

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50428976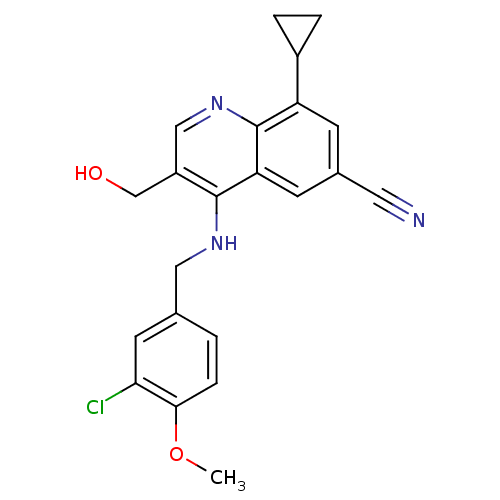 (CHEMBL2333219) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.277 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of PDE5 (unknown origin) using FAM-cGMP as substrate after 60 mins by fluorescence assay | Eur J Med Chem 60: 285-94 (2013) Article DOI: 10.1016/j.ejmech.2012.12.009 BindingDB Entry DOI: 10.7270/Q22F7PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50428975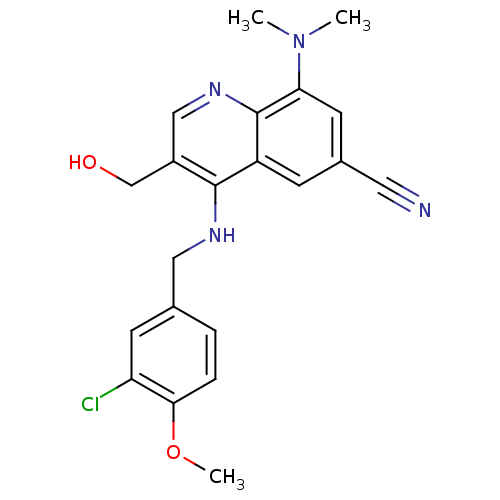 (CHEMBL2333220) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of PDE5 (unknown origin) using FAM-cGMP as substrate after 60 mins by fluorescence assay | Eur J Med Chem 60: 285-94 (2013) Article DOI: 10.1016/j.ejmech.2012.12.009 BindingDB Entry DOI: 10.7270/Q22F7PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202150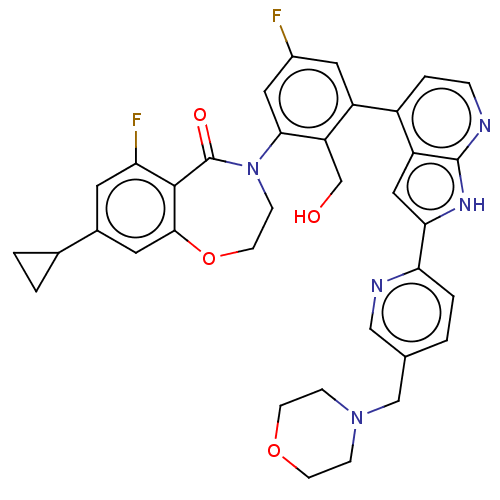 (US9233983, I-20) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description The inhibition assays were performed using the generic tyrosine kinase assay kit from C is Bio (HTRF KinEASE-TK #62TK0PEB). The assay is based on t... | US Patent US9233983 (2016) BindingDB Entry DOI: 10.7270/Q22J69PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143373 (US9682953, 20.A-10 | US9682953, 20.A-9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50428971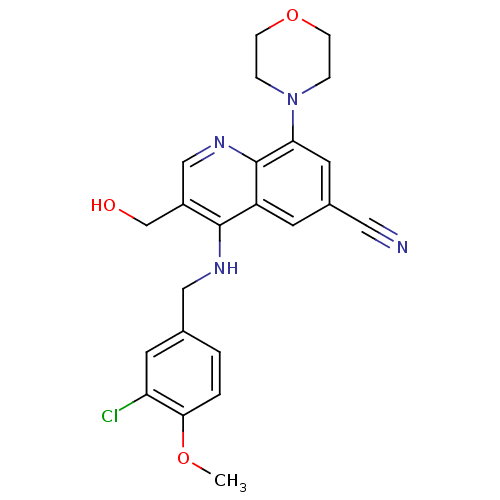 (CHEMBL2333224) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of PDE5 (unknown origin) using FAM-cGMP as substrate after 60 mins by fluorescence assay | Eur J Med Chem 60: 285-94 (2013) Article DOI: 10.1016/j.ejmech.2012.12.009 BindingDB Entry DOI: 10.7270/Q22F7PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202149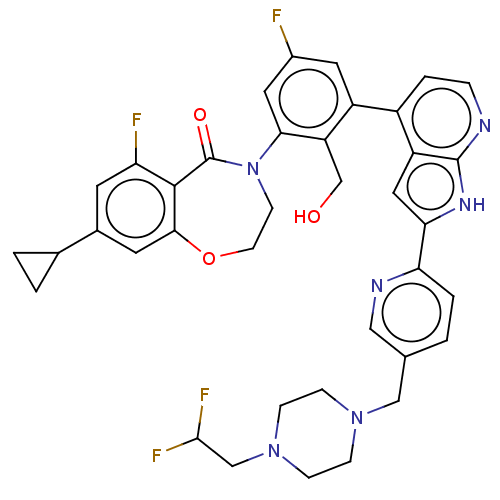 (US9233983, I-19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description The inhibition assays were performed using the generic tyrosine kinase assay kit from C is Bio (HTRF KinEASE-TK #62TK0PEB). The assay is based on t... | US Patent US9233983 (2016) BindingDB Entry DOI: 10.7270/Q22J69PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143358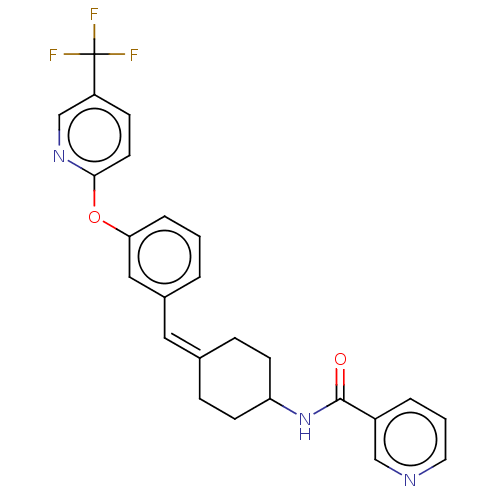 (US9682953, 20.A-2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202226 (US9233983, R-5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description The inhibition assays were performed using the generic tyrosine kinase assay kit from C is Bio (HTRF KinEASE-TK #62TK0PEB). The assay is based on t... | US Patent US9233983 (2016) BindingDB Entry DOI: 10.7270/Q22J69PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143449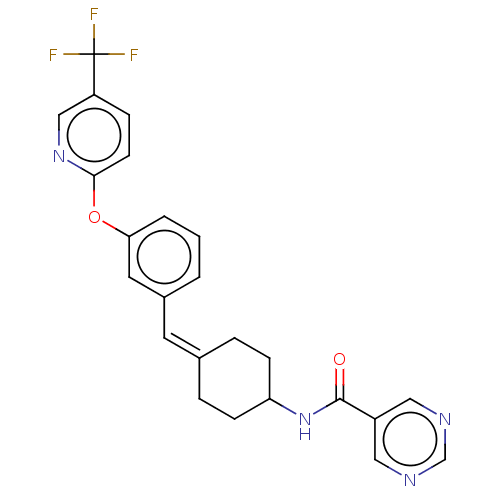 (US9682953, 20.A-19 | US9682953, 20.A-20) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14776 (2-{2-ethoxy-5-[(4-ethylpiperazine-1-)sulfonyl]phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of PDE5 (unknown origin) using FAM-cGMP as substrate after 60 mins by fluorescence assay | Eur J Med Chem 60: 285-94 (2013) Article DOI: 10.1016/j.ejmech.2012.12.009 BindingDB Entry DOI: 10.7270/Q22F7PSZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202200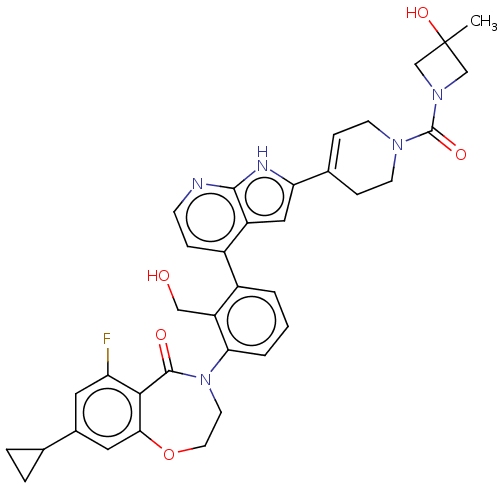 (US9233983, P-15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description The inhibition assays were performed using the generic tyrosine kinase assay kit from C is Bio (HTRF KinEASE-TK #62TK0PEB). The assay is based on t... | US Patent US9233983 (2016) BindingDB Entry DOI: 10.7270/Q22J69PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202199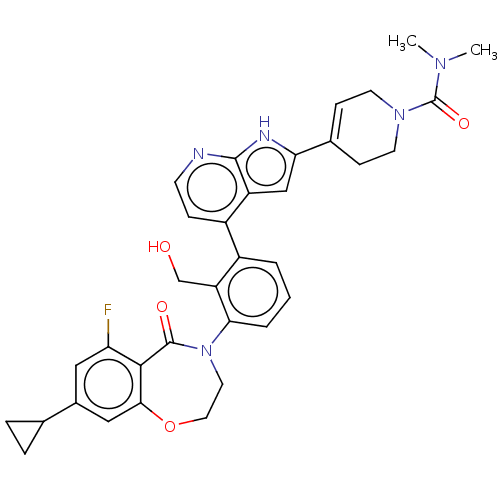 (US9233983, P-14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description The inhibition assays were performed using the generic tyrosine kinase assay kit from C is Bio (HTRF KinEASE-TK #62TK0PEB). The assay is based on t... | US Patent US9233983 (2016) BindingDB Entry DOI: 10.7270/Q22J69PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202198 (US9233983, P-13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description The inhibition assays were performed using the generic tyrosine kinase