

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM248955 (US9434725, 186) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins in presence of ATP by caliper microfluidic mobility shift as... | J Med Chem 60: 4458-4473 (2017) Article DOI: 10.1021/acs.jmedchem.7b00418 BindingDB Entry DOI: 10.7270/Q2H997PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM248955 (US9434725, 186) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins in presence of ATP by caliper microfluidic mobility shift as... | J Med Chem 60: 4458-4473 (2017) Article DOI: 10.1021/acs.jmedchem.7b00418 BindingDB Entry DOI: 10.7270/Q2H997PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50266626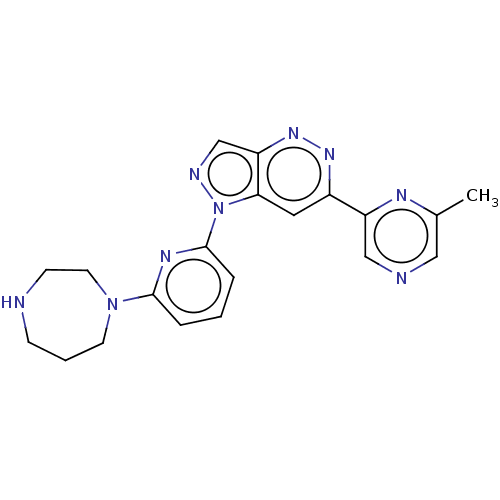 (CHEMBL4091687) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins in presence of ATP by caliper microfluidic mobility shift as... | J Med Chem 60: 4458-4473 (2017) Article DOI: 10.1021/acs.jmedchem.7b00418 BindingDB Entry DOI: 10.7270/Q2H997PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206757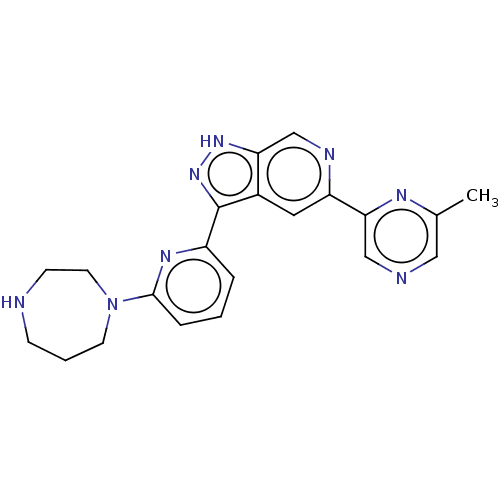 (US9260425, 522) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins in presence of ATP by caliper microfluidic mobility shift as... | J Med Chem 60: 4458-4473 (2017) Article DOI: 10.1021/acs.jmedchem.7b00418 BindingDB Entry DOI: 10.7270/Q2H997PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50266631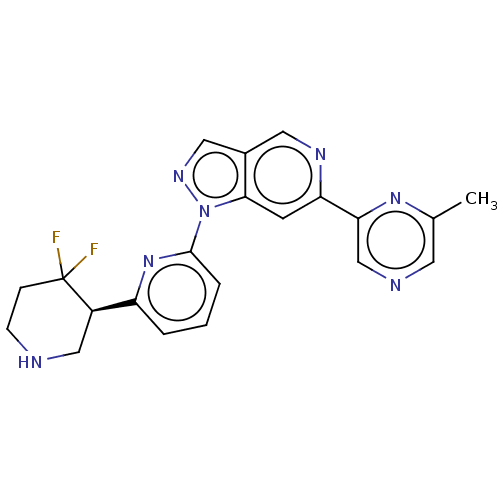 (CHEMBL4076913) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins in presence of ATP by caliper microfluidic mobility shift as... | J Med Chem 60: 4458-4473 (2017) Article DOI: 10.1021/acs.jmedchem.7b00418 BindingDB Entry DOI: 10.7270/Q2H997PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM248907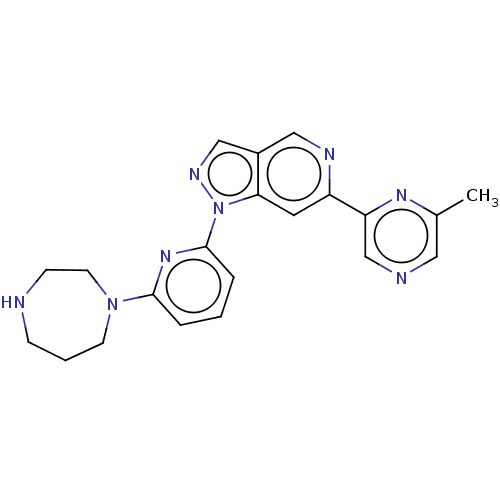 (US9434725, 138) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins in presence of ATP by caliper microfluidic mobility shift as... | J Med Chem 60: 4458-4473 (2017) Article DOI: 10.1021/acs.jmedchem.7b00418 BindingDB Entry DOI: 