 Found 2876 hits with Last Name = 'tric' and Initial = 'b'
Found 2876 hits with Last Name = 'tric' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17428
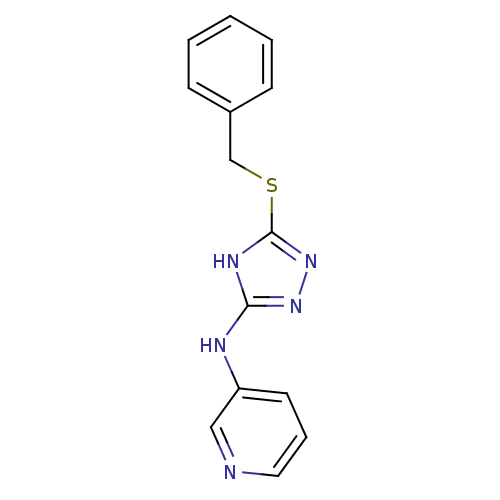
(1,2,4-Triazole Compound, 86 | N-[5-(benzylsulfanyl...)Show InChI InChI=1S/C14H13N5S/c1-2-5-11(6-3-1)10-20-14-17-13(18-19-14)16-12-7-4-8-15-9-12/h1-9H,10H2,(H2,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17355
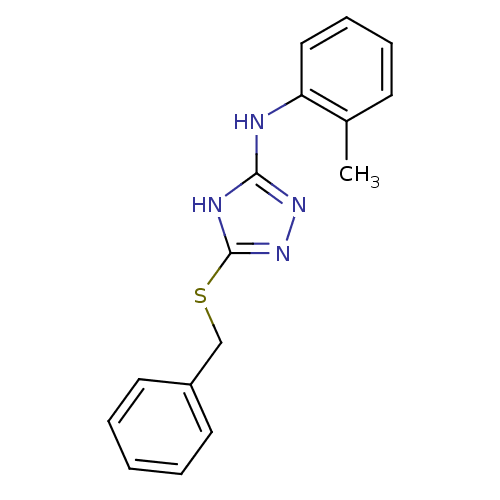
(1,2,4-Triazole Compound, 13 | 5-(benzylsulfanyl)-N...)Show InChI InChI=1S/C16H16N4S/c1-12-7-5-6-10-14(12)17-15-18-16(20-19-15)21-11-13-8-3-2-4-9-13/h2-10H,11H2,1H3,(H2,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17388
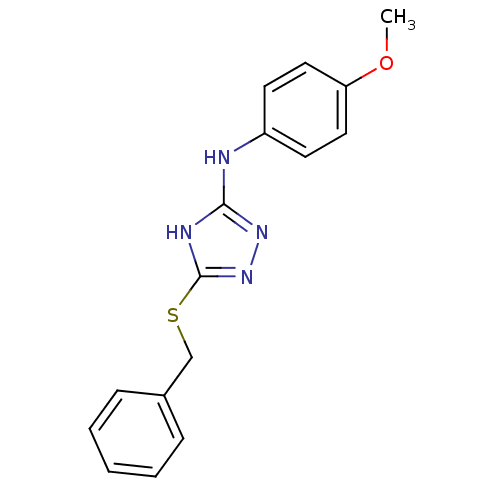
(1,2,4-Triazole Compound, 46 | 5-(benzylsulfanyl)-N...)Show InChI InChI=1S/C16H16N4OS/c1-21-14-9-7-13(8-10-14)17-15-18-16(20-19-15)22-11-12-5-3-2-4-6-12/h2-10H,11H2,1H3,(H2,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17365
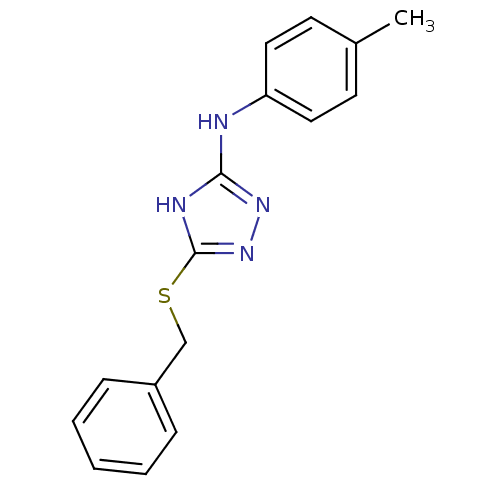
(1,2,4-Triazole Compound, 23 | 5-(benzylsulfanyl)-N...)Show InChI InChI=1S/C16H16N4S/c1-12-7-9-14(10-8-12)17-15-18-16(20-19-15)21-11-13-5-3-2-4-6-13/h2-10H,11H2,1H3,(H2,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | -57.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17390
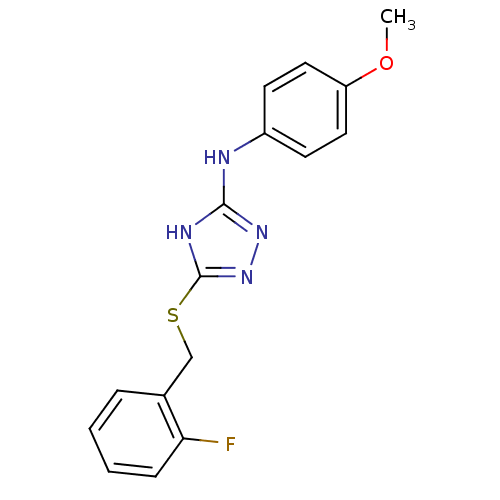
(1,2,4-Triazole Compound, 48 | 5-{[(2-fluorophenyl)...)Show InChI InChI=1S/C16H15FN4OS/c1-22-13-8-6-12(7-9-13)18-15-19-16(21-20-15)23-10-11-4-2-3-5-14(11)17/h2-9H,10H2,1H3,(H2,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50292694
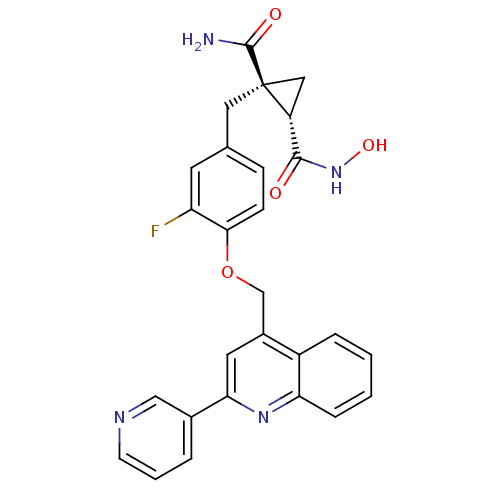
((1R,2S)-1-(3-fluoro-4-((2-(pyridin-3-yl)quinolin-4...)Show SMILES NC(=O)[C@@]1(Cc2ccc(OCc3cc(nc4ccccc34)-c3cccnc3)c(F)c2)C[C@@H]1C(=O)NO |r| Show InChI InChI=1S/C27H23FN4O4/c28-21-10-16(12-27(26(29)34)13-20(27)25(33)32-35)7-8-24(21)36-15-18-11-23(17-4-3-9-30-14-17)31-22-6-2-1-5-19(18)22/h1-11,14,20,35H,12-13,15H2,(H2,29,34)(H,32,33)/t20-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE |
Bioorg Med Chem Lett 18: 5809-14 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.045
BindingDB Entry DOI: 10.7270/Q2N29WZX |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM26524

((1R,2S)-1-({3-fluoro-4-[(2-phenylquinolin-4-yl)met...)Show SMILES NC(=O)[C@@]1(Cc2ccc(OCc3cc(nc4ccccc34)-c3ccccc3)c(F)c2)C[C@@H]1C(=O)NO |r| Show InChI InChI=1S/C28H24FN3O4/c29-22-12-17(14-28(27(30)34)15-21(28)26(33)32-35)10-11-25(22)36-16-19-13-24(18-6-2-1-3-7-18)31-23-9-5-4-8-20(19)23/h1-13,21,35H,14-16H2,(H2,30,34)(H,32,33)/t21-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE |
Bioorg Med Chem Lett 18: 5809-14 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.045
BindingDB Entry DOI: 10.7270/Q2N29WZX |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17362