assay kit from C is Bio (HTRF KinEASE-TK #62TK0PEB). The assay is based on t... | US Patent US9233983 (2016) BindingDB Entry DOI: 10.7270/Q22J69PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202131 (US9233983, I-2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description The inhibition assays were performed using the generic tyrosine kinase assay kit from C is Bio (HTRF KinEASE-TK #62TK0PEB). The assay is based on t... | US Patent US9233983 (2016) BindingDB Entry DOI: 10.7270/Q22J69PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143391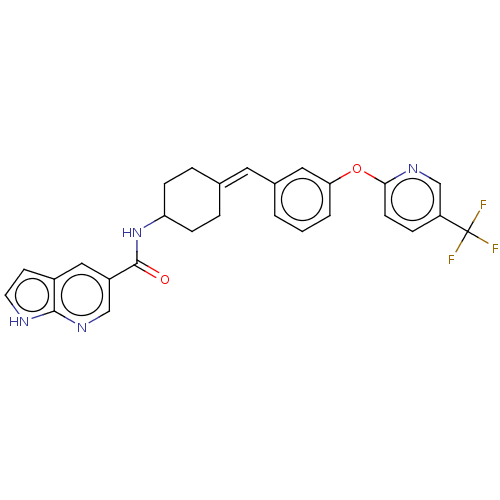 (US9682953, 20.A-14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143368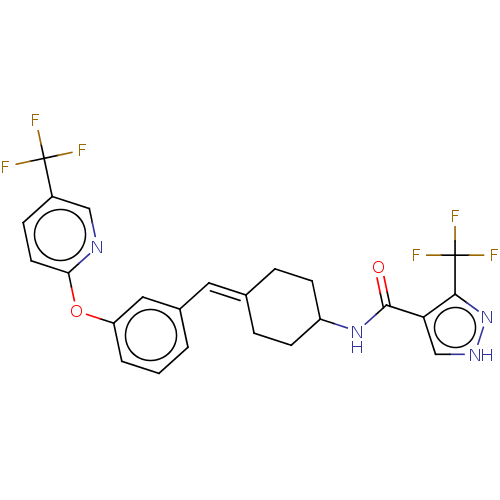 (US9682953, 20.A-8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143366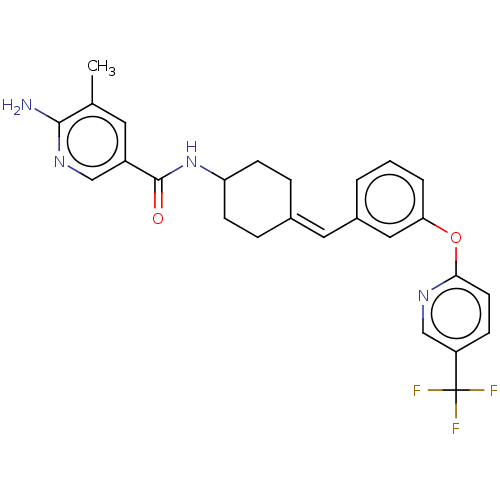 (US9682953, 20.A-6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143361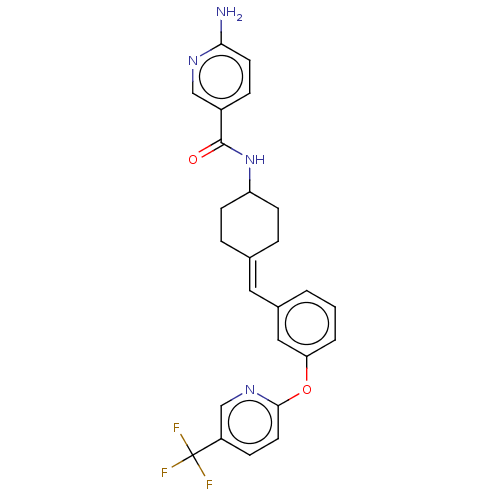 (US9682953, 20.A-4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143291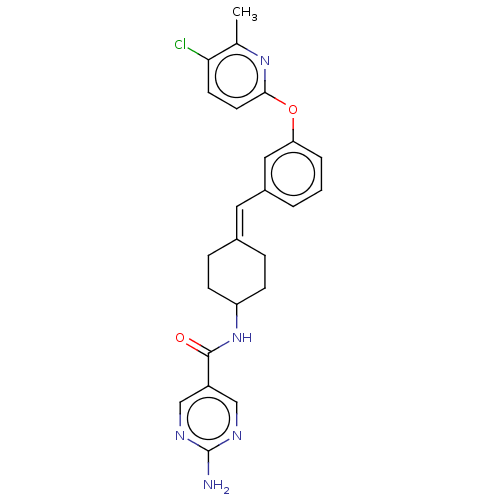 (US9682953, 2.E-27) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143274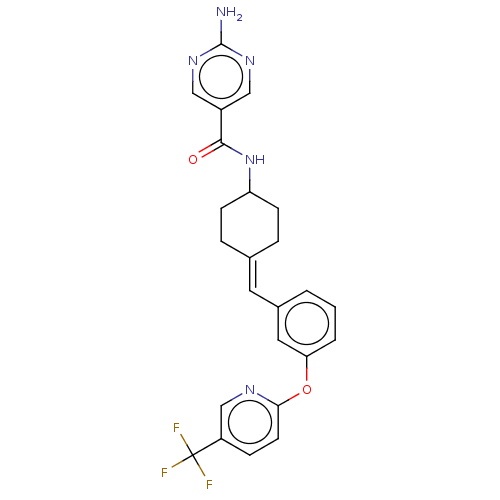 (US9682953, 2.C-6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143352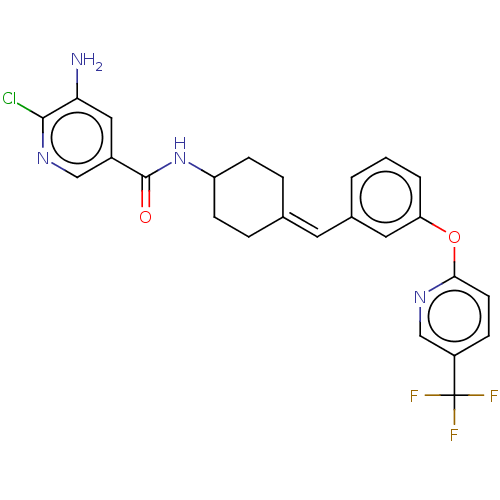 (US9682953, 10.1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202215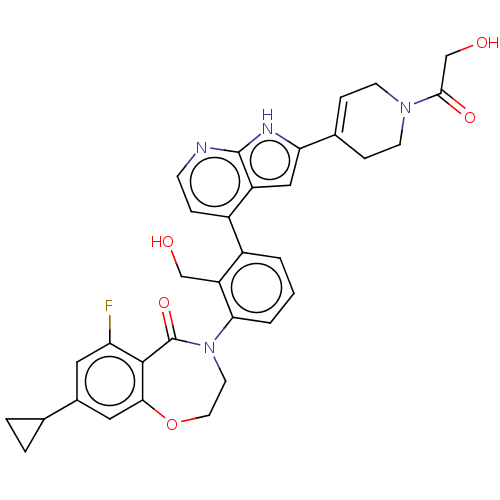 (US9233983, P-30) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description The inhibition assays were performed using the generic tyrosine kinase assay kit from C is Bio (HTRF KinEASE-TK #62TK0PEB). The assay is based on t... | US Patent US9233983 (2016) BindingDB Entry DOI: 10.7270/Q22J69PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202131 (US9233983, I-2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human full length BTK preincubated for 10 mins followed by Tk-biotin peptide substrate addition after 90 mins by TR-FRET as... | Bioorg Med Chem Lett 27: 1867-1873 (2017) Article