10.7270/Q2H997PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50131266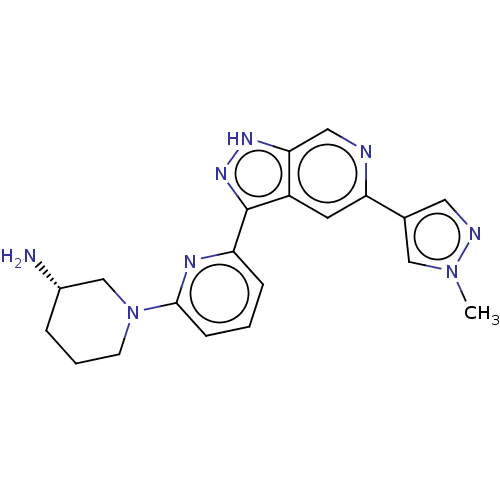 (CHEMBL3634758 | US9260425, 173) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins in presence of ATP by caliper microfluidic mobility shift as... | J Med Chem 60: 4458-4473 (2017) Article DOI: 10.1021/acs.jmedchem.7b00418 BindingDB Entry DOI: 10.7270/Q2H997PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM248907 (US9434725, 138) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins in presence of ATP by caliper microfluidic mobility shift as... | J Med Chem 60: 4458-4473 (2017) Article DOI: 10.1021/acs.jmedchem.7b00418 BindingDB Entry DOI: 10.7270/Q2H997PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM206420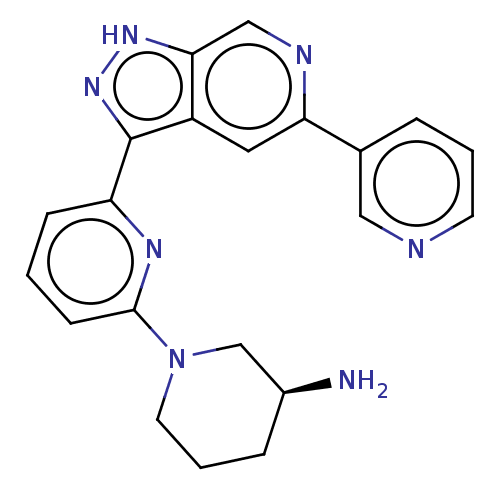 (US9260425, 161) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins in presence of ATP by caliper microfluidic mobility shift as... | J Med Chem 60: 4458-4473 (2017) Article DOI: 10.1021/acs.jmedchem.7b00418 BindingDB Entry DOI: 10.7270/Q2H997PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50266626 (CHEMBL4091687) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins in presence of ATP by caliper microfluidic mobility shift as... | J Med Chem 60: 4458-4473 (2017) Article DOI: 10.1021/acs.jmedchem.7b00418 BindingDB Entry DOI: 10.7270/Q2H997PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM206757 (US9260425, 522) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins in presence of ATP by caliper microfluidic mobility shift as... | J Med Chem 60: 4458-4473 (2017) Article DOI: 10.1021/acs.jmedchem.7b00418 BindingDB Entry DOI: 10.7270/Q2H997PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206420 (US9260425, 161) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins in presence of ATP by caliper microfluidic mobility shift as... | J Med Chem 60: 4458-4473 (2017) Article DOI: 10.1021/acs.jmedchem.7b00418 BindingDB Entry DOI: 10.7270/Q2H997PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50131266 (CHEMBL3634758 | US9260425, 173) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins in presence of ATP by caliper microfluidic mobility shift as... | J Med Chem 60: 4458-4473 (2017) Article DOI: 10.1021/acs.jmedchem.7b00418 BindingDB Entry DOI: 10.7270/Q2H997PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50266631 (CHEMBL4076913) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins in presence of ATP by caliper microfluidic mobility shift as... | J Med Chem 60: 4458-4473 (2017) Article DOI: 10.1021/acs.jmedchem.7b00418 BindingDB Entry DOI: 10.7270/Q2H997PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50094929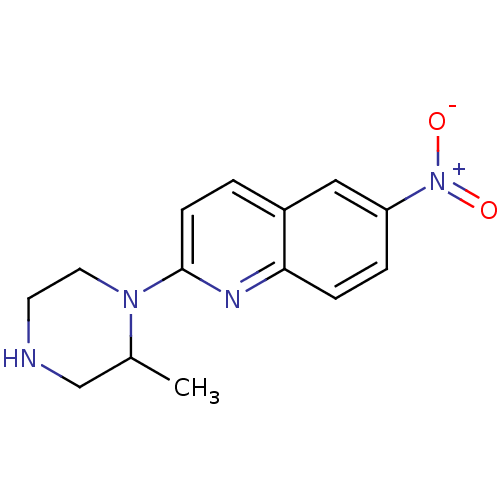 (2-(2-Methyl-piperazin-1-yl)-6-nitro-quinoline | CH...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Washington University Curated by ChEMBL | Assay Description In vitro radioligand [3H]-paroxetine from rat cortical Serotonin transporter | Bioorg Med Chem Lett 10: 2643-6 (2000) BindingDB Entry DOI: 10.7270/Q2W37VJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM206614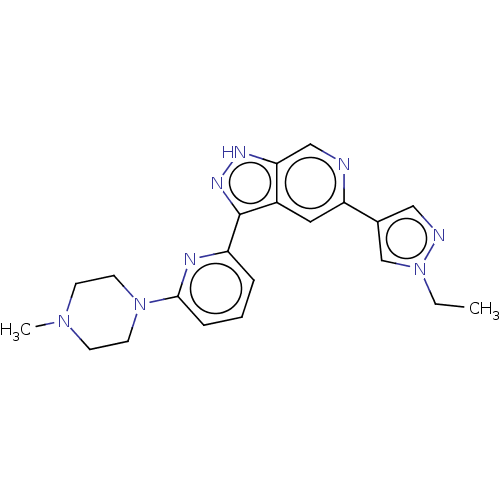 (US9260425, 372) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins in presence of ATP by caliper microfluidic mobility shift as... | J Med Chem 60: 4458-4473 (2017) Article DOI: 10.1021/acs.jmedchem.7b00418 BindingDB Entry DOI: 10.7270/Q2H997PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM206757 (US9260425, 522) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins in presence of ATP by caliper microfluidic mobility shift as... | J Med Chem 60: 4458-4473 (2017) Article DOI: 10.1021/acs.jmedchem.7b00418 BindingDB Entry DOI: 10.7270/Q2H997PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM248955 (US9434725, 186) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins in presence of ATP by caliper microfluidic mobility shift as... | J Med Chem 60: 4458-4473 (2017) Article DOI: 10.1021/acs.jmedchem.7b00418 BindingDB Entry DOI: 