(1,2,4-Triazole Compound, 20 | 5-[(3-methylbut-2-en...)Show SMILES CC(C)=CCSc1nnc(Nc2ccccc2C)[nH]1 |(5.94,4.3,;4.59,5.03,;4.55,6.57,;3.28,4.22,;1.81,4.7,;.67,3.67,;-.8,4.15,;-1.27,5.61,;-2.81,5.61,;-3.29,4.15,;-4.75,3.67,;-5.9,4.7,;-5.13,6.03,;-5.9,7.37,;-7.44,7.37,;-8.21,6.03,;-7.44,4.7,;-8.21,3.37,;-2.04,3.24,)| Show InChI InChI=1S/C14H18N4S/c1-10(2)8-9-19-14-16-13(17-18-14)15-12-7-5-4-6-11(12)3/h4-8H,9H2,1-3H3,(H2,15,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50292691

((1R,2S)-1-(3-fluoro-4-((2-(pyrrolidin-1-yl)quinoli...)Show SMILES NC(=O)[C@@]1(Cc2ccc(OCc3cc(nc4ccccc34)N3CCCC3)c(F)c2)C[C@@H]1C(=O)NO |r| Show InChI InChI=1S/C26H27FN4O4/c27-20-11-16(13-26(25(28)33)14-19(26)24(32)30-34)7-8-22(20)35-15-17-12-23(31-9-3-4-10-31)29-21-6-2-1-5-18(17)21/h1-2,5-8,11-12,19,34H,3-4,9-10,13-15H2,(H2,28,33)(H,30,32)/t19-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE |
Bioorg Med Chem Lett 18: 5809-14 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.045
BindingDB Entry DOI: 10.7270/Q2N29WZX |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50292693
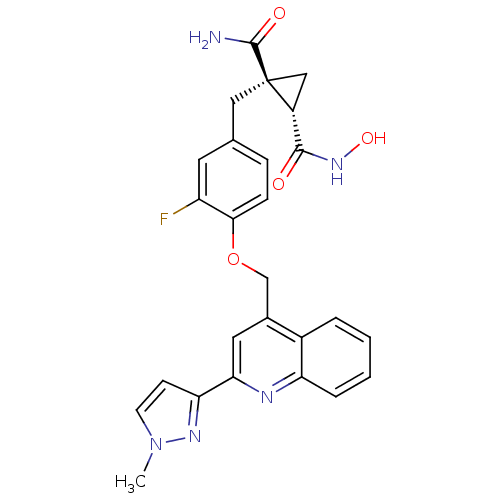
((1R,2S)-1-(3-fluoro-4-((2-(1-methyl-1H-pyrazol-3-y...)Show SMILES Cn1ccc(n1)-c1cc(COc2ccc(C[C@@]3(C[C@@H]3C(=O)NO)C(N)=O)cc2F)c2ccccc2n1 |r| Show InChI InChI=1S/C26H24FN5O4/c1-32-9-8-21(30-32)22-11-16(17-4-2-3-5-20(17)29-22)14-36-23-7-6-15(10-19(23)27)12-26(25(28)34)13-18(26)24(33)31-35/h2-11,18,35H,12-14H2,1H3,(H2,28,34)(H,31,33)/t18-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE |
Bioorg Med Chem Lett 18: 5809-14 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.045
BindingDB Entry DOI: 10.7270/Q2N29WZX |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17395
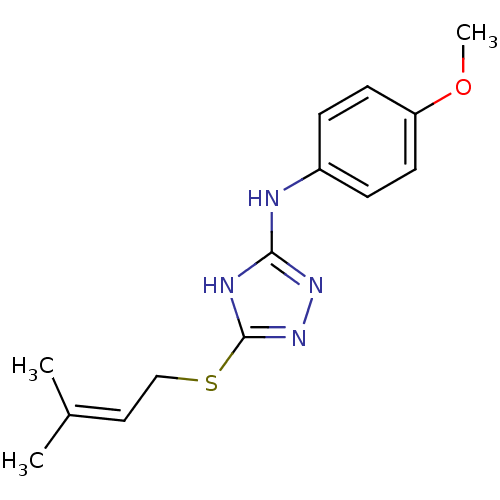
(1,2,4-Triazole Compound, 53 | N-(4-methoxyphenyl)-...)Show SMILES COc1ccc(Nc2nnc(SCC=C(C)C)[nH]2)cc1 |(-9.75,8.7,;-8.21,8.7,;-7.44,7.37,;-8.21,6.03,;-7.44,4.7,;-5.9,4.7,;-4.75,3.67,;-3.29,4.15,;-2.81,5.61,;-1.27,5.61,;-.8,4.15,;.67,3.67,;1.81,4.7,;3.28,4.22,;4.51,5.15,;5.93,4.55,;4.32,6.68,;-2.04,3.24,;-5.13,6.03,;-5.9,7.37,)| Show InChI InChI=1S/C14H18N4OS/c1-10(2)8-9-20-14-16-13(17-18-14)15-11-4-6-12(19-3)7-5-11/h4-8H,9H2,1-3H3,(H2,15,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM26525
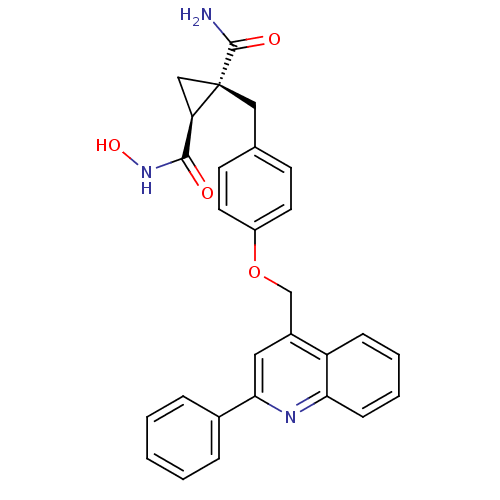
((1S,2R)-1-N-hydroxy-2-({4-[(2-phenylquinolin-4-yl)...)Show SMILES NC(=O)[C@@]1(Cc2ccc(OCc3cc(nc4ccccc34)-c3ccccc3)cc2)C[C@@H]1C(=O)NO |r| Show InChI InChI=1S/C28H25N3O4/c29-27(33)28(16-23(28)26(32)31-34)15-18-10-12-21(13-11-18)35-17-20-14-25(19-6-2-1-3-7-19)30-24-9-5-4-8-22(20)24/h1-14,23,34H,15-17H2,(H2,29,33)(H,31,32)/t23-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE |
Bioorg Med Chem Lett 18: 5809-14 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.045
BindingDB Entry DOI: 10.7270/Q2N29WZX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306314

((R)-N-(4-(7-chloro-8-hydroxy-3-methyl-2,3,4,5-tetr...)Show SMILES CN1CCc2cc(Cl)c(O)cc2[C@H](C1)c1ccc(CN(C2CCC2)C(C)=O)cc1 |r| Show InChI InChI=1S/C24H29ClN2O2/c1-16(28)27(20-4-3-5-20)14-17-6-8-18(9-7-17)22-15-26(2)11-10-19-12-23(25)24(29)13-21(19)22/h6-9,12-13,20,22,29H,3-5,10-11,14-15H2,1-2H3/t22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50343977
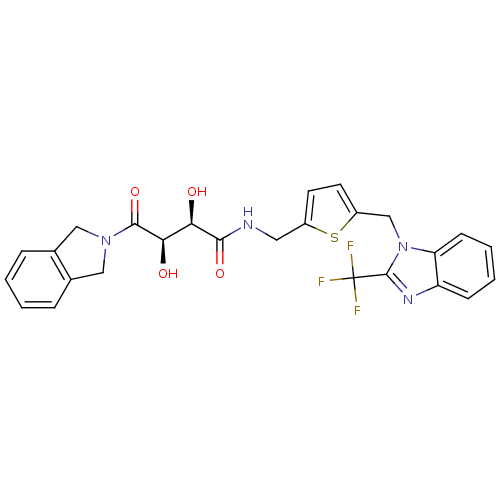
((2R,3R)-2,3-dihydroxy-4-(isoindolin-2-yl)-4-oxo-N-...)Show SMILES O[C@H]([C@@H](O)C(=O)N1Cc2ccccc2C1)C(=O)NCc1ccc(Cn2c(nc3ccccc23)C(F)(F)F)s1 |r| Show InChI InChI=1S/C26H23F3N4O4S/c27-26(28,29)25-31-19-7-3-4-8-20(19)33(25)14-18-10-9-17(38-18)11-30-23(36)21(34)22(35)24(37)32-12-15-5-1-2-6-16(15)13-32/h1-10,21-22,34-35H,11-14H2,(H,30,36)/t21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 4812-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.104
BindingDB Entry DOI: 10.7270/Q2GX4BWK |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50292690

((1R,2S)-1-(3-fluoro-4-((2-morpholinoquinolin-4-yl)...)Show SMILES NC(=O)[C@@]1(Cc2ccc(OCc3cc(nc4ccccc34)N3CCOCC3)c(F)c2)C[C@@H]1C(=O)NO |r| Show InChI InChI=1S/C26H27FN4O5/c27-20-11-16(13-26(25(28)33)14-19(26)24(32)30-34)5-6-22(20)36-15-17-12-23(31-7-9-35-10-8-31)29-21-4-2-1-3-18(17)21/h1-6,11-12,19,34H,7-10,13-15H2,(H2,28,33)(H,30,32)/t19-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE |
Bioorg Med Chem Lett 18: 5809-14 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.045
BindingDB Entry DOI: 10.7270/Q2N29WZX |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17358
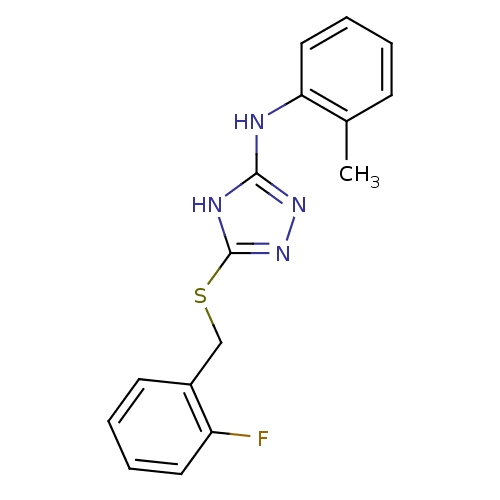
(1,2,4-Triazole Compound, 16 | 5-{[(2-fluorophenyl)...)Show InChI InChI=1S/C16H15FN4S/c1-11-6-2-5-9-14(11)18-15-19-16(21-20-15)22-10-12-7-3-4-8-13(12)17/h2-9H,10H2,1H3,(H2,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | -54.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50292688
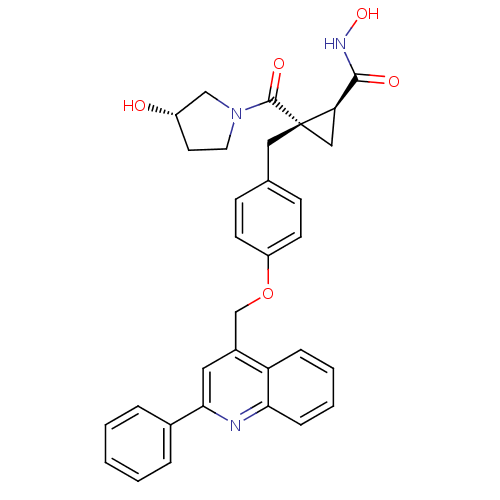
((1S,2R)-N-hydroxy-2-((S)-3-hydroxypyrrolidine-1-ca...)Show SMILES ONC(=O)[C@H]1C[C@]1(Cc1ccc(OCc2cc(nc3ccccc23)-c2ccccc2)cc1)C(=O)N1CC[C@H](O)C1 |r| Show InChI InChI=1S/C32H31N3O5/c36-24-14-15-35(19-24)31(38)32(18-27(32)30(37)34-39)17-21-10-12-25(13-11-21)40-20-23-16-29(22-6-2-1-3-7-22)33-28-9-5-4-8-26(23)28/h1-13,16,24,27,36,39H,14-15,17-20H2,(H,34,37)/t24-,27+,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE |
Bioorg Med Chem Lett 18: 5809-14 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.045
BindingDB Entry DOI: 10.7270/Q2N29WZX |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50292689