DOI: 10.1016/j.bmcl.2017.02.026 BindingDB Entry DOI: 10.7270/Q2K35X74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202199 (US9233983, P-14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human full length BTK preincubated for 10 mins followed by Tk-biotin peptide substrate addition after 90 mins by TR-FRET as... | Bioorg Med Chem Lett 27: 1867-1873 (2017) Article DOI: 10.1016/j.bmcl.2017.02.026 BindingDB Entry DOI: 10.7270/Q2K35X74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143456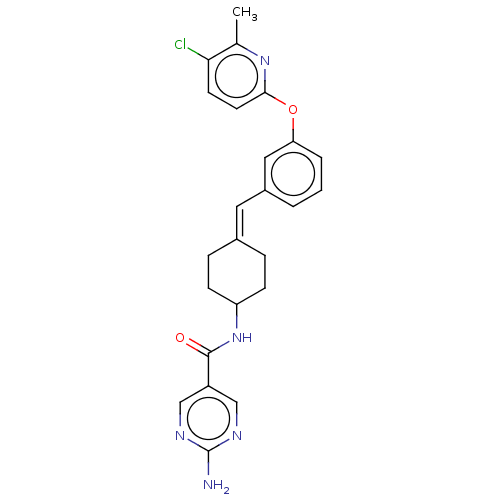 (US9682953, 20.A-21 | US9682953, 20.A-22) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202200 (US9233983, P-15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human full length BTK preincubated for 10 mins followed by Tk-biotin peptide substrate addition after 90 mins by TR-FRET as... | Bioorg Med Chem Lett 27: 1867-1873 (2017) Article DOI: 10.1016/j.bmcl.2017.02.026 BindingDB Entry DOI: 10.7270/Q2K35X74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202220 (US9233983, Q-3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description The inhibition assays were performed using the generic tyrosine kinase assay kit from C is Bio (HTRF KinEASE-TK #62TK0PEB). The assay is based on t... | US Patent US9233983 (2016) BindingDB Entry DOI: 10.7270/Q22J69PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14777 ((2R,8R)-2-(2H-1,3-benzodioxol-5-yl)-6-methyl-3,6,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of PDE5 (unknown origin) using FAM-cGMP as substrate after 60 mins by fluorescence assay | Eur J Med Chem 60: 285-94 (2013) Article DOI: 10.1016/j.ejmech.2012.12.009 BindingDB Entry DOI: 10.7270/Q22F7PSZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50450836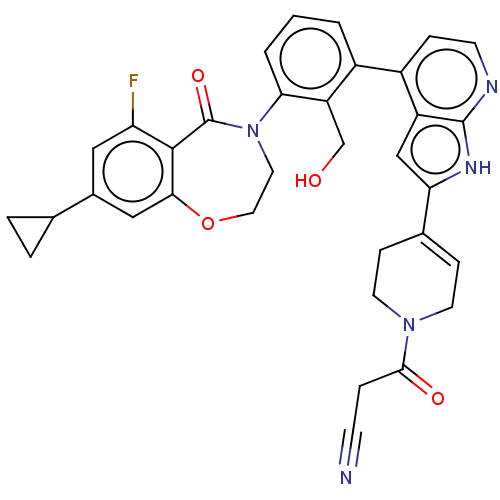 (CHEMBL4216939) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human full length BTK preincubated for 10 mins followed by Tk-biotin peptide substrate addition after 90 mins by TR-FRET as... | Bioorg Med Chem Lett 27: 1867-1873 (2017) Article DOI: 10.1016/j.bmcl.2017.02.026 BindingDB Entry DOI: 10.7270/Q2K35X74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202207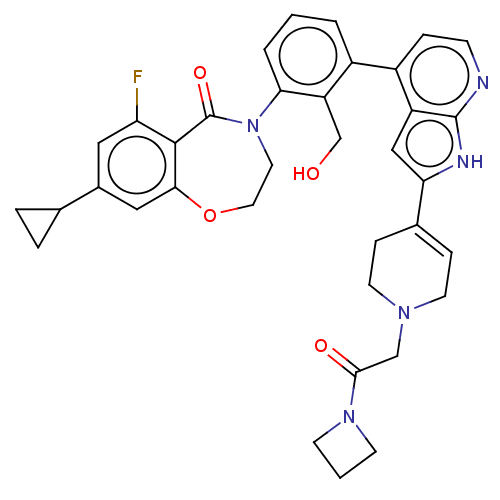 (US9233983, P-22) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description The inhibition assays were performed using the generic tyrosine kinase assay kit from C is Bio (HTRF KinEASE-TK #62TK0PEB). The assay is based on t... | US Patent US9233983 (2016) BindingDB Entry DOI: 10.7270/Q22J69PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202212 (US9233983, P-27) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description The inhibition assays were performed using the generic tyrosine kinase assay kit from C is Bio (HTRF KinEASE-TK #62TK0PEB). The assay is based on t... | US Patent US9233983 (2016) BindingDB Entry DOI: 10.7270/Q22J69PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202214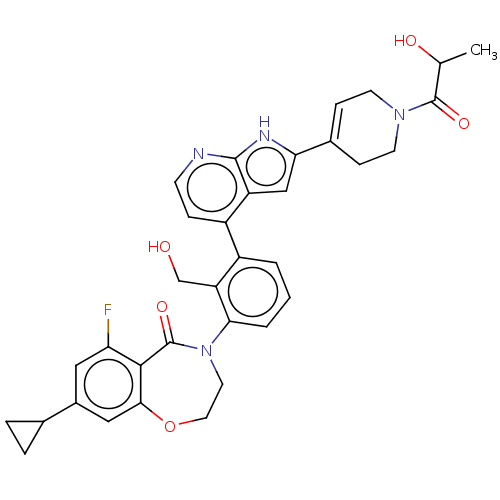 (US9233983, P-29) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description The inhibition assays were performed using the generic tyrosine kinase assay kit from C is Bio (HTRF KinEASE-TK #62TK0PEB). The assay is based on t... | US Patent US9233983 (2016) BindingDB Entry DOI: 10.7270/Q22J69PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202201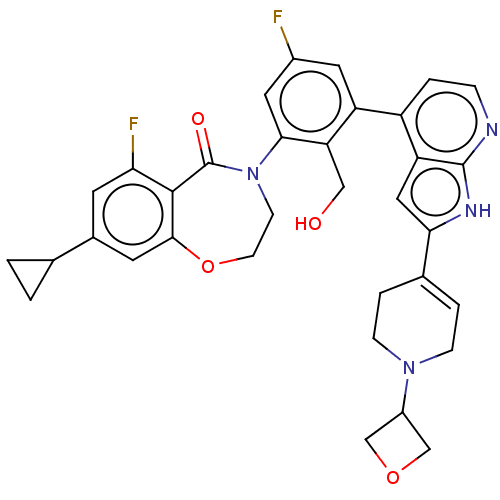 (US9233983, P-16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description The inhibition assays were performed using the generic tyrosine kinase assay kit from C is Bio (HTRF KinEASE-TK #62TK0PEB). The assay is based on t... | US Patent US9233983 (2016) BindingDB Entry DOI: 10.7270/Q22J69PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202147 (US9233983, I-17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description The inhibition assays were performed using the generic tyrosine kinase assay kit from C is Bio (HTRF KinEASE-TK #62TK0PEB). The assay is based on t... | US Patent US9233983 (2016) BindingDB Entry DOI: 