

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093310 (3-{4-[5-(2,6-Dimethyl-piperidin-1-yl)-pentyl]-3-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of coagulation factor Xa. | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093310 (3-{4-[5-(2,6-Dimethyl-piperidin-1-yl)-pentyl]-3-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against Prothrombinase | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093302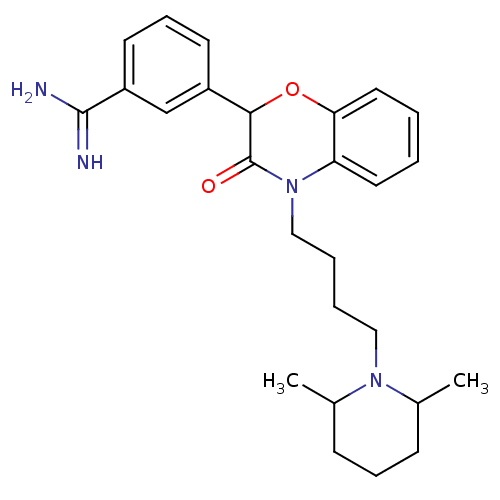 (3-{4-[4-(2,6-Dimethyl-piperidin-1-yl)-butyl]-3-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of coagulation factor Xa. | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093306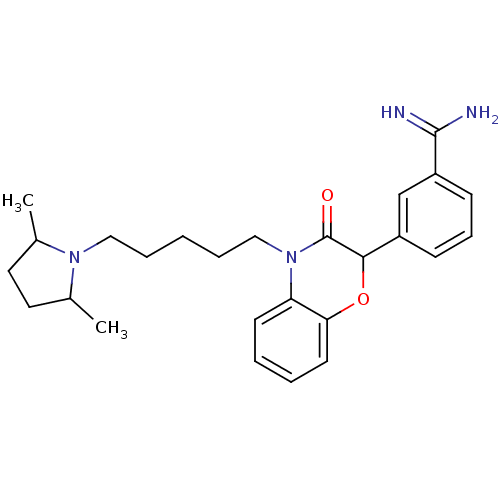 (3-{4-[5-(2,5-Dimethyl-pyrrolidin-1-yl)-pentyl]-3-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of coagulation factor Xa. | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093313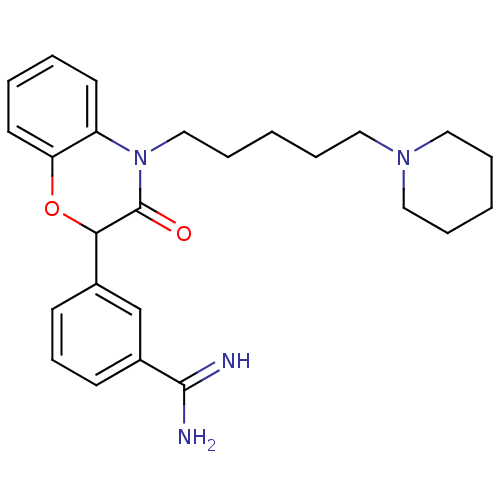 (3-[3-Oxo-4-(5-piperidin-1-yl-pentyl)-3,4-dihydro-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of coagulation factor Xa. | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093312 (3-{4-[6-(2,6-Dimethyl-piperidin-1-yl)-hexyl]-3-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of coagulation factor Xa. | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093309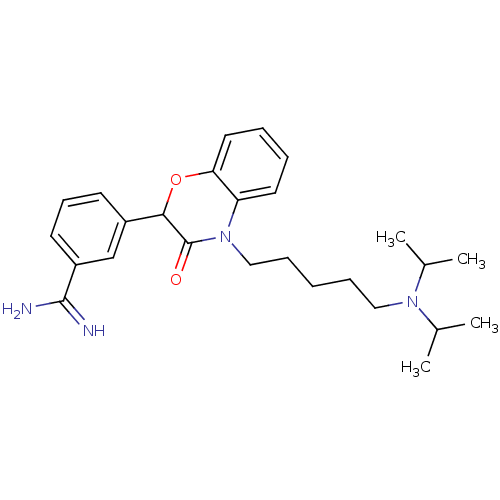 (3-[4-(5-Diisopropylamino-pentyl)-3-oxo-3,4-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of coagulation factor Xa. | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093299 (3-[4-(5-Morpholin-4-yl-pentyl)-3-oxo-3,4-dihydro-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of coagulation factor Xa. | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50093302 (3-{4-[4-(2,6-Dimethyl-piperidin-1-yl)-butyl]-3-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against trypsin | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50093310 (3-{4-[5-(2,6-Dimethyl-piperidin-1-yl)-pentyl]-3-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against trypsin | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50093306 (3-{4-[5-(2,5-Dimethyl-pyrrolidin-1-yl)-pentyl]-3-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against trypsin | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093308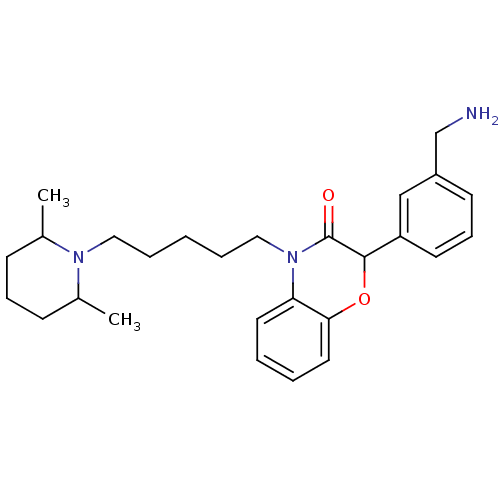 (2-(3-Aminomethyl-phenyl)-4-[5-(2,6-dimethyl-piperi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of coagulation factor Xa. | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50093310 (3-{4-[5-(2,6-Dimethyl-piperidin-1-yl)-pentyl]-3-ox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against trypsin | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50093306 (3-{4-[5-(2,5-Dimethyl-pyrrolidin-1-yl)-pentyl]-3-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50093313 (3-[3-Oxo-4-(5-piperidin-1-yl-pentyl)-3,4-dihydro-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against trypsin | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50093302 (3-{4-[4-(2,6-Dimethyl-piperidin-1-yl)-butyl]-3-oxo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50093299 (3-[4-(5-Morpholin-4-yl-pentyl)-3-oxo-3,4-dihydro-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against trypsin | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50093309 (3-[4-(5-Diisopropylamino-pentyl)-3-oxo-3,4-dihydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50093309 (3-[4-(5-Diisopropylamino-pentyl)-3-oxo-3,4-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against trypsin | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093320 (3-{4-[5-(2,6-Dimethyl-piperidin-1-yl)-pentyl]-3-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of coagulation factor Xa. | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093301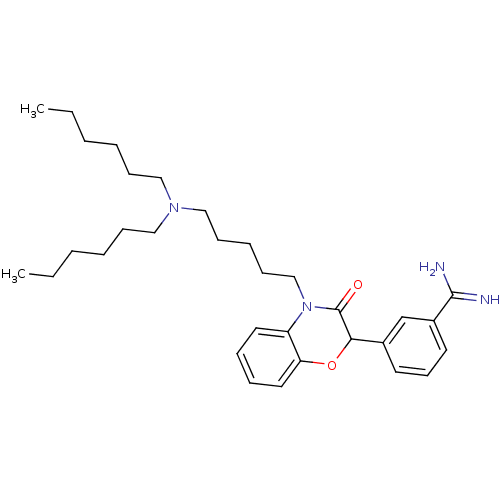 (3-[4-(5-Dihexylamino-pentyl)-3-oxo-3,4-dihydro-2H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of coagulation factor Xa. | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50093318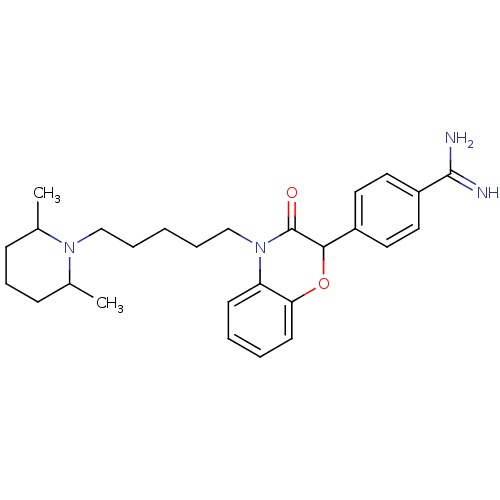 (4-{4-[5-(2,6-Dimethyl-piperidin-1-yl)-pentyl]-3-ox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50093301 (3-[4-(5-Dihexylamino-pentyl)-3-oxo-3,4-dihydro-2H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50093312 (3-{4-[6-(2,6-Dimethyl-piperidin-1-yl)-hexyl]-3-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against trypsin | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50093299 (3-[4-(5-Morpholin-4-yl-pentyl)-3-oxo-3,4-dihydro-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50093301 (3-[4-(5-Dihexylamino-pentyl)-3-oxo-3,4-dihydro-2H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against plasmin | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50093301 (3-[4-(5-Dihexylamino-pentyl)-3-oxo-3,4-dihydro-2H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against trypsin | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093311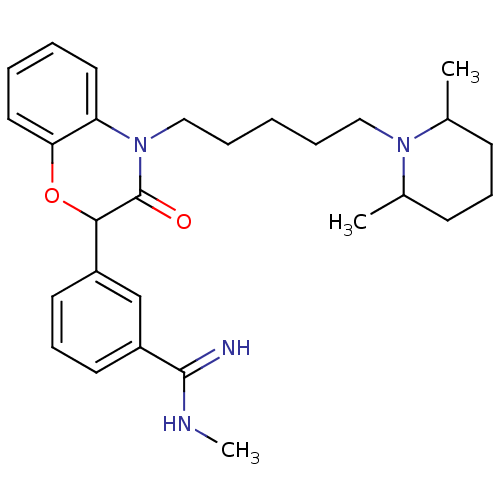 (3-{4-[5-(2,6-Dimethyl-piperidin-1-yl)-pentyl]-3-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of coagulation factor Xa. | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50093313 (3-[3-Oxo-4-(5-piperidin-1-yl-pentyl)-3,4-dihydro-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50093317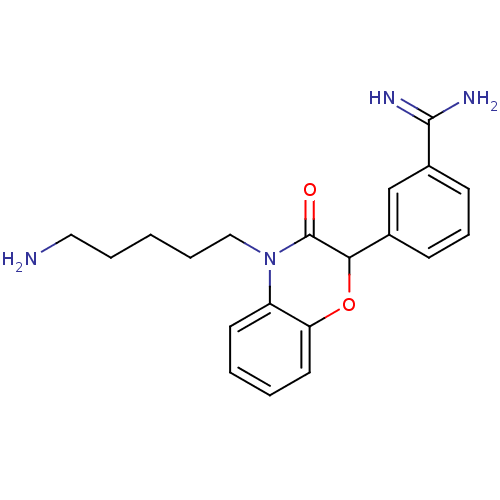 (3-[4-(5-Amino-pentyl)-3-oxo-3,4-dihydro-2H-benzo[1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against trypsin | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093314 (4-[5-(2,6-Dimethyl-piperidin-1-yl)-pentyl]-2-(4-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of coagulation factor Xa. | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50093312 (3-{4-[6-(2,6-Dimethyl-piperidin-1-yl)-hexyl]-3-oxo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093319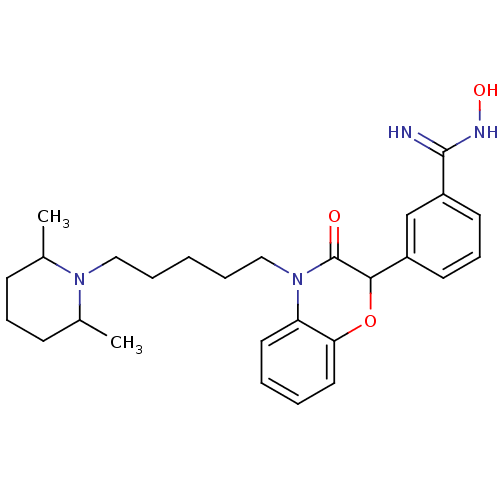 (3-{4-[5-(2,6-Dimethyl-piperidin-1-yl)-pentyl]-3-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of coagulation factor Xa. | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093317 (3-[4-(5-Amino-pentyl)-3-oxo-3,4-dihydro-2H-benzo[1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of coagulation factor Xa. | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50093308 (2-(3-Aminomethyl-phenyl)-4-[5-(2,6-dimethyl-piperi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against trypsin | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093318 (4-{4-[5-(2,6-Dimethyl-piperidin-1-yl)-pentyl]-3-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of coagulation factor Xa. | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50093310 (3-{4-[5-(2,6-Dimethyl-piperidin-1-yl)-pentyl]-3-ox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against plasmin | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50093312 (3-{4-[6-(2,6-Dimethyl-piperidin-1-yl)-hexyl]-3-oxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against plasmin | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50093318 (4-{4-[5-(2,6-Dimethyl-piperidin-1-yl)-pentyl]-3-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against trypsin | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093304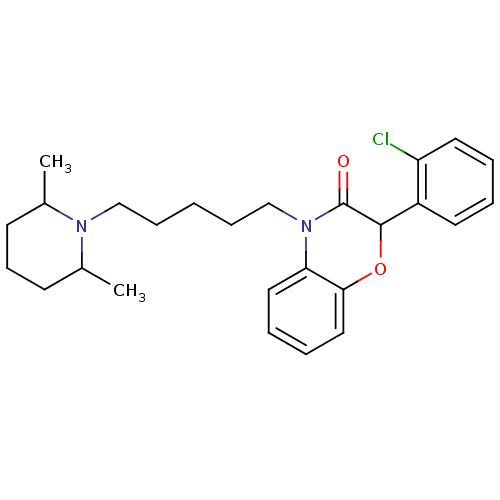 (2-(2-Chloro-phenyl)-4-[5-(2,6-dimethyl-piperidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of coagulation factor Xa. | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093303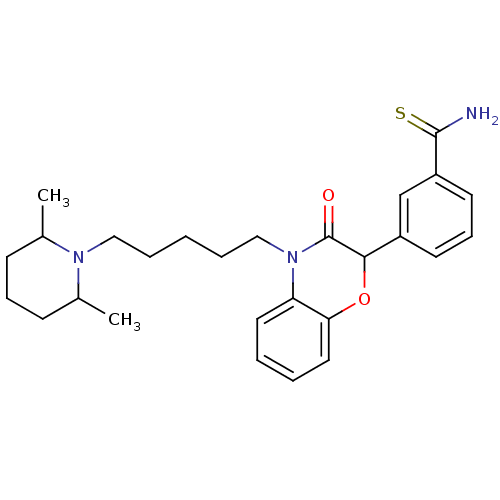 (3-{4-[5-(2,6-Dimethyl-piperidin-1-yl)-pentyl]-3-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of coagulation factor Xa. | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50093313 (3-[3-Oxo-4-(5-piperidin-1-yl-pentyl)-3,4-dihydro-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against