10.7270/Q2H997PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50247054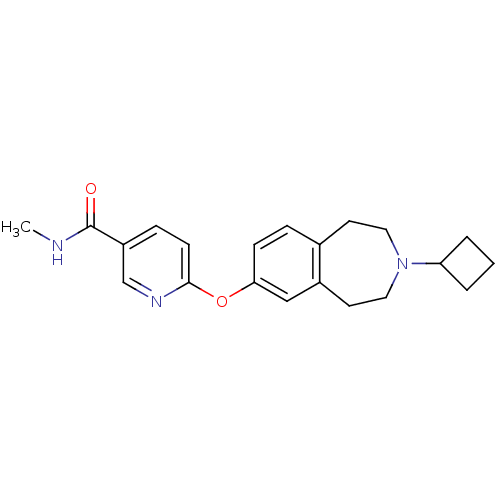 (6-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Binding affinity to histamine H3 receptor | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50247053 (1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in HEK293 cells by [35S]gammaS binding assay | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50063266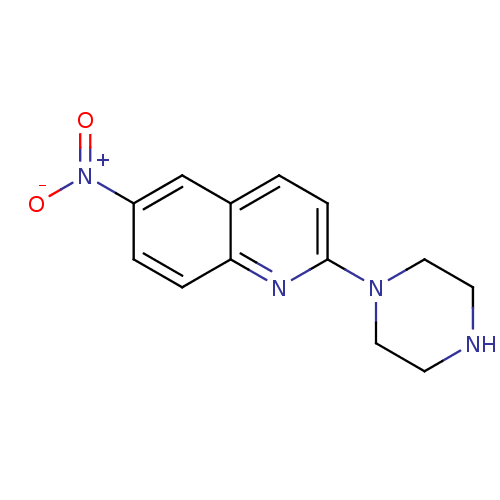 (6-Nitro-2-piperazin-1-yl-quinoline | 6-nitroquipaz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Washington University Curated by ChEMBL | Assay Description In vitro radioligand [3H]-paroxetine from rat cortical Serotonin transporter | Bioorg Med Chem Lett 10: 2643-6 (2000) BindingDB Entry DOI: 10.7270/Q2W37VJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50063266 (6-Nitro-2-piperazin-1-yl-quinoline | 6-nitroquipaz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Washington University Curated by ChEMBL | Assay Description In vitro radioligand [3H]-paroxetine from rat cortical Serotonin transporter | Bioorg Med Chem Lett 10: 2643-6 (2000) BindingDB Entry DOI: 10.7270/Q2W37VJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50094930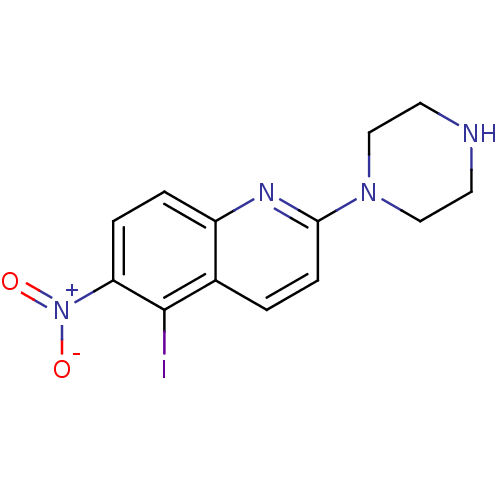 (5-Iodo-6-nitro-2-piperazin-1-yl-quinoline | CHEMBL...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Washington University Curated by ChEMBL | Assay Description In vitro radioligand [3H]-paroxetine from rat cortical Serotonin transporter | Bioorg Med Chem Lett 10: 2643-6 (2000) BindingDB Entry DOI: 10.7270/Q2W37VJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50266642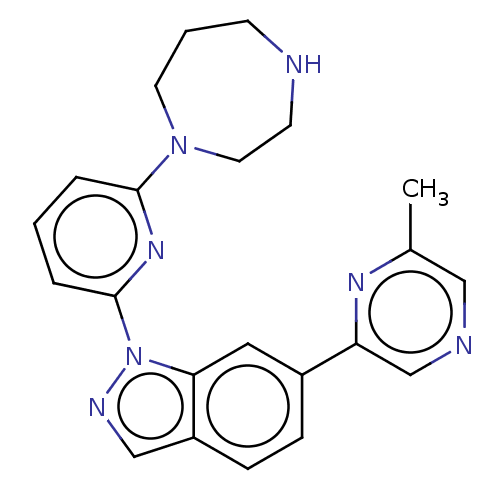 (CHEMBL4097308) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins in presence of ATP by caliper microfluidic mobility shift as... | J Med Chem 60: 4458-4473 (2017) Article DOI: 10.1021/acs.jmedchem.7b00418 BindingDB Entry DOI: 10.7270/Q2H997PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM206420 (US9260425, 161) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins in presence of ATP by caliper microfluidic mobility shift as... | J Med Chem 60: 4458-4473 (2017) Article DOI: 10.1021/acs.jmedchem.7b00418 BindingDB Entry DOI: 10.7270/Q2H997PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50268293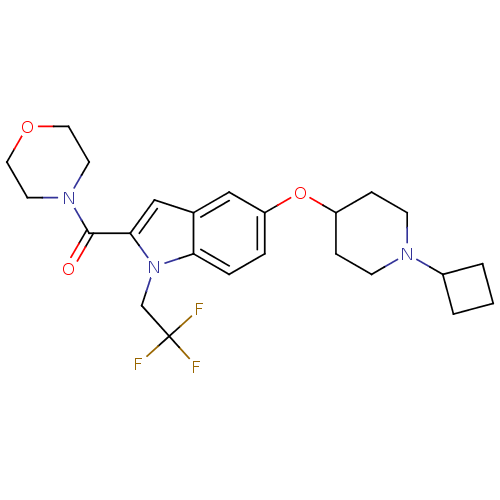 ((5-(1-cyclobutylpiperidin-4-yloxy)-1-(2,2,2-triflu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50131266 (CHEMBL3634758 | US9260425, 173) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins in presence of ATP by caliper microfluidic mobility shift as... | J Med Chem 60: 4458-4473 (2017) Article DOI: 10.1021/acs.jmedchem.7b00418 BindingDB Entry DOI: 10.7270/Q2H997PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM206689 (US9260425, 450) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins in presence of ATP by caliper microfluidic mobility shift as... | J Med Chem 60: 4458-4473 (2017) Article DOI: 10.1021/acs.jmedchem.7b00418 BindingDB Entry DOI: 10.7270/Q2H997PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50189257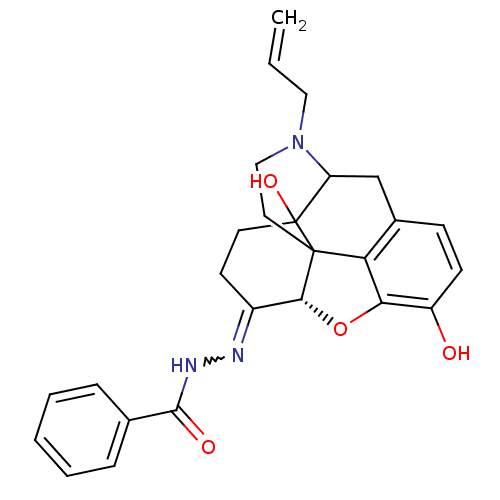 (CHEMBL378753 | NalBzOH) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Displacement of radiolabeled DAMGO from mu opioid receptor in Hartley guinea pig brain | Bioorg Med Chem Lett 16: 4291-5 (2006) Article DOI: 10.1016/j.bmcl.2006.05.060 BindingDB Entry DOI: 10.7270/Q2XG9RXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50266631 (CHEMBL4076913) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins in presence of ATP by caliper microfluidic mobility shift as... | J Med Chem 60: 4458-4473 (2017) Article DOI: 10.1021/acs.jmedchem.7b00418 BindingDB Entry DOI: 10.7270/Q2H997PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50169264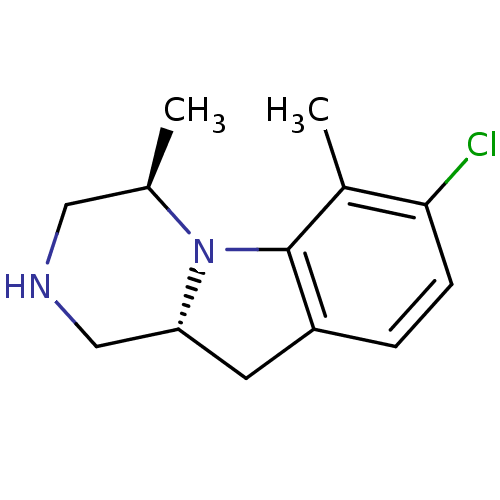 ((4R,10aR)-7-Chloro-4,6-dimethyl-1,2,3,4,10,10a-hex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Research Ltd. Curated by ChEMBL | Assay Description Binding affinity toward 5-HT2C receptor evaluated by displacement