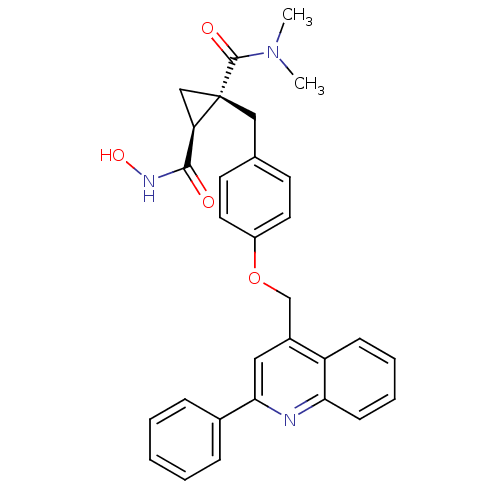
((1R,2S)-N2-hydroxy-N1,N1-dimethyl-1-(4-((2-phenylq...)Show SMILES CN(C)C(=O)[C@@]1(Cc2ccc(OCc3cc(nc4ccccc34)-c3ccccc3)cc2)C[C@@H]1C(=O)NO |r| Show InChI InChI=1S/C30H29N3O4/c1-33(2)29(35)30(18-25(30)28(34)32-36)17-20-12-14-23(15-13-20)37-19-22-16-27(21-8-4-3-5-9-21)31-26-11-7-6-10-24(22)26/h3-16,25,36H,17-19H2,1-2H3,(H,32,34)/t25-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE |
Bioorg Med Chem Lett 18: 5809-14 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.045
BindingDB Entry DOI: 10.7270/Q2N29WZX |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50292681
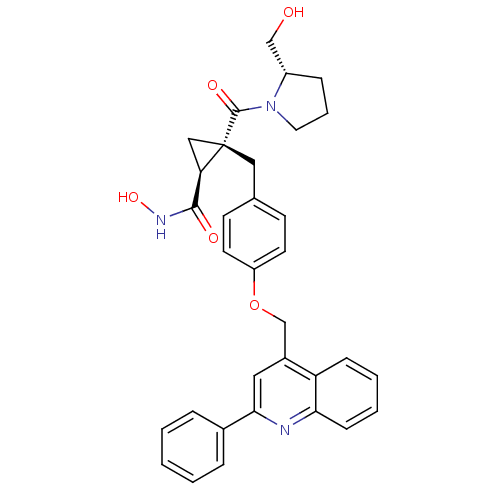
((1S,2R)-N-hydroxy-2-((S)-2-(hydroxymethyl)pyrrolid...)Show SMILES OC[C@@H]1CCCN1C(=O)[C@@]1(Cc2ccc(OCc3cc(nc4ccccc34)-c3ccccc3)cc2)C[C@@H]1C(=O)NO |r| Show InChI InChI=1S/C33H33N3O5/c37-20-25-9-6-16-36(25)32(39)33(19-28(33)31(38)35-40)18-22-12-14-26(15-13-22)41-21-24-17-30(23-7-2-1-3-8-23)34-29-11-5-4-10-27(24)29/h1-5,7-8,10-15,17,25,28,37,40H,6,9,16,18-21H2,(H,35,38)/t25-,28+,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE |
Bioorg Med Chem Lett 18: 5809-14 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.045
BindingDB Entry DOI: 10.7270/Q2N29WZX |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17430
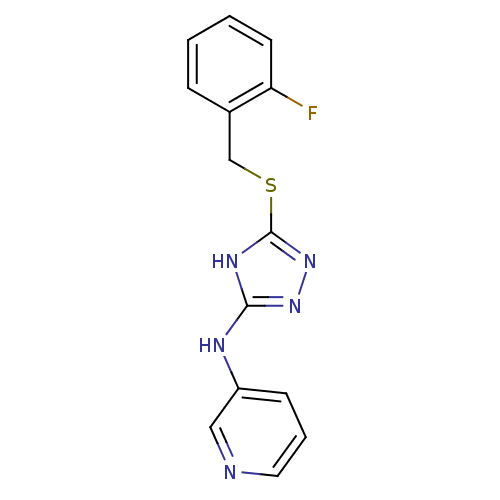
(1,2,4-Triazole Compound, 88 | N-(5-{[(2-fluorophen...)Show InChI InChI=1S/C14H12FN5S/c15-12-6-2-1-4-10(12)9-21-14-18-13(19-20-14)17-11-5-3-7-16-8-11/h1-8H,9H2,(H2,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50292682
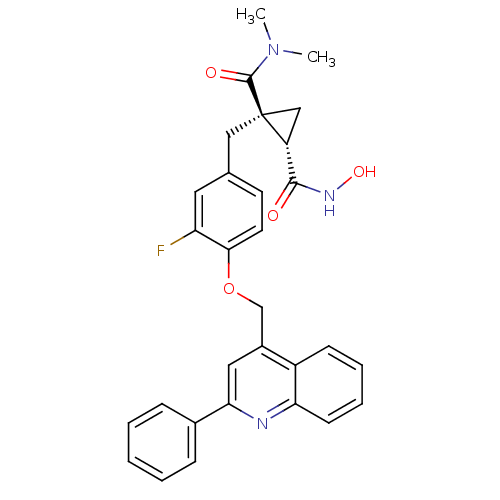
((1R,2S)-1-(3-fluoro-4-((2-phenylquinolin-4-yl)meth...)Show SMILES CN(C)C(=O)[C@@]1(Cc2ccc(OCc3cc(nc4ccccc34)-c3ccccc3)c(F)c2)C[C@@H]1C(=O)NO |r| Show InChI InChI=1S/C30H28FN3O4/c1-34(2)29(36)30(17-23(30)28(35)33-37)16-19-12-13-27(24(31)14-19)38-18-21-15-26(20-8-4-3-5-9-20)32-25-11-7-6-10-22(21)25/h3-15,23,37H,16-18H2,1-2H3,(H,33,35)/t23-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE |
Bioorg Med Chem Lett 18: 5809-14 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.045
BindingDB Entry DOI: 10.7270/Q2N29WZX |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50292687
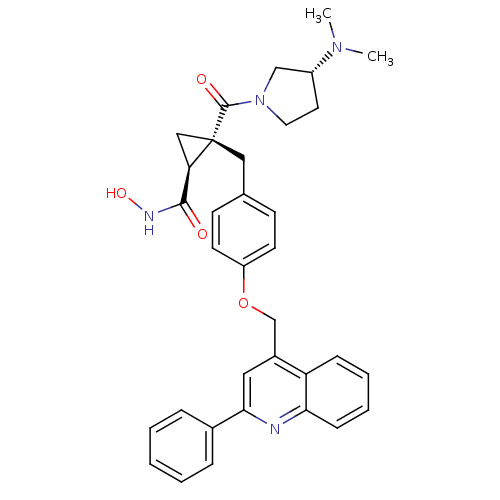
((1S,2R)-2-((R)-3-(dimethylamino)pyrrolidine-1-carb...)Show SMILES CN(C)[C@@H]1CCN(C1)C(=O)[C@@]1(Cc2ccc(OCc3cc(nc4ccccc34)-c3ccccc3)cc2)C[C@@H]1C(=O)NO |r| Show InChI InChI=1S/C34H36N4O4/c1-37(2)26-16-17-38(21-26)33(40)34(20-29(34)32(39)36-41)19-23-12-14-27(15-13-23)42-22-25-18-31(24-8-4-3-5-9-24)35-30-11-7-6-10-28(25)30/h3-15,18,26,29,41H,16-17,19-22H2,1-2H3,(H,36,39)/t26-,29-,34+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE |
Bioorg Med Chem Lett 18: 5809-14 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.045