plasmin | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K-dependent protein C (Homo sapiens (Human)) | BDBM50093310 (3-{4-[5-(2,6-Dimethyl-piperidin-1-yl)-pentyl]-3-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against activated protein C | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093305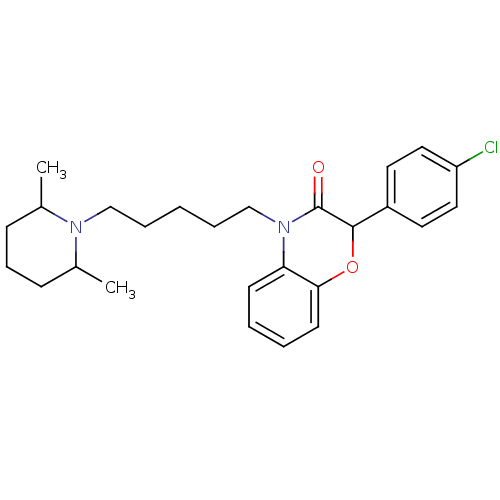 (2-(4-Chloro-phenyl)-4-[5-(2,6-dimethyl-piperidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of coagulation factor Xa. | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50093308 (2-(3-Aminomethyl-phenyl)-4-[5-(2,6-dimethyl-piperi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against plasmin | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093315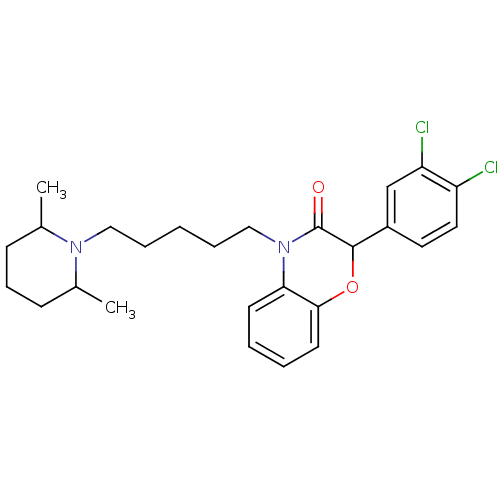 (2-(3,4-Dichloro-phenyl)-4-[5-(2,6-dimethyl-piperid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of coagulation factor Xa. | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093300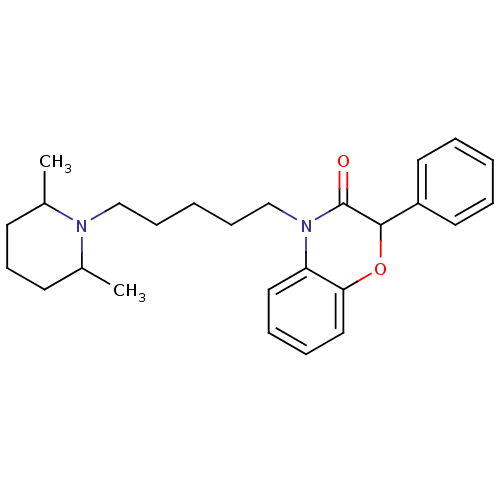 (4-[5-(2,6-Dimethyl-piperidin-1-yl)-pentyl]-2-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of coagulation factor Xa. | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093316 (4-[5-(2,6-Dimethyl-piperidin-1-yl)-pentyl]-2-p-tol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of coagulation factor Xa. | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093307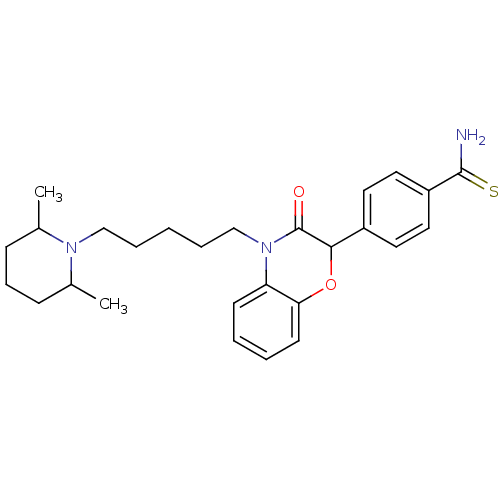 (4-{4-[5-(2,6-Dimethyl-piperidin-1-yl)-pentyl]-3-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of coagulation factor Xa. | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50093302 (3-{4-[4-(2,6-Dimethyl-piperidin-1-yl)-butyl]-3-oxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibitory activity against plasmin | J Med Chem 43: 4063-70 (2000) BindingDB Entry DOI: 10.7270/Q2W66K1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 53 total ) | Next | Last >> |