

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50288098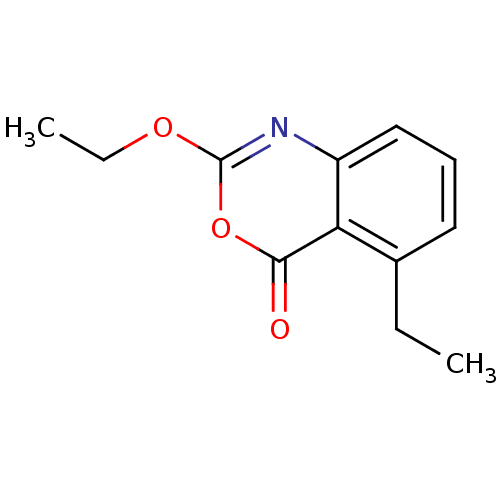 (2-Ethoxy-5-ethyl-benzo[d][1,3]oxazin-4-one | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0430 | n/a | n/a | n/a | n/a | 0.0000145 | 3.39E+5 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406288 (CHEMBL344066) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0680 | n/a | n/a | n/a | n/a | 0.0000759 | 1.12E+6 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219919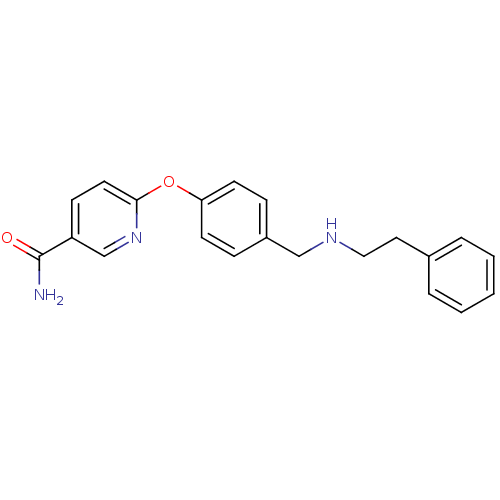 (6-(4-((phenethylamino)methyl)phenoxy)nicotinamide ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406293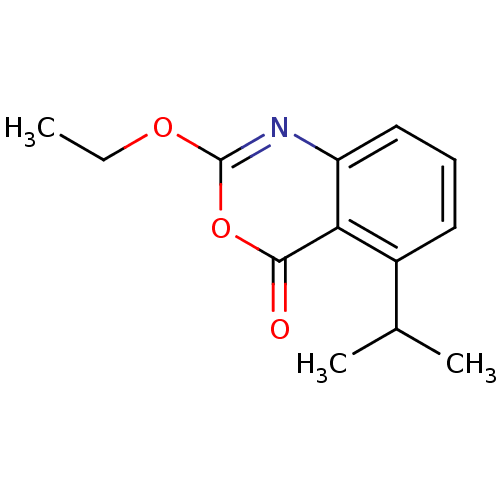 (CHEMBL356120) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0710 | n/a | n/a | n/a | n/a | 0.0000550 | 7.76E+5 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406217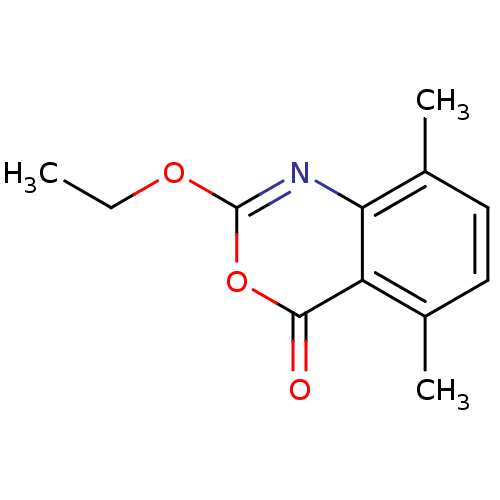 (CHEMBL145002) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | 0.00000617 | 6.46E+4 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50288087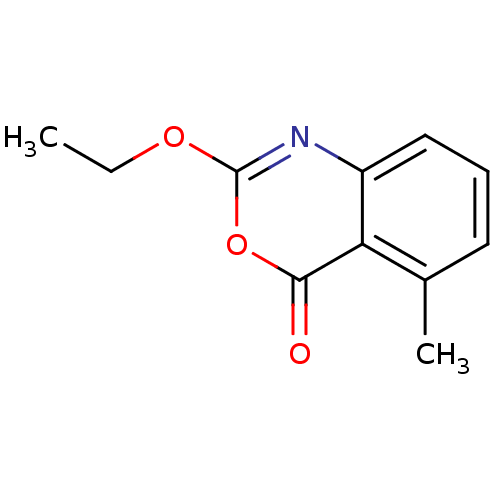 (2-Ethoxy-5-methyl-benzo[d][1,3]oxazin-4-one | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.126 | n/a | n/a | n/a | n/a | 0.0000117 | 1.02E+5 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from cloned human mu opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 6841-6 (2007) Article DOI: 10.1016/j.bmcl.2007.10.025 BindingDB Entry DOI: 10.7270/Q2125TGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219921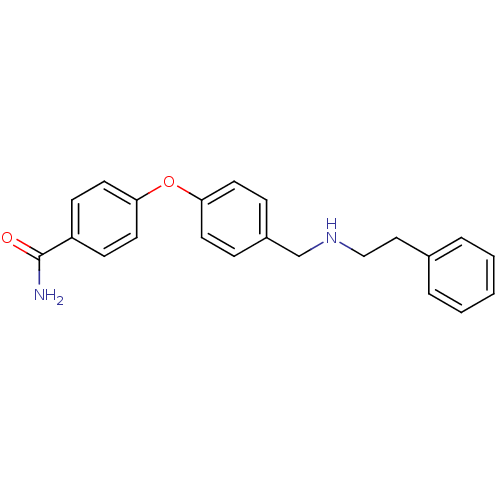 (4-(4-((phenethylamino)methyl)phenoxy)benzamide | C...