BindingDB Entry DOI: 10.7270/Q2N29WZX |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17411

(1,2,4-Triazole Compound, 69 | methyl 4-[(5-{[(2-fl...)Show InChI InChI=1S/C17H15FN4O2S/c1-24-15(23)11-6-8-13(9-7-11)19-16-20-17(22-21-16)25-10-12-4-2-3-5-14(12)18/h2-9H,10H2,1H3,(H2,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17377

(1,2,4-Triazole Compound, 35 | 5-(benzylsulfanyl)-N...)Show InChI InChI=1S/C15H13ClN4S/c16-12-6-8-13(9-7-12)17-14-18-15(20-19-14)21-10-11-4-2-1-3-5-11/h1-9H,10H2,(H2,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306315
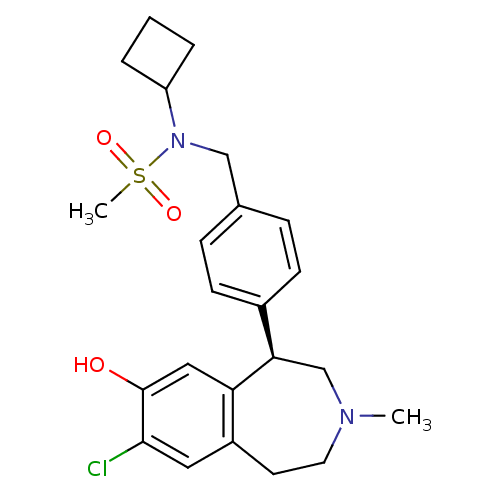
((R)-N-(4-(7-chloro-8-hydroxy-3-methyl-2,3,4,5-tetr...)Show SMILES CN1CCc2cc(Cl)c(O)cc2[C@H](C1)c1ccc(CN(C2CCC2)S(C)(=O)=O)cc1 |r| Show InChI InChI=1S/C23H29ClN2O3S/c1-25-11-10-18-12-22(24)23(27)13-20(18)21(15-25)17-8-6-16(7-9-17)14-26(30(2,28)29)19-4-3-5-19/h6-9,12-13,19,21,27H,3-5,10-11,14-15H2,1-2H3/t21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17431
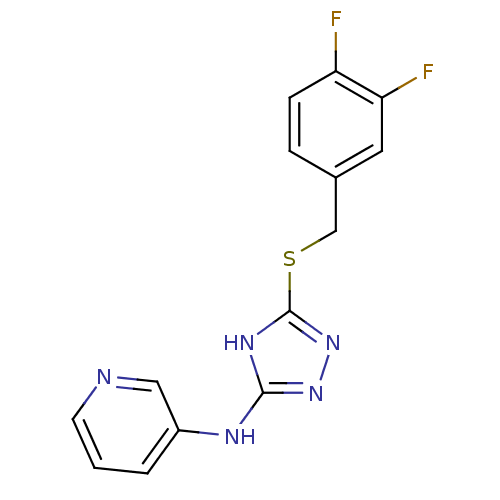
(1,2,4-Triazole Compound, 89 | N-(5-{[(3,4-difluoro...)Show InChI InChI=1S/C14H11F2N5S/c15-11-4-3-9(6-12(11)16)8-22-14-19-13(20-21-14)18-10-2-1-5-17-7-10/h1-7H,8H2,(H2,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306316
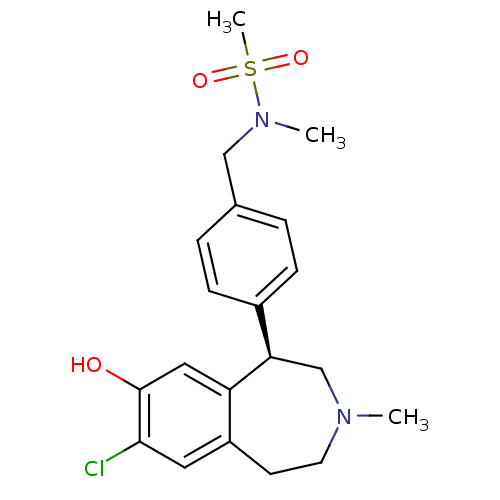
((R)-N-(4-(7-chloro-8-hydroxy-3-methyl-2,3,4,5-tetr...)Show SMILES CN(Cc1ccc(cc1)[C@H]1CN(C)CCc2cc(Cl)c(O)cc12)S(C)(=O)=O |r| Show InChI InChI=1S/C20H25ClN2O3S/c1-22-9-8-16-10-19(21)20(24)11-17(16)18(13-22)15-6-4-14(5-7-15)12-23(2)27(3,25)26/h4-7,10-11,18,24H,8-9,12-13H2,1-3H3/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17416
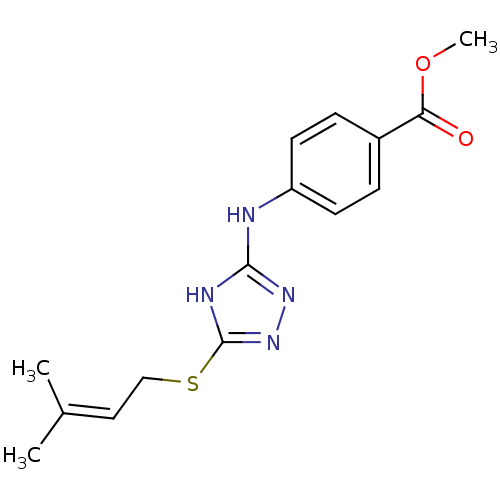
(1,2,4-Triazole Compound, 74 | methyl 4-({5-[(3-met...)Show SMILES COC(=O)c1ccc(Nc2nnc(SCC=C(C)C)[nH]2)cc1 |(-10.52,10.03,;-9.75,8.7,;-8.21,8.7,;-7.44,10.03,;-7.44,7.37,;-8.21,6.03,;-7.44,4.7,;-5.9,4.7,;-4.75,3.67,;-3.29,4.15,;-2.81,5.61,;-1.27,5.61,;-.8,4.15,;.67,3.67,;1.81,4.7,;3.28,4.22,;4.42,5.25,;5.89,4.78,;4.1,6.76,;-2.04,3.24,;-5.13,6.03,;-5.9,7.37,)| Show InChI InChI=1S/C15H18N4O2S/c1-10(2)8-9-22-15-17-14(18-19-15)16-12-6-4-11(5-7-12)13(20)21-3/h4-8H,9H2,1-3H3,(H2,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306322
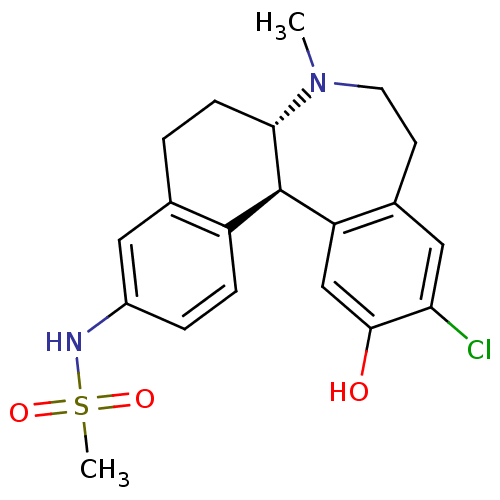
(CHEMBL597909 | N-((6aS,13bR)-11-chloro-12-hydroxy-...)Show SMILES CN1CCc2cc(Cl)c(O)cc2[C@@H]2[C@@H]1CCc1cc(NS(C)(=O)=O)ccc21 |r| Show InChI InChI=1S/C20H23ClN2O3S/c1-23-8-7-13-10-17(21)19(24)11-16(13)20-15-5-4-14(22-27(2,25)26)9-12(15)3-6-18(20)23/h4-5,9-11,18,20,22,24H,3,6-8H2,1-2H3/t18-,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50343976
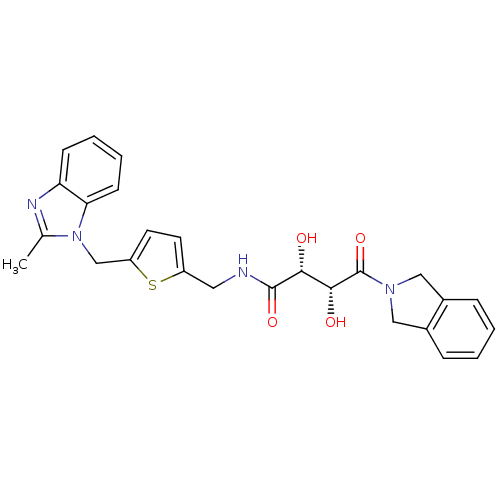