of [3H]-5-HT radioligand | Bioorg Med Chem Lett 15: 3604-8 (2005) Article DOI: 10.1016/j.bmcl.2005.05.074 BindingDB Entry DOI: 10.7270/Q2GQ6X87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50068133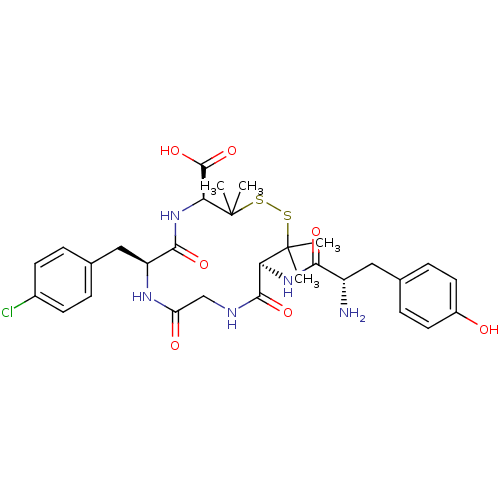 ((4S,7S,13S)-13-[(S)-2-Amino-3-(4-hydroxy-phenyl)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Displacement of radiolabeled DPDPE-Cl from delta opioid receptor in Hartley guinea pig brain | Bioorg Med Chem Lett 16: 4291-5 (2006) Article DOI: 10.1016/j.bmcl.2006.05.060 BindingDB Entry DOI: 10.7270/Q2XG9RXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50169264 ((4R,10aR)-7-Chloro-4,6-dimethyl-1,2,3,4,10,10a-hex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-5HT from human recombinant 5HT2C receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 1207-11 (2006) Article DOI: 10.1016/j.bmcl.2005.11.083 BindingDB Entry DOI: 10.7270/Q21R6Q3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Cavia porcellus) | BDBM50189257 (CHEMBL378753 | NalBzOH) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Displacement of radiolabeled NalBzOH from kappa3 opioid receptor in Hartley guinea pig brain | Bioorg Med Chem Lett 16: 4291-5 (2006) Article DOI: 10.1016/j.bmcl.2006.05.060 BindingDB Entry DOI: 10.7270/Q2XG9RXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50268291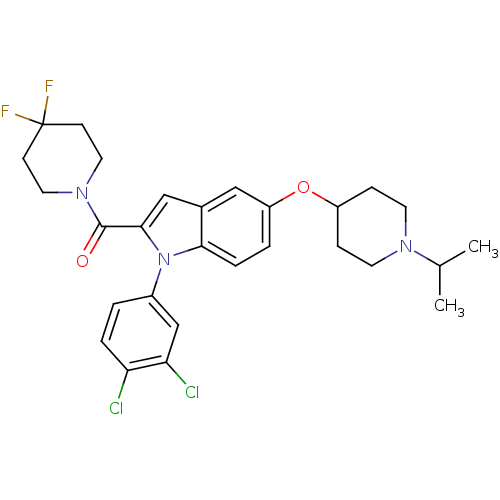 ((1-(3,4-dichlorophenyl)-5-(1-isopropylpiperidin-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM206614 (US9260425, 372) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins in presence of ATP by caliper microfluidic mobility shift as... | J Med Chem 60: 4458-4473 (2017) Article DOI: 10.1021/acs.jmedchem.7b00418 BindingDB Entry DOI: 10.7270/Q2H997PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50244490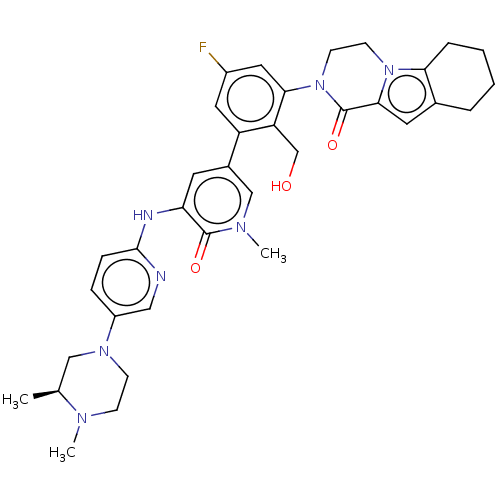 (CHEMBL4102992) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... | J Med Chem 61: 2227-2245 (2018) Article DOI: 10.1021/acs.jmedchem.7b01712 BindingDB Entry DOI: 10.7270/Q2H134F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50050915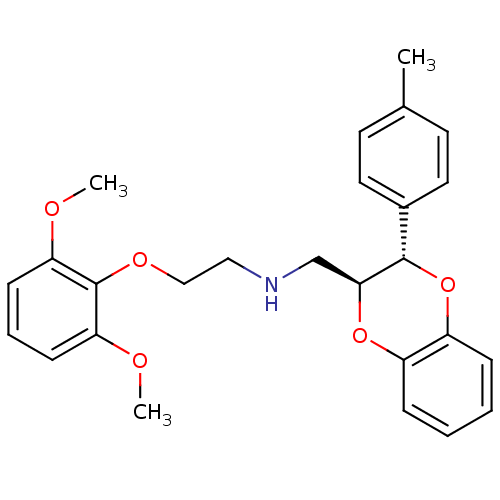 (CHEMBL72657 | [2-(2,6-Dimethoxy-phenoxy)-ethyl]-((...