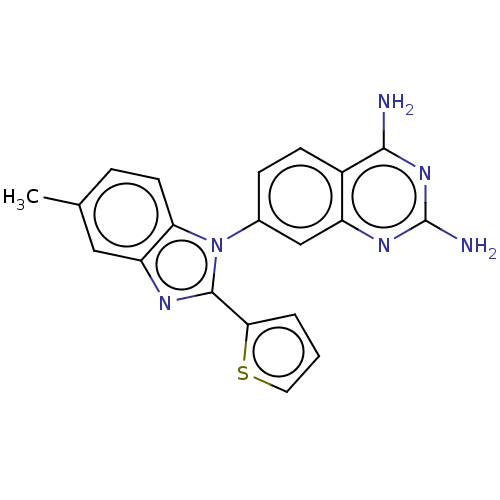 (US8835445, 36) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448759 (CHEMBL3128023 | US8835445, 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448744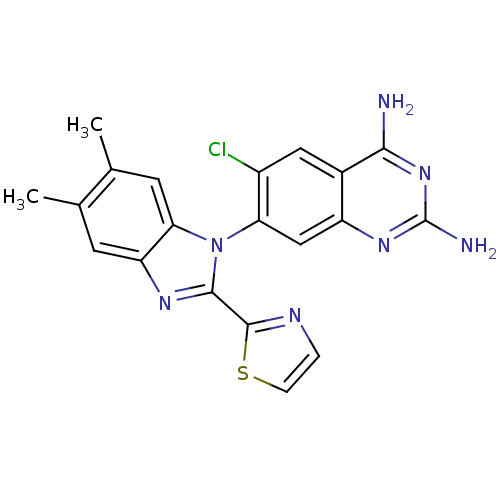 (CHEMBL3128014 | US8835445, 25 | US8835445, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131845 (US8835445, 23) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448756 (CHEMBL3128026 | US8835445, 21) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448744 (CHEMBL3128014 | US8835445, 25 | US8835445, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131846 (US8835445, 24) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50351175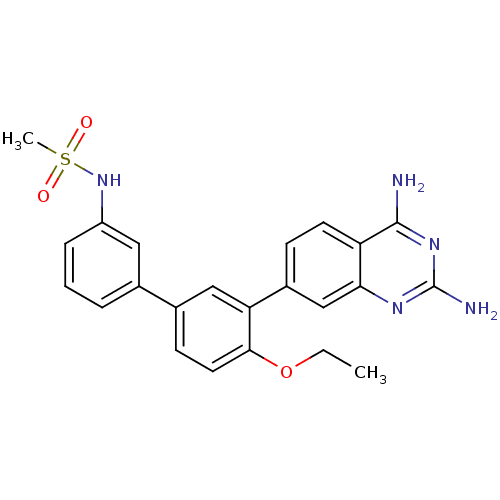 (CHEMBL1818127 | US8835445, 30) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | PDB US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131850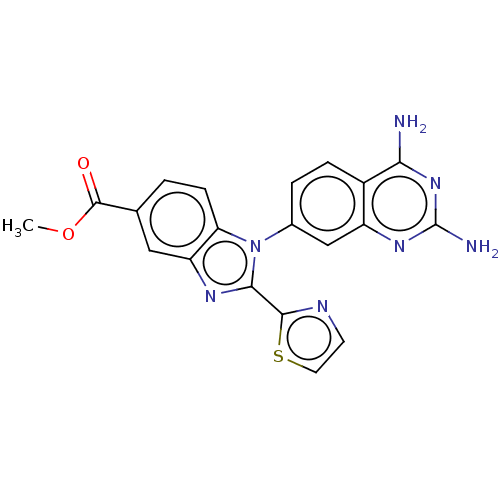 (US8835445, 32) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448753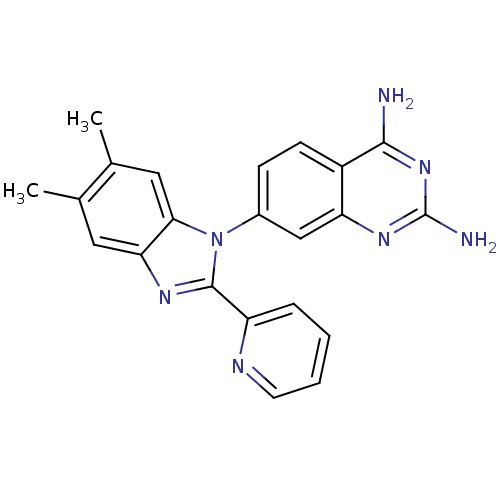 (CHEMBL3127912 | US8835445, 16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50351173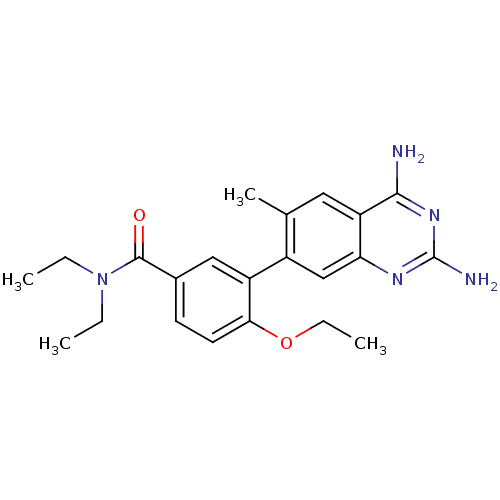 (CHEMBL1818129 | US8835445, 40) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50351174 (CHEMBL1818128 | US8835445, 31) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448745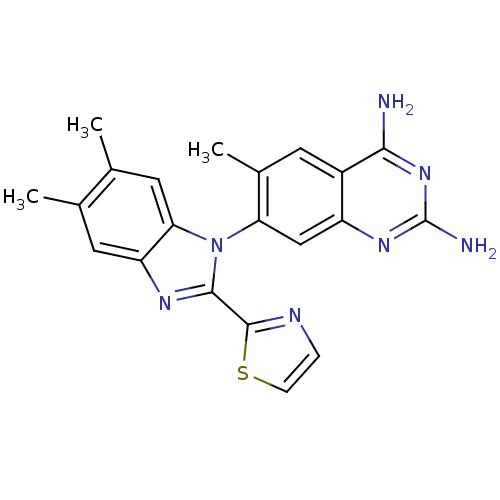 (CHEMBL3128013 | US8835445, 26) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131854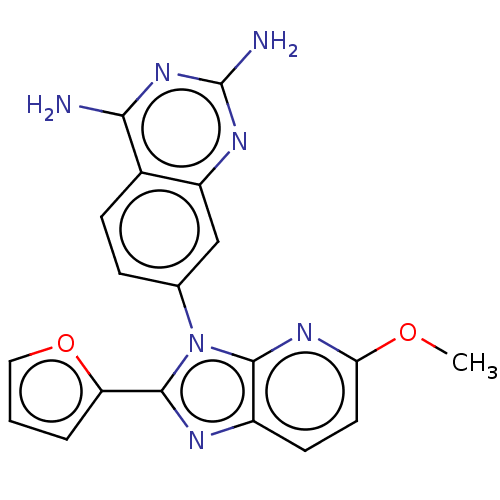 (US8835445, 37) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131857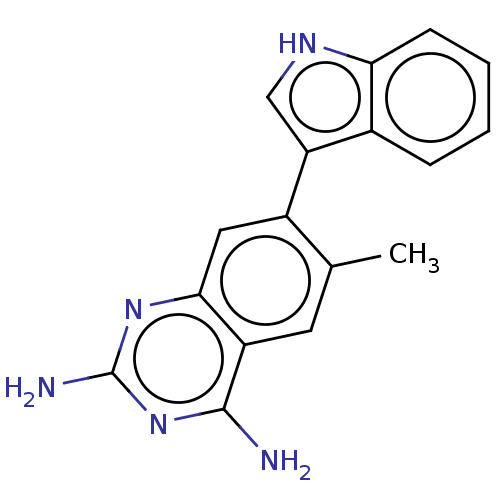 (US8835445, 41) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131844 (US8835445, 20) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448749 (CHEMBL3127916 | US8835445, 15) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131852 (US8835445, 35) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131856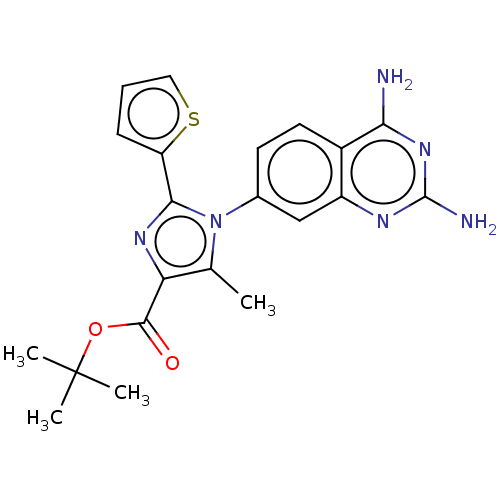 (US8835445, 39) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131849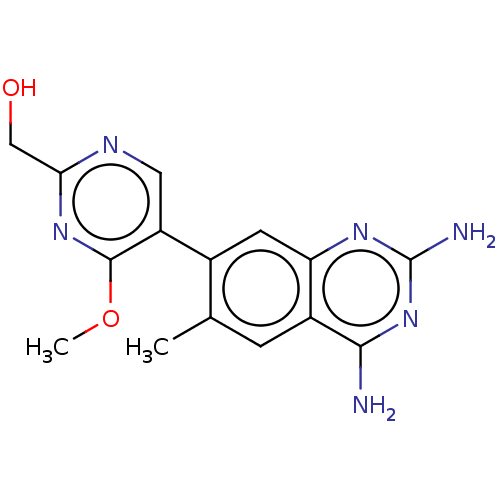 (US8835445, 29) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50351180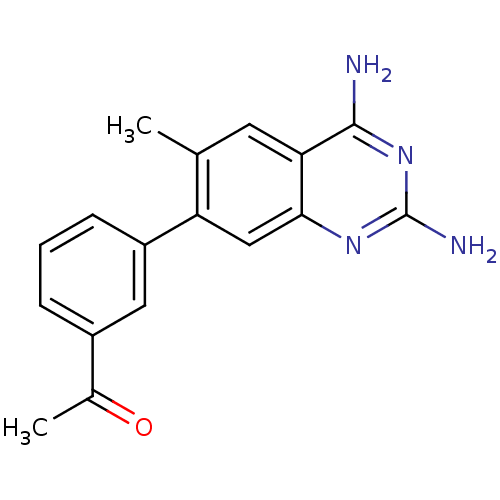 (CHEMBL1818120 | US8835445, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131842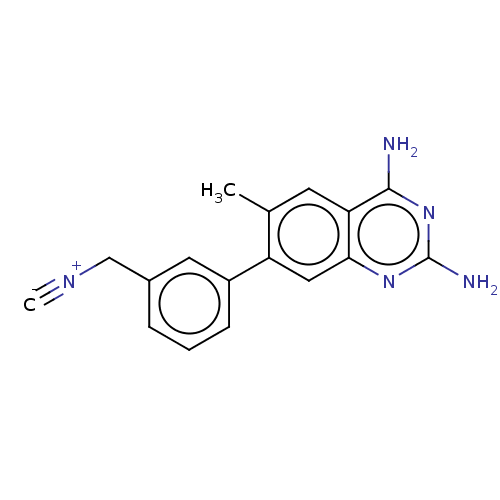 (US8835445, 13) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131839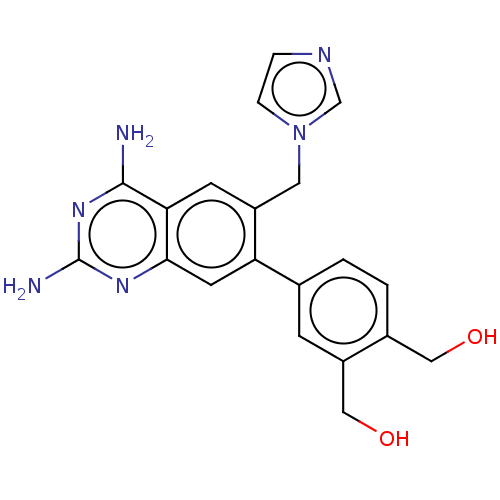 (US8835445, 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448748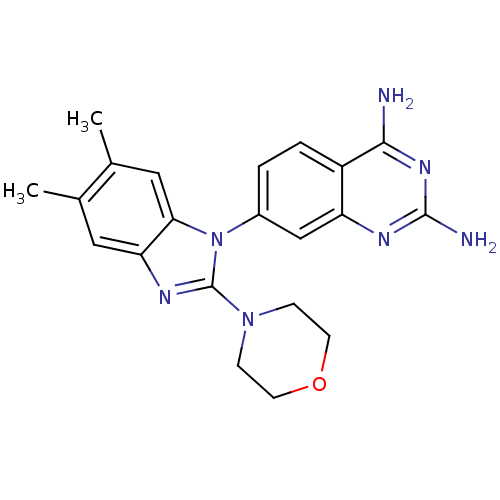 (CHEMBL3127917 | US8835445, 19) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131838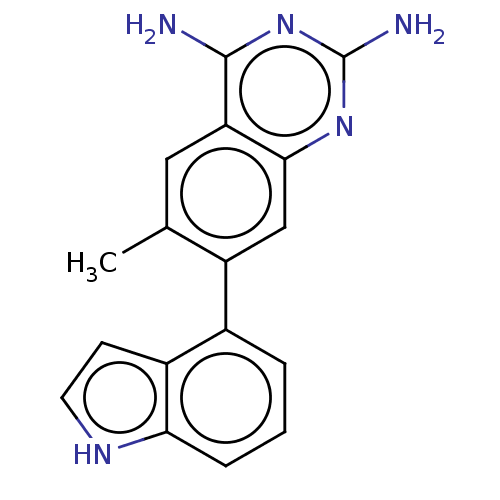 (US8835445, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131841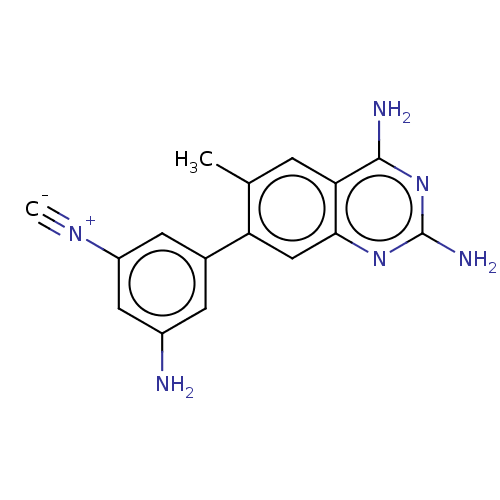 (US8835445, 12) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131837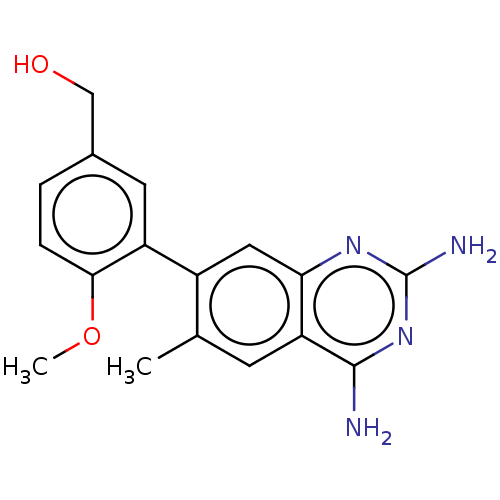 (US8835445, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131848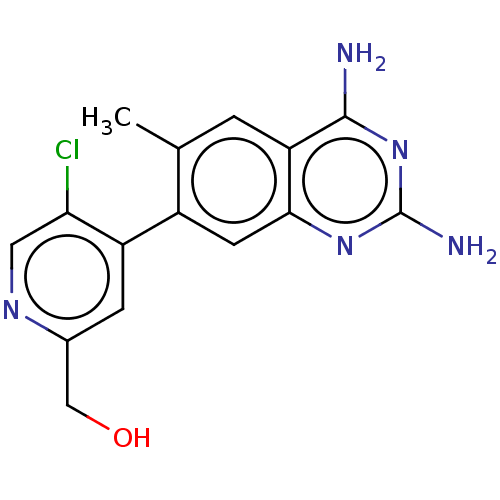 (US8835445, 28) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50351190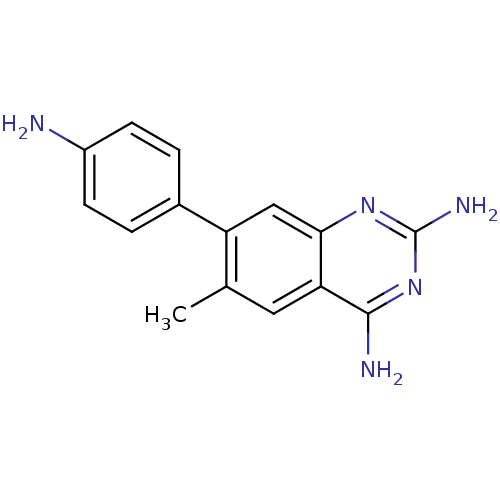 (CHEMBL1818110 | US8835445, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131851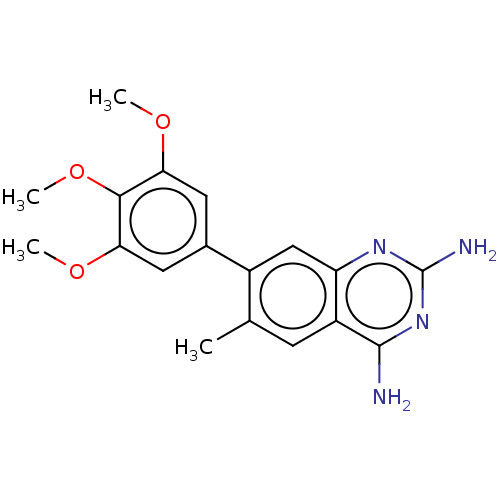 (US8835445, 33) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131840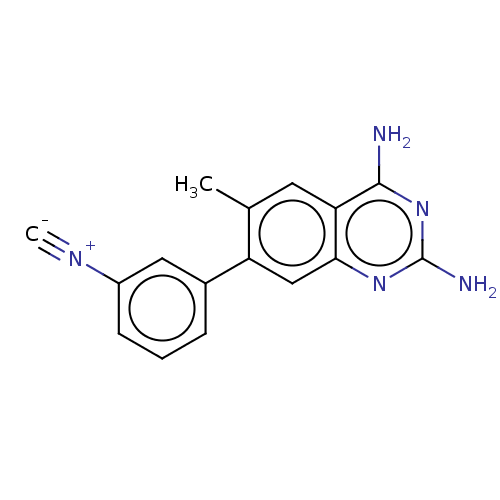 (US8835445, 11) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131855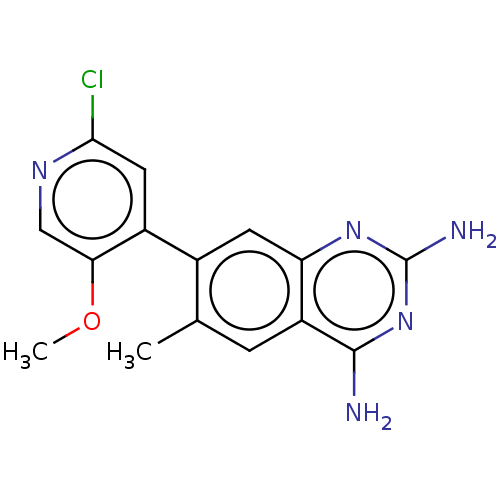 (US8835445, 38) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131843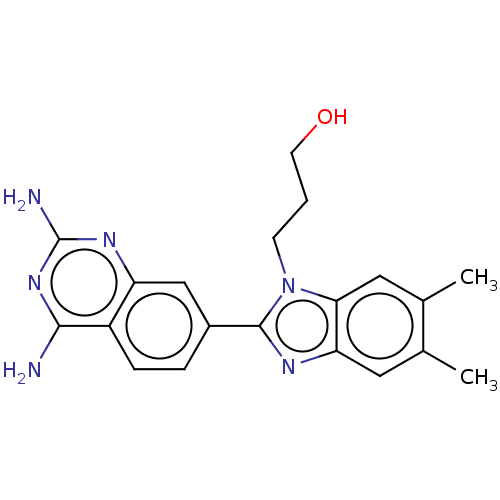 (US8835445, 14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131847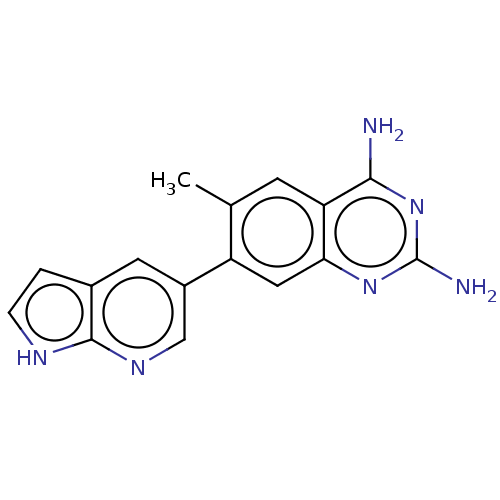 (US8835445, 27) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50351191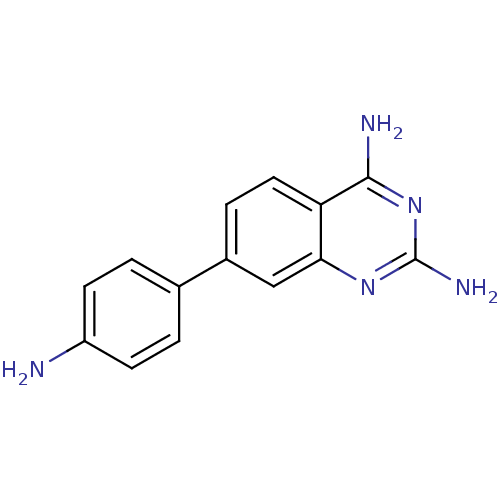 (CHEMBL1818109 | US8835445, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50351188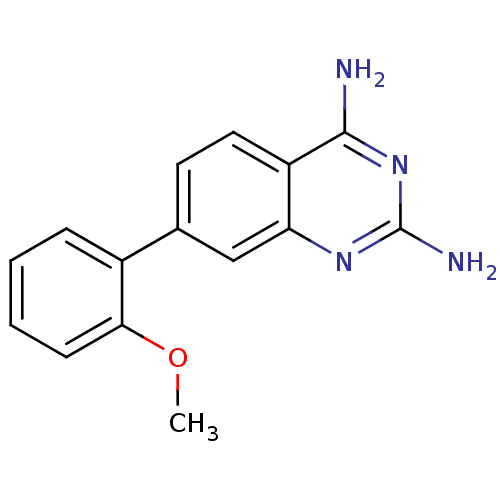 (CHEMBL1818113 | US8835445, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50448745 (CHEMBL3128013 | US8835445, 26) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131836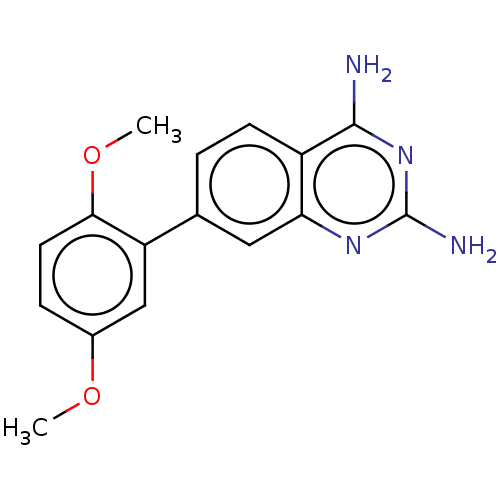 (US8835445, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50351181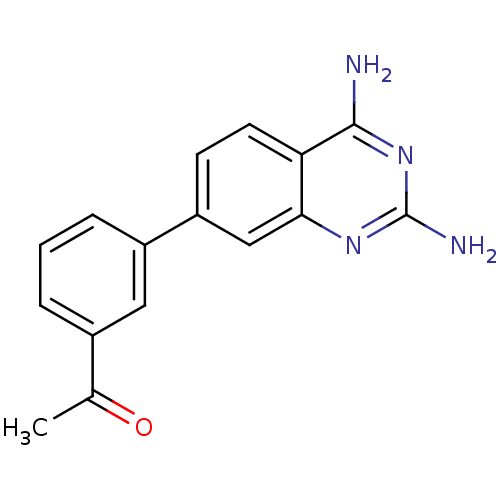 (CHEMBL1818119 | US8835445, 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50351174 (CHEMBL1818128 | US8835445, 31) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | US Patent | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50351175 (CHEMBL1818127 | US8835445, 30) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | US Patent | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50448744 (CHEMBL3128014 | US8835445, 25 | US8835445, 34) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | US Patent | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50448744 (CHEMBL3128014 | US8835445, 25 | US8835445, 34) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | US Patent | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131835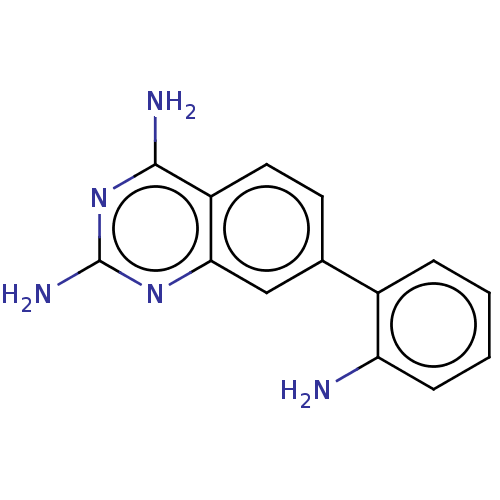 (US8835445, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 15.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50351180 (CHEMBL1818120 | US8835445, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | US Patent | 26.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM131837 (US8835445, 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 27.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50351173 (CHEMBL1818129 | US8835445, 40) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM131842 (US8835445, 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 368 total ) | Next | Last >> |