((2R,3R)-2,3-dihydroxy-4-(isoindolin-2-yl)-N-((5-((...)Show SMILES Cc1nc2ccccc2n1Cc1ccc(CNC(=O)[C@H](O)[C@@H](O)C(=O)N2Cc3ccccc3C2)s1 |r| Show InChI InChI=1S/C26H26N4O4S/c1-16-28-21-8-4-5-9-22(21)30(16)15-20-11-10-19(35-20)12-27-25(33)23(31)24(32)26(34)29-13-17-6-2-3-7-18(17)14-29/h2-11,23-24,31-32H,12-15H2,1H3,(H,27,33)/t23-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 4812-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.104
BindingDB Entry DOI: 10.7270/Q2GX4BWK |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17352
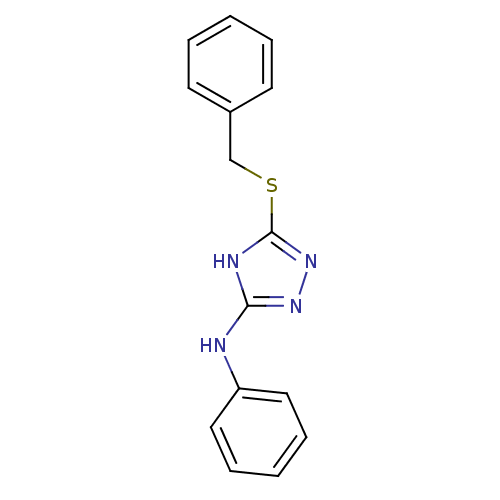
(1,2,4-Triazole Compound, 6 | 5-(benzylsulfanyl)-N-...)Show InChI InChI=1S/C15H14N4S/c1-3-7-12(8-4-1)11-20-15-17-14(18-19-15)16-13-9-5-2-6-10-13/h1-10H,11H2,(H2,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | -52.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50362726
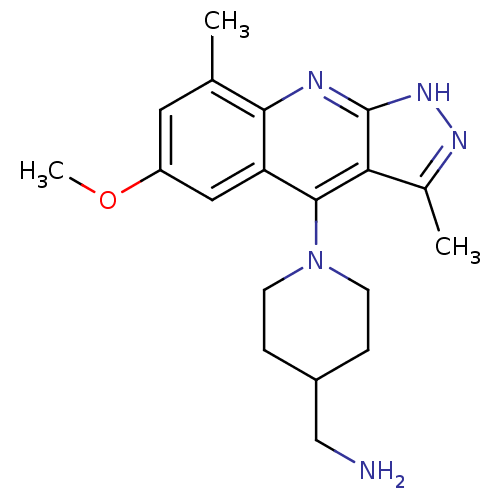
(CHEMBL1939796)Show SMILES COc1cc(C)c2nc3[nH]nc(C)c3c(N3CCC(CN)CC3)c2c1 Show InChI InChI=1S/C19H25N5O/c1-11-8-14(25-3)9-15-17(11)21-19-16(12(2)22-23-19)18(15)24-6-4-13(10-20)5-7-24/h8-9,13H,4-7,10,20H2,1-3H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PDE10A using [3H]cAMP as substrate by scintillation proximity assay |
Bioorg Med Chem Lett 22: 1019-22 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.127
BindingDB Entry DOI: 10.7270/Q2BV7H3J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17385

(1,2,4-Triazole Compound, 43 | N-(4-chlorophenyl)-5...)Show SMILES CC(C)=CCSc1nnc(Nc2ccc(Cl)cc2)[nH]1 |(5.94,4.35,;4.57,5.06,;4.5,6.59,;3.28,4.22,;1.81,4.7,;.67,3.67,;-.8,4.15,;-1.27,5.61,;-2.81,5.61,;-3.29,4.15,;-4.75,3.67,;-5.9,4.7,;-5.13,6.03,;-5.9,7.37,;-7.44,7.37,;-8.21,8.7,;-8.21,6.03,;-7.44,4.7,;-2.04,3.24,)| Show InChI InChI=1S/C13H15ClN4S/c1-9(2)7-8-19-13-16-12(17-18-13)15-11-5-3-10(14)4-6-11/h3-7H,8H2,1-2H3,(H2,15,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50292686

((1S,2R)-2-((S)-3-(dimethylamino)pyrrolidine-1-carb...)Show SMILES CN(C)[C@H]1CCN(C1)C(=O)[C@@]1(Cc2ccc(OCc3cc(nc4ccccc34)-c3ccccc3)cc2)C[C@@H]1C(=O)NO |r| Show InChI InChI=1S/C34H36N4O4/c1-37(2)26-16-17-38(21-26)33(40)34(20-29(34)32(39)36-41)19-23-12-14-27(15-13-23)42-22-25-18-31(24-8-4-3-5-9-24)35-30-11-7-6-10-28(25)30/h3-15,18,26,29,41H,16-17,19-22H2,1-2H3,(H,36,39)/t26-,29+,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE |
Bioorg Med Chem Lett 18: 5809-14 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.045
BindingDB Entry DOI: 10.7270/Q2N29WZX |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Arg8-vasopressin from human vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter |
Bioorg Med Chem Lett 19: 6018-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.050
BindingDB Entry DOI: 10.7270/Q2J9679W |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065428