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Binding affinity of the compound towards Alpha-1A adrenergic receptor in bovine brainusing [3H]-prazosin as radioligand | J Med Chem 39: 2253-8 (1996) Article DOI: 10.1021/jm960069a BindingDB Entry DOI: 10.7270/Q22Z14NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111951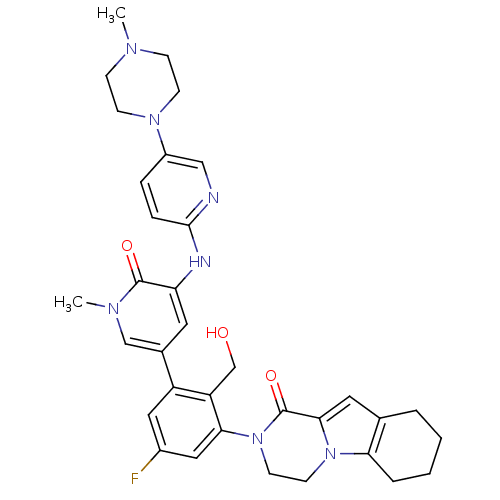 (US8618107, 197) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... | J Med Chem 61: 2227-2245 (2018) Article DOI: 10.1021/acs.jmedchem.7b01712 BindingDB Entry DOI: 10.7270/Q2H134F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50244489 (CHEMBL4095379) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... | J Med Chem 61: 2227-2245 (2018) Article DOI: 10.1021/acs.jmedchem.7b01712 BindingDB Entry DOI: 10.7270/Q2H134F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50453799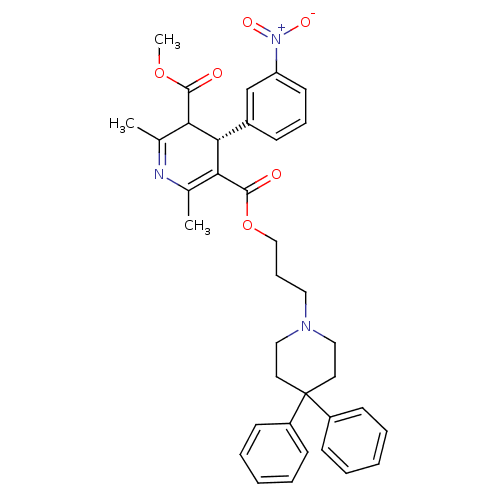 (Niguldipine) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Binding affinity of the compound towards Alpha-1A adrenergic receptor in bovine brainusing [3H]-prazosin as radioligand | J Med Chem 39: 2253-8 (1996) Article DOI: 10.1021/jm960069a BindingDB Entry DOI: 10.7270/Q22Z14NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50266628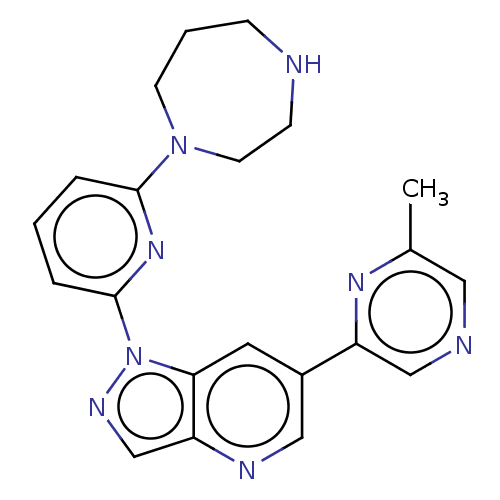 (CHEMBL4089586) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins in presence of ATP by caliper microfluidic mobility shift as... | J Med Chem 60: 4458-4473 (2017) Article DOI: 10.1021/acs.jmedchem.7b00418 BindingDB Entry DOI: 10.7270/Q2H997PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139391 ((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Binding affinity to histamine H3 receptor | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50266629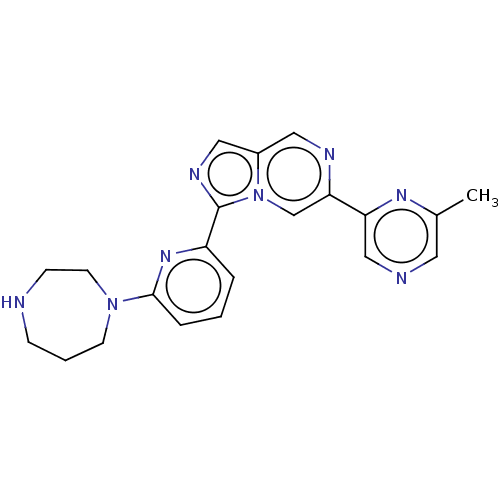 (CHEMBL4061456) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins in presence of ATP by caliper microfluidic mobility shift as... | J Med Chem 60: 4458-4473 (2017) Article DOI: 10.1021/acs.jmedchem.7b00418 BindingDB Entry DOI: 10.7270/Q2H997PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50268323 ((5-(1-cyclopropylpiperidin-4-yloxy)-1-(2,2,2-trifl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50428131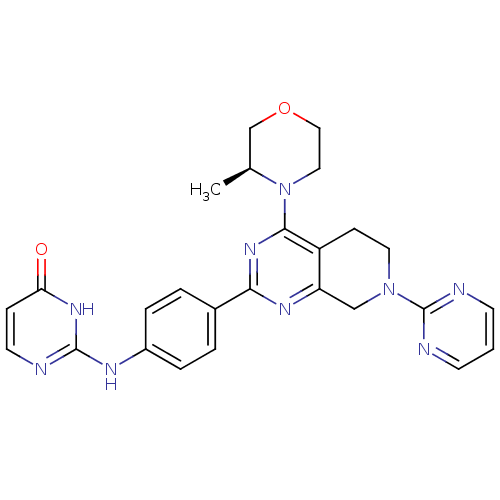 (CHEMBL2331687) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant mTOR (1360 to 2549)+GBL (unknown origin) using GFP-4E-BP1 as substrate after 30 mins by FRET assay | ACS Med Chem Lett 4: 103-7 (2013) Article DOI: 10.1021/ml3003132 BindingDB Entry DOI: 10.7270/Q28C9XK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50266642 (CHEMBL4097308) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins in presence of ATP by caliper microfluidic mobility shift as... | J Med Chem 60: 4458-4473 (2017) Article DOI: 10.1021/acs.jmedchem.7b00418 BindingDB Entry DOI: 10.7270/Q2H997PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50189257 (CHEMBL378753 | NalBzOH) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Displacement of radiolabeled U69593 from kappa1 opioid receptor in Hartley guinea pig brain | Bioorg Med Chem Lett 16: 4291-5 (2006) Article DOI: 10.1016/j.bmcl.2006.05.060 BindingDB Entry DOI: 10.7270/Q2XG9RXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50244491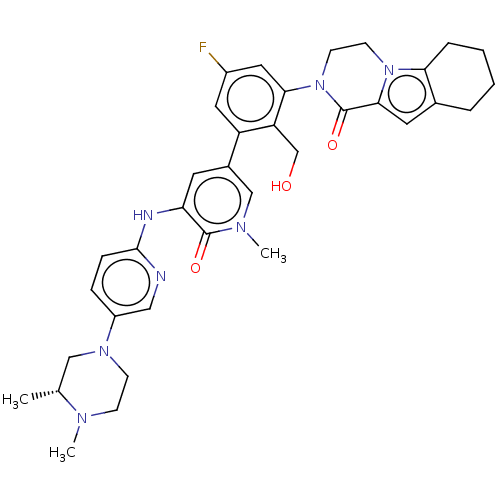 (CHEMBL4092794) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... | J Med Chem 61: 2227-2245 (2018) Article DOI: 10.1021/acs.jmedchem.7b01712 BindingDB Entry DOI: 10.7270/Q2H134F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50244488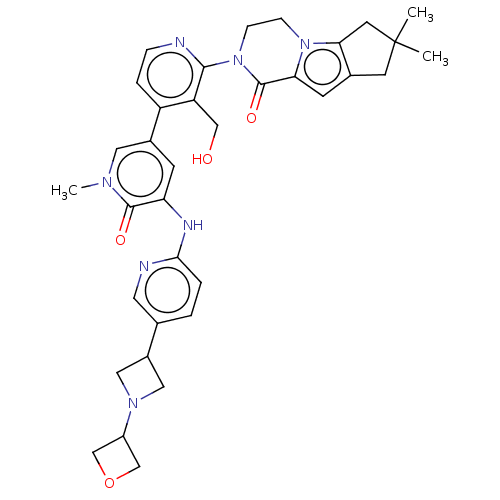 (CHEMBL4069790) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... | J Med Chem 61: 2227-2245 (2018) Article DOI: 10.1021/acs.jmedchem.7b01712 BindingDB Entry DOI: 10.7270/Q2H134F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5408 total ) | Next | Last >> |