10.7270/Q22J69PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202208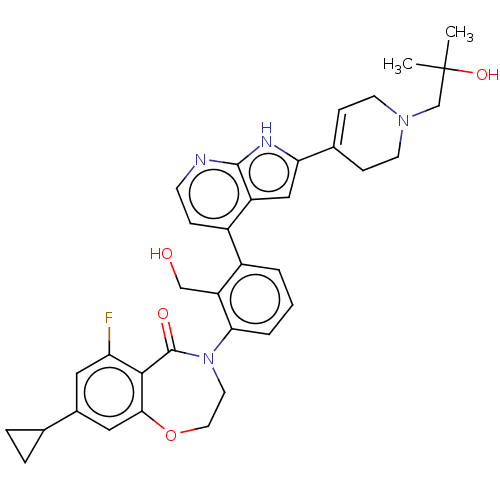 (US9233983, P-23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description The inhibition assays were performed using the generic tyrosine kinase assay kit from C is Bio (HTRF KinEASE-TK #62TK0PEB). The assay is based on t... | US Patent US9233983 (2016) BindingDB Entry DOI: 10.7270/Q22J69PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202208 (US9233983, P-23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human full length BTK preincubated for 10 mins followed by Tk-biotin peptide substrate addition after 90 mins by TR-FRET as... | Bioorg Med Chem Lett 27: 1867-1873 (2017) Article DOI: 10.1016/j.bmcl.2017.02.026 BindingDB Entry DOI: 10.7270/Q2K35X74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202144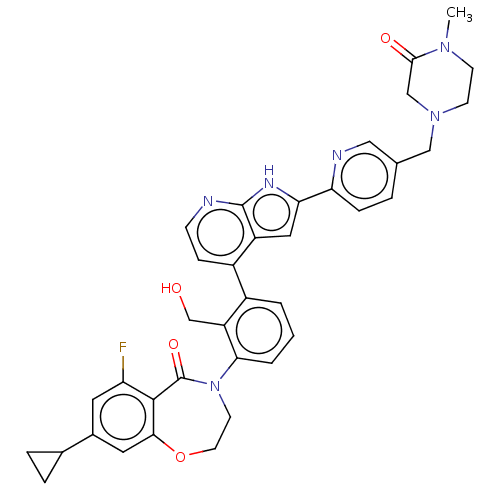 (US9233983, I-15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description The inhibition assays were performed using the generic tyrosine kinase assay kit from C is Bio (HTRF KinEASE-TK #62TK0PEB). The assay is based on t... | US Patent US9233983 (2016) BindingDB Entry DOI: 10.7270/Q22J69PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202223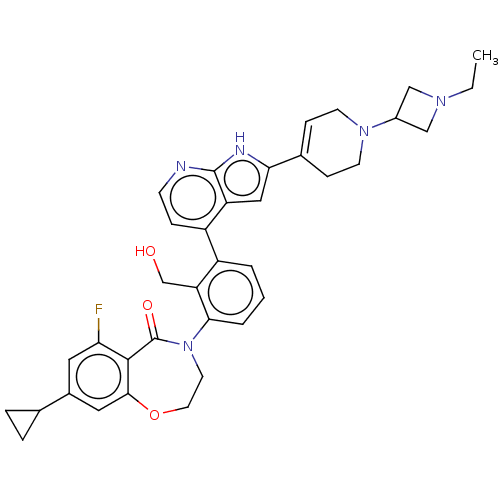 (US9233983, R-2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description The inhibition assays were performed using the generic tyrosine kinase assay kit from C is Bio (HTRF KinEASE-TK #62TK0PEB). The assay is based on t... | US Patent US9233983 (2016) BindingDB Entry DOI: 10.7270/Q22J69PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202145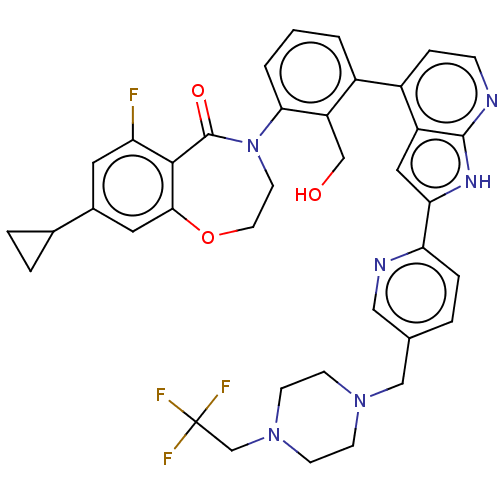 (US9233983, I-16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description The inhibition assays were performed using the generic tyrosine kinase assay kit from C is Bio (HTRF KinEASE-TK #62TK0PEB). The assay is based on t... | US Patent US9233983 (2016) BindingDB Entry DOI: 10.7270/Q22J69PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202225 (US9233983, R-4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description The inhibition assays were performed using the generic tyrosine kinase assay kit from C is Bio (HTRF KinEASE-TK #62TK0PEB). The assay is based on t... | US Patent US9233983 (2016) BindingDB Entry DOI: 10.7270/Q22J69PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202203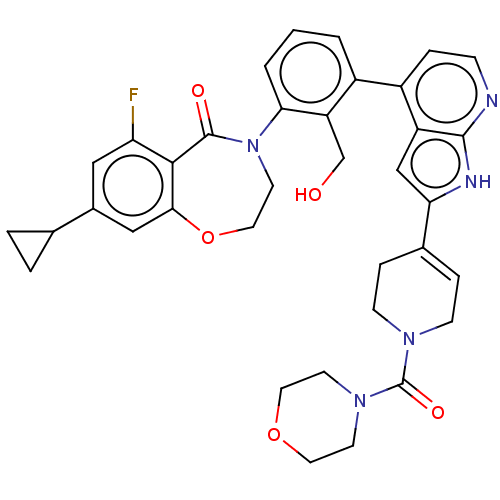 (US9233983, P-18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description The inhibition assays were performed using the generic tyrosine kinase assay kit from C is Bio (HTRF KinEASE-TK #62TK0PEB). The assay is based on t... | US Patent US9233983 (2016) BindingDB Entry DOI: 10.7270/Q22J69PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202122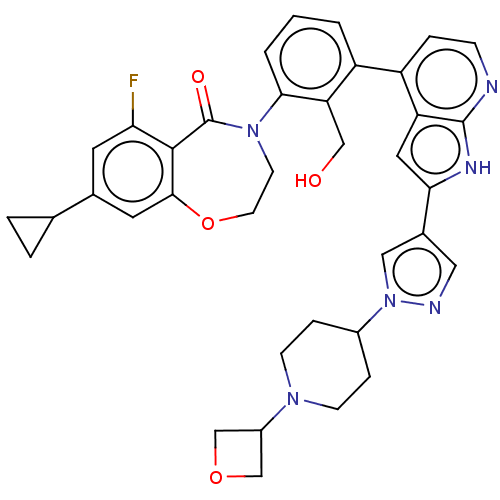 (US9233983, D-14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description The inhibition assays were performed using the generic tyrosine kinase assay kit from C is Bio (HTRF KinEASE-TK #62TK0PEB). The assay is based on t... | US Patent US9233983 (2016) BindingDB Entry DOI: 10.7270/Q22J69PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202143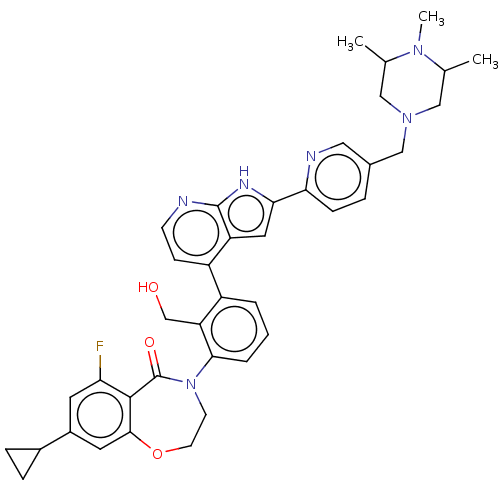 (US9233983, I-14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description The inhibition assays were performed using the generic tyrosine kinase assay kit from C is Bio (HTRF KinEASE-TK #62TK0PEB). The assay is based on t... | US Patent US9233983 (2016) BindingDB Entry DOI: 10.7270/Q22J69PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143375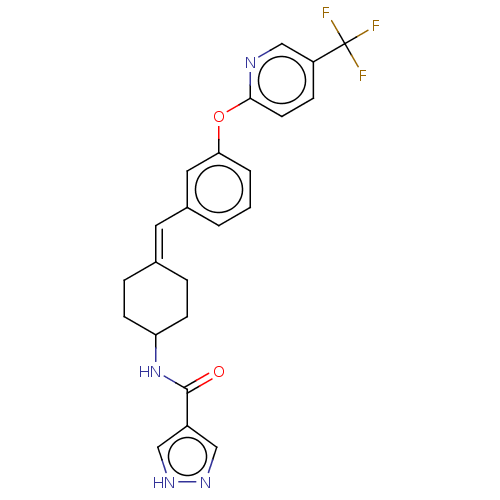 (US9682953, 20.A-11 | US9682953, 20.A-12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202122 (US9233983, D-14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human full length BTK preincubated for 10 mins followed by Tk-biotin peptide substrate addition after 90 mins by TR-FRET as... | Bioorg Med Chem Lett 27: 1867-1873 (2017) Article DOI: 10.1016/j.bmcl.2017.02.026 BindingDB Entry DOI: 10.7270/Q2K35X74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202148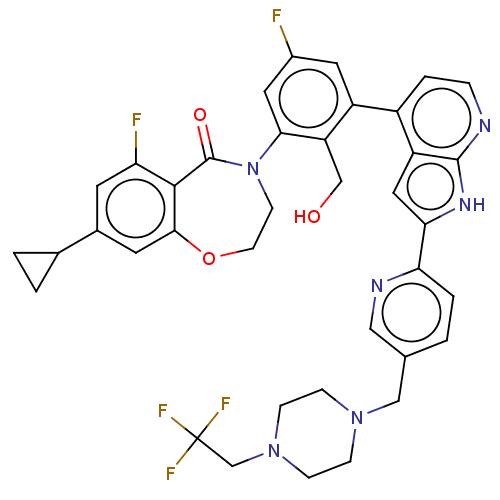 (US9233983, I-18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description The inhibition assays were performed using the generic tyrosine kinase assay kit from C is Bio (HTRF KinEASE-TK #62TK0PEB). The assay is based on t... | US Patent US9233983 (2016) BindingDB Entry DOI: 10.7270/Q22J69PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM143265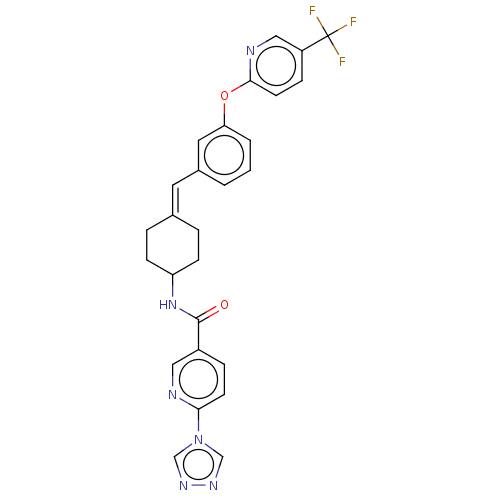 (US9682953, 2.B-13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description All reagents were purchased from Sigma-Aldrich unless specified. Human and Rat Fatty Acid Amide Hydrolase (FAAH) genes used in assay have been descri... | US Patent US9682953 (2017) BindingDB Entry DOI: 10.7270/Q2BR8QBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202189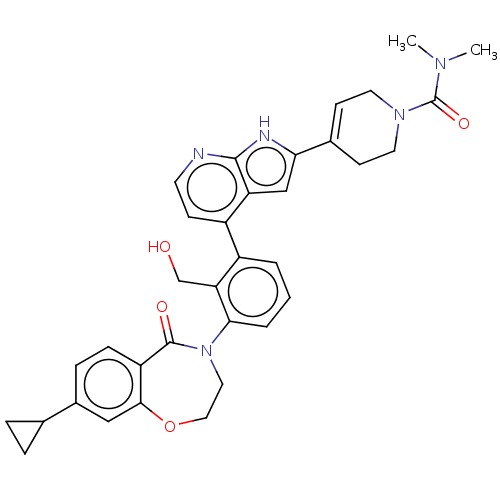 (US9233983, P-5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description The inhibition assays were performed using the generic tyrosine kinase assay kit from C is Bio (HTRF KinEASE-TK #62TK0PEB). The assay is based on t... | US Patent US9233983 (2016) BindingDB Entry DOI: 10.7270/Q22J69PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202174 (US9233983, K-22) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description The inhibition assays were performed using the generic tyrosine kinase assay kit from C is Bio (HTRF KinEASE-TK #62TK0PEB). The assay is based on t... | US Patent US9233983 (2016) BindingDB Entry DOI: 10.7270/Q22J69PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202142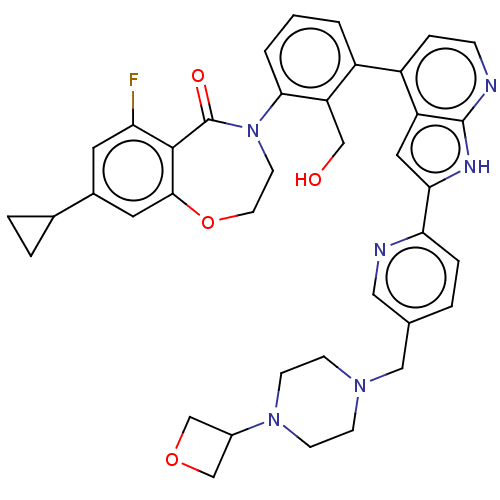 (US9233983, I-13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ADVINUS THERAPEUTICS LIMITED US Patent | Assay Description The inhibition assays were performed using the generic tyrosine kinase assay kit from C is Bio (HTRF KinEASE-TK #62TK0PEB). The assay is based on t... | US Patent US9233983 (2016) BindingDB Entry DOI: 10.7270/Q22J69PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 385 total ) | Next | Last >> |