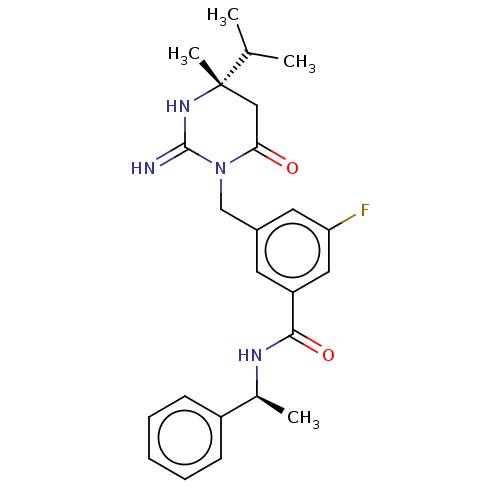
(CHEMBL3401350)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cc(F)cc(c2)C(=O)N[C@@H](C)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H29FN4O2/c1-15(2)24(4)13-21(30)29(23(26)28-24)14-17-10-19(12-20(25)11-17)22(31)27-16(3)18-8-6-5-7-9-18/h5-12,15-16H,13-14H2,1-4H3,(H2,26,28)(H,27,31)/t16-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306317
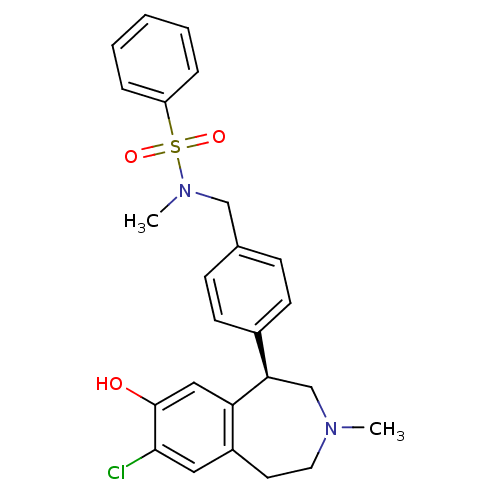
((R)-N-(4-(7-chloro-8-hydroxy-3-methyl-2,3,4,5-tetr...)Show SMILES CN(Cc1ccc(cc1)[C@H]1CN(C)CCc2cc(Cl)c(O)cc12)S(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C25H27ClN2O3S/c1-27-13-12-20-14-24(26)25(29)15-22(20)23(17-27)19-10-8-18(9-11-19)16-28(2)32(30,31)21-6-4-3-5-7-21/h3-11,14-15,23,29H,12-13,16-17H2,1-2H3/t23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50362722
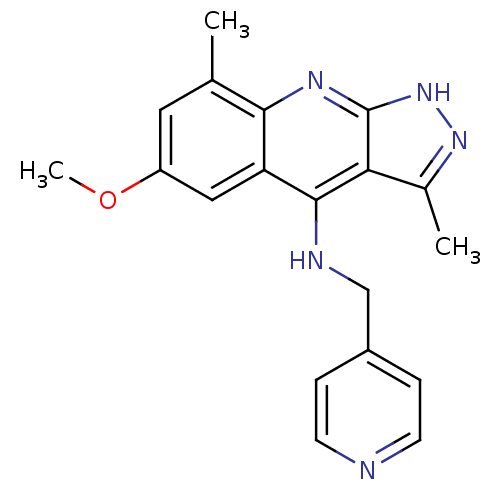
(CHEMBL1939800)Show InChI InChI=1S/C19H19N5O/c1-11-8-14(25-3)9-15-17(11)22-19-16(12(2)23-24-19)18(15)21-10-13-4-6-20-7-5-13/h4-9H,10H2,1-3H3,(H2,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PDE10A using [3H]cAMP as substrate by scintillation proximity assay |
Bioorg Med Chem Lett 22: 1019-22 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.127
BindingDB Entry DOI: 10.7270/Q2BV7H3J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17380
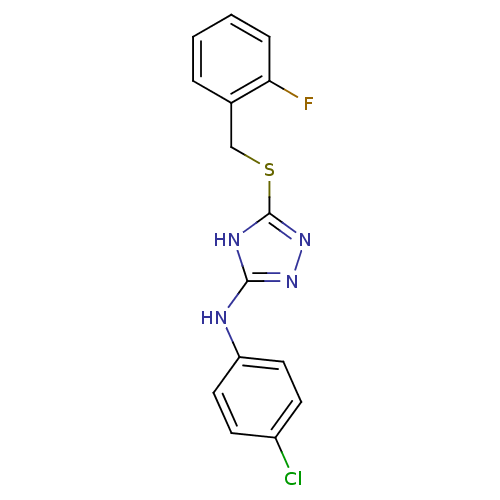
(1,2,4-Triazole Compound, 38 | N-(4-chlorophenyl)-5...)Show InChI InChI=1S/C15H12ClFN4S/c16-11-5-7-12(8-6-11)18-14-19-15(21-20-14)22-9-10-3-1-2-4-13(10)17/h1-8H,9H2,(H2,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50292685

((1R,2S)-N2-hydroxy-1-(4-((2-phenylquinolin-4-yl)me...)Show SMILES ONC(=O)[C@H]1C[C@]1(Cc1ccc(OCc2cc(nc3ccccc23)-c2ccccc2)cc1)C(=O)NCC1CCNCC1 |r| Show InChI InChI=1S/C34H36N4O4/c39-32(38-41)29-20-34(29,33(40)36-21-24-14-16-35-17-15-24)19-23-10-12-27(13-11-23)42-22-26-18-31(25-6-2-1-3-7-25)37-30-9-5-4-8-28(26)30/h1-13,18,24,29,35,41H,14-17,19-22H2,(H,36,40)(H,38,39)/t29-,34+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE |
Bioorg Med Chem Lett 18: 5809-14 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.045
BindingDB Entry DOI: 10.7270/Q2N29WZX |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17368
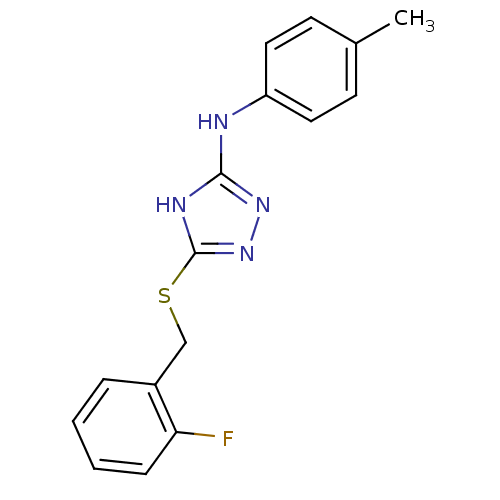
(1,2,4-Triazole Compound, 26 | 5-{[(2-fluorophenyl)...)Show InChI InChI=1S/C16H15FN4S/c1-11-6-8-13(9-7-11)18-15-19-16(21-20-15)22-10-12-4-2-3-5-14(12)17/h2-9H,10H2,1H3,(H2,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | -51.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306325