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219919 (6-(4-((phenethylamino)methyl)phenoxy)nicotinamide ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human mu opioid receptor expressed in CHO cells in presence of high sodium by SPA | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219919 (6-(4-((phenethylamino)methyl)phenoxy)nicotinamide ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from cloned human mu opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 6841-6 (2007) Article DOI: 10.1016/j.bmcl.2007.10.025 BindingDB Entry DOI: 10.7270/Q2125TGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406234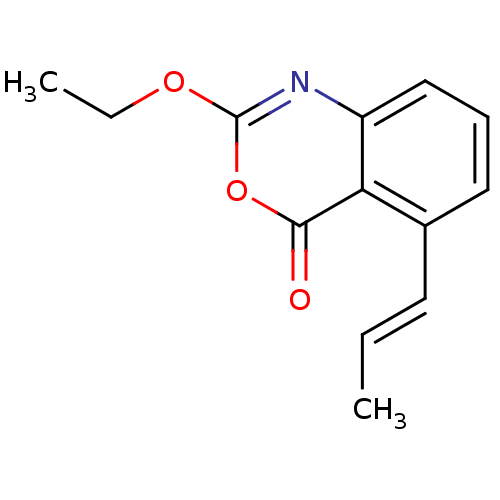 (CHEMBL145774) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | 0.0000661 | 3.47E+5 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406239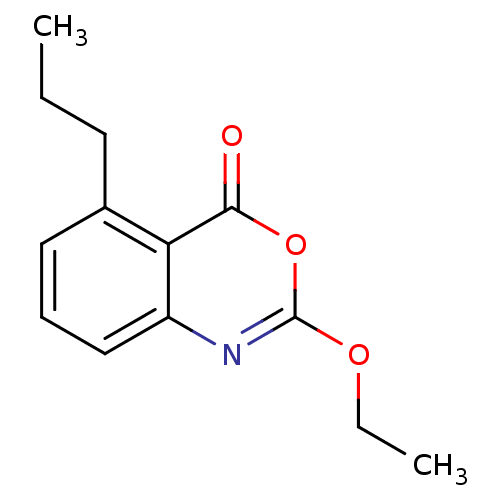 (CHEMBL357464) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.251 | n/a | n/a | n/a | n/a | 0.0000708 | 3.09E+5 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219921 (4-(4-((phenethylamino)methyl)phenoxy)benzamide | C...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human mu opioid receptor expressed in CHO cells in presence of high sodium by SPA | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406240 (CHEMBL347760) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.398 | n/a | n/a | n/a | n/a | 0.0000182 | 4.57E+4 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50005688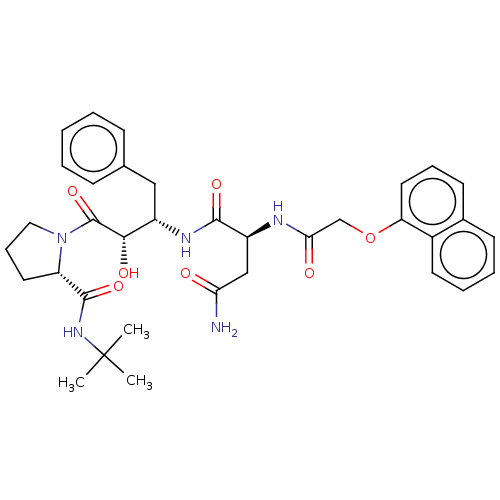 (CHEMBL3085518 | N*1*-[1-Benzyl-3-(2-tert-butylcarb...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Binding affinity against HIV Protease enzyme.(by Dixon analysis) | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219918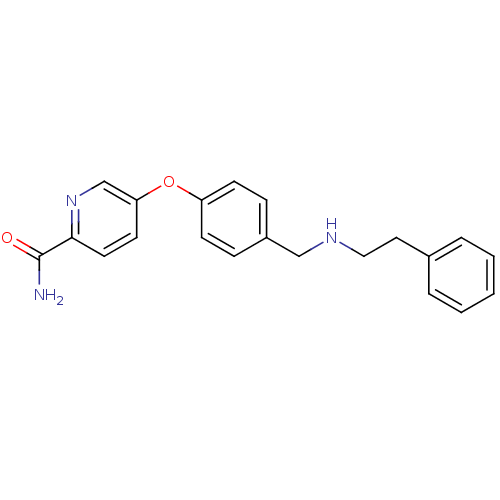 (5-(4-((phenethylamino)methyl)phenoxy)picolinamide ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406245 (CHEMBL145592) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.501 | n/a | n/a | n/a | n/a | 0.00468 | 1.00E+7 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human mu opioid receptor expressed in CHO cells in presence of high sodium by SPA | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from cloned human mu opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 6841-6 (2007) Article DOI: 10.1016/j.bmcl.2007.10.025 BindingDB Entry DOI: 10.7270/Q2125TGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50005701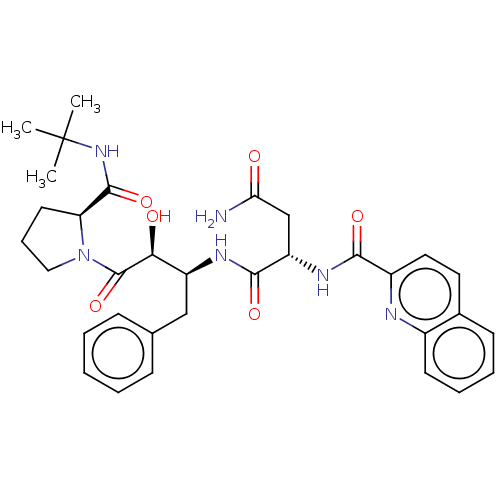 (CHEMBL337283 | N*1*-[1-Benzyl-3-(2-tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Binding affinity against HIV protease enzyme.(by Dixon analysis) | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50219921 (4-(4-((phenethylamino)methyl)phenoxy)benzamide | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50219919 (6-(4-((phenethylamino)methyl)phenoxy)nicotinamide ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406185 (CHEMBL144906) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | 0.00141 | 1.51E+6 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291760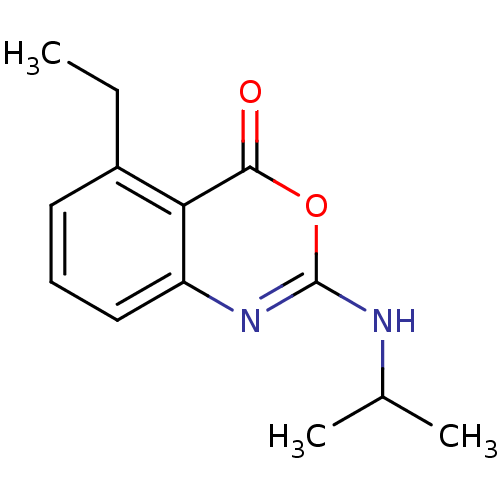 (5-Ethyl-2-isopropylamino-benzo[d][1,3]oxazin-4-one...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | 0.0000661 | 7.08E+4 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50219919 (6-(4-((phenethylamino)methyl)phenoxy)nicotinamide ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from cloned human delta opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 6841-6 (2007) Article DOI: 10.1016/j.bmcl.2007.10.025 BindingDB Entry DOI: 10.7270/Q2125TGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50219919 (6-(4-((phenethylamino)methyl)phenoxy)nicotinamide ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from human delta opioid receptor expressed in CHO cells in presence of high sodium by SPA | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50219921 (4-(4-((phenethylamino)methyl)phenoxy)benzamide | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50219919 (6-(4-((phenethylamino)methyl)phenoxy)nicotinamide ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406260 (CHEMBL143793) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.41 | n/a | n/a | n/a | n/a | 0.000355 | 2.51E+5 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50063719 (2-Isopropylamino-5-methyl-benzo[d][1,3]oxazin-4-on...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.58 | n/a | n/a | n/a | n/a | 0.0000178 | 1.18E+4 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406221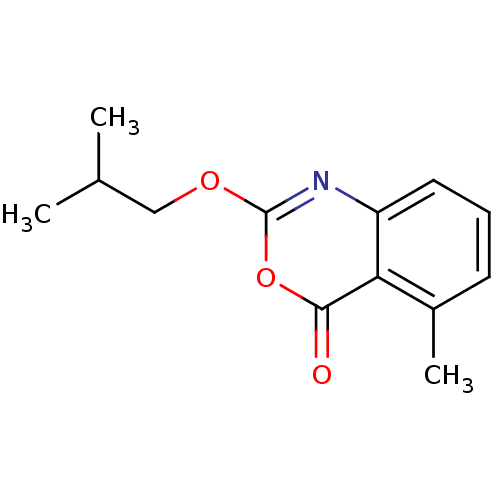 (CHEMBL145729) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.58 | n/a | n/a | n/a | n/a | 0.0000112 | 7.08E+3 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219918 (5-(4-((phenethylamino)methyl)phenoxy)picolinamide ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50219921 (4-(4-((phenethylamino)methyl)phenoxy)benzamide | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from human delta opioid receptor expressed in CHO cells in presence of high sodium by SPA | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219925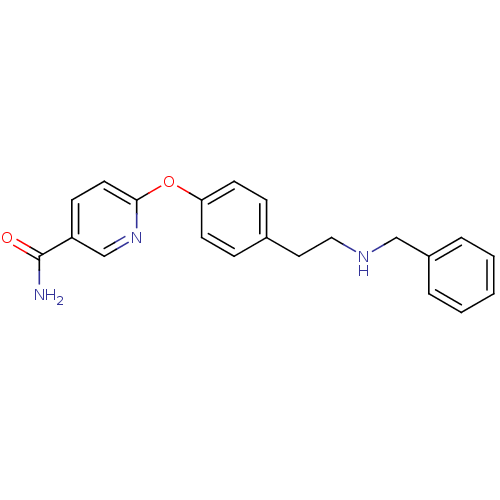 (6-(4-(2-(benzylamino)ethyl)phenoxy)nicotinamide | ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50219916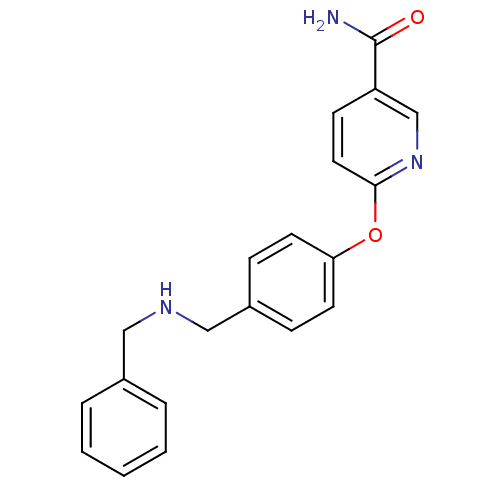 (6-(4-((benzylamino)methyl)phenoxy)nicotinamide | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406198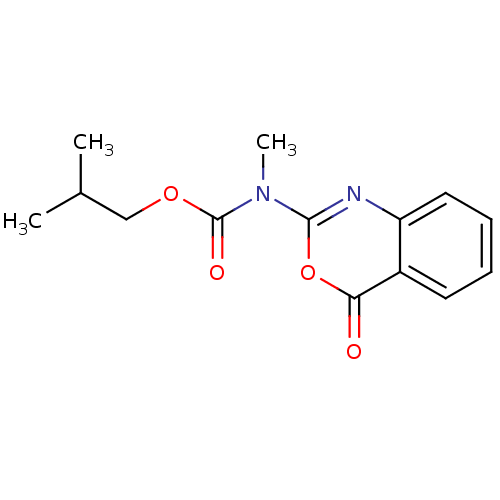 (CHEMBL145035) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.51 | n/a | n/a | n/a | n/a | 0.000813 | 3.02E+5 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50219918 (5-(4-((phenethylamino)methyl)phenoxy)picolinamide ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291763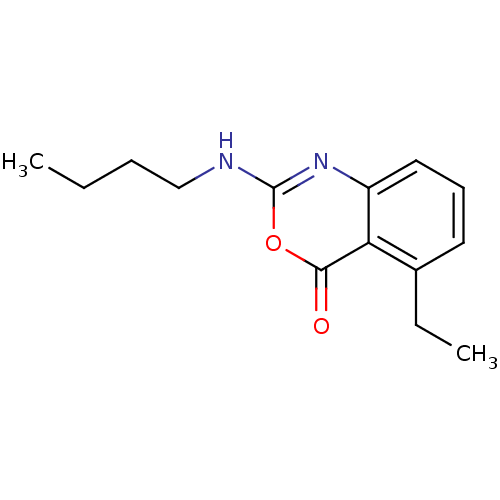 (2-Butylamino-5-ethyl-benzo[d][1,3]oxazin-4-one | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.98 | n/a | n/a | n/a | n/a | 0.000537 | 1.29E+5 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4215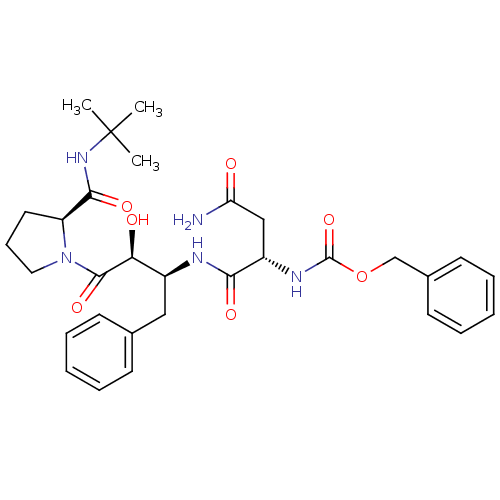 (AHPBA 1a | benzyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-2-(t...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Binding affinity against HIV Protease enzyme.(by Dixon analysis) | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219916 (6-(4-((benzylamino)methyl)phenoxy)nicotinamide | C...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from cloned human kappa opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 6841-6 (2007) Article DOI: 10.1016/j.bmcl.2007.10.025 BindingDB Entry DOI: 10.7270/Q2125TGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cells in presence of high sodium by SPA | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from human delta opioid receptor expressed in CHO cells in presence of high sodium by SPA | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]bremazocine from cloned human delta opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 6841-6 (2007) Article DOI: 10.1016/j.bmcl.2007.10.025 BindingDB Entry DOI: 10.7270/Q2125TGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 377 total ) | Next | Last >> |