(1-((6aS,13bR)-11-chloro-12-hydroxy-7-methyl-6,6a,7...)Show SMILES CN1CCc2cc(Cl)c(O)cc2[C@@H]2[C@@H]1CCc1cc(NC(=O)Nc3c(Cl)cccc3Cl)ccc21 |r| Show InChI InChI=1S/C26H24Cl3N3O2/c1-32-10-9-15-12-21(29)23(33)13-18(15)24-17-7-6-16(11-14(17)5-8-22(24)32)30-26(34)31-25-19(27)3-2-4-20(25)28/h2-4,6-7,11-13,22,24,33H,5,8-10H2,1H3,(H2,30,31,34)/t22-,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17408
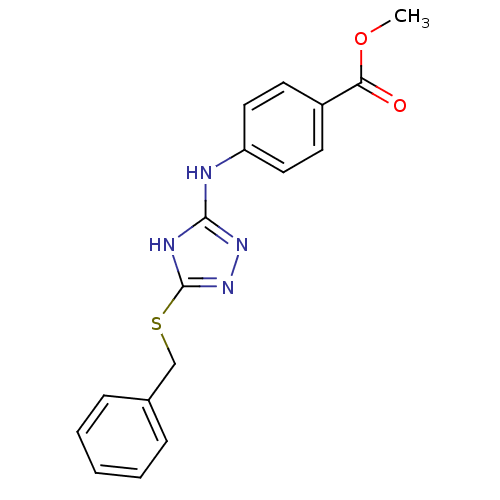
(1,2,4-Triazole Compound, 66 | methyl 4-{[5-(benzyl...)Show InChI InChI=1S/C17H16N4O2S/c1-23-15(22)13-7-9-14(10-8-13)18-16-19-17(21-20-16)24-11-12-5-3-2-4-6-12/h2-10H,11H2,1H3,(H2,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306323
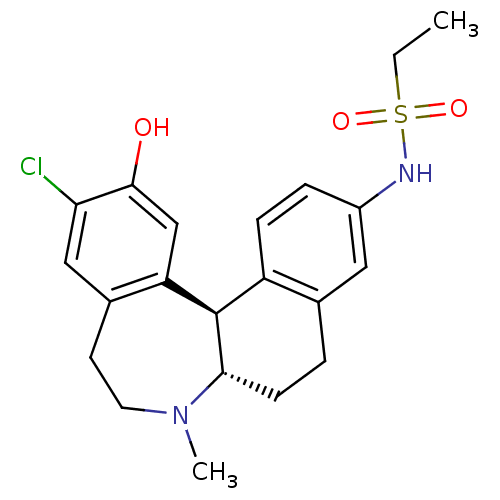
(CHEMBL600986 | N-((6aS,13bR)-11-chloro-12-hydroxy-...)Show SMILES CCS(=O)(=O)Nc1ccc2[C@H]3[C@H](CCc2c1)N(C)CCc1cc(Cl)c(O)cc31 |r| Show InChI InChI=1S/C21H25ClN2O3S/c1-3-28(26,27)23-15-5-6-16-13(10-15)4-7-19-21(16)17-12-20(25)18(22)11-14(17)8-9-24(19)2/h5-6,10-12,19,21,23,25H,3-4,7-9H2,1-2H3/t19-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50111336
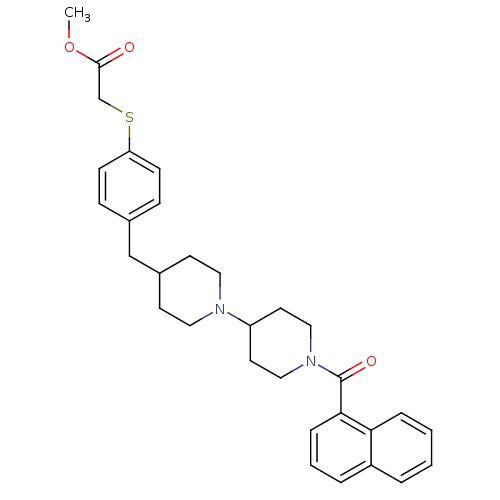
(CHEMBL11892 | {4-[1'-(Naphthalene-1-carbonyl)-[1,4...)Show SMILES COC(=O)CSc1ccc(CC2CCN(CC2)C2CCN(CC2)C(=O)c2cccc3ccccc23)cc1 Show InChI InChI=1S/C31H36N2O3S/c1-36-30(34)22-37-27-11-9-23(10-12-27)21-24-13-17-32(18-14-24)26-15-19-33(20-16-26)31(35)29-8-4-6-25-5-2-3-7-28(25)29/h2-12,24,26H,13-22H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic acetylcholine receptor M2 stably expressed in CHO-K1 cells using [3H]-QNB as radioligand. |
Bioorg Med Chem Lett 12: 1087-91 (2002)
BindingDB Entry DOI: 10.7270/Q2MS3S2P |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306319
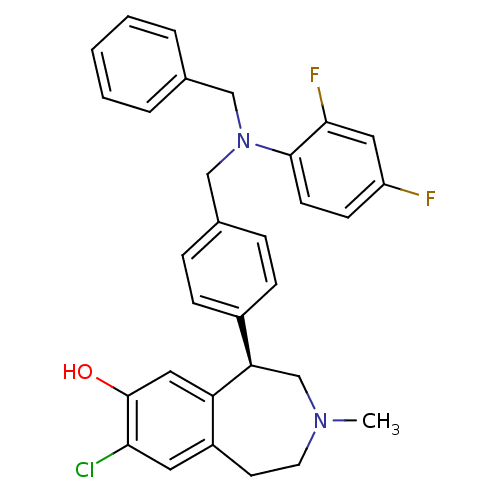
((R)-5-(4-((benzyl(2,4-difluorophenyl)amino)methyl)...)Show SMILES CN1CCc2cc(Cl)c(O)cc2[C@H](C1)c1ccc(CN(Cc2ccccc2)c2ccc(F)cc2F)cc1 |r| Show InChI InChI=1S/C31H29ClF2N2O/c1-35-14-13-24-15-28(32)31(37)17-26(24)27(20-35)23-9-7-22(8-10-23)19-36(18-21-5-3-2-4-6-21)30-12-11-25(33)16-29(30)34/h2-12,15-17,27,37H,13-14,18-20H2,1H3/t27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17354
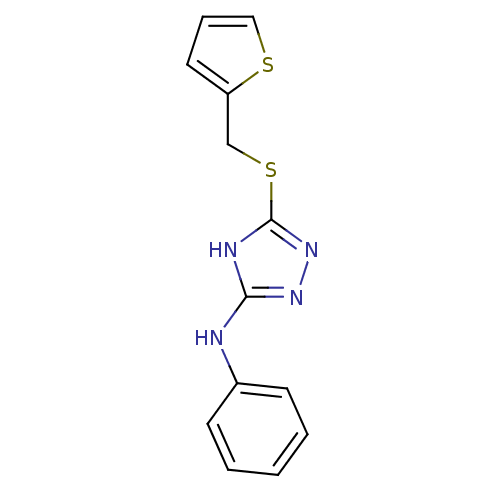
(1,2,4-Triazole Compound, 12 | N-phenyl-5-[(thiophe...)Show InChI InChI=1S/C13H12N4S2/c1-2-5-10(6-3-1)14-12-15-13(17-16-12)19-9-11-7-4-8-18-11/h1-8H,9H2,(H2,14,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.75 | -51.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50292692
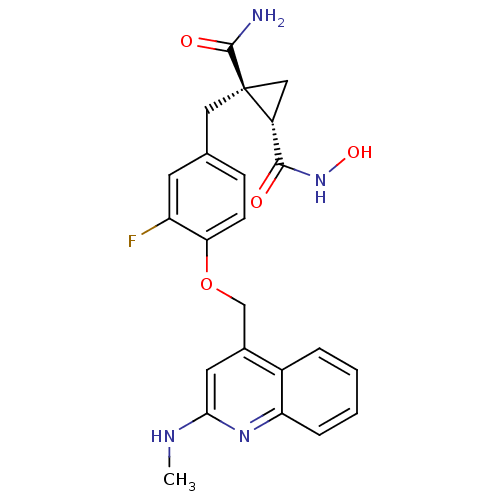
((1R,2S)-1-(3-fluoro-4-((2-(methylamino)quinolin-4-...)Show SMILES CNc1cc(COc2ccc(C[C@@]3(C[C@@H]3C(=O)NO)C(N)=O)cc2F)c2ccccc2n1 |r| Show InChI InChI=1S/C23H23FN4O4/c1-26-20-9-14(15-4-2-3-5-18(15)27-20)12-32-19-7-6-13(8-17(19)24)10-23(22(25)30)11-16(23)21(29)28-31/h2-9,16,31H,10-12H2,1H3,(H2,25,30)(H,26,27)(H,28,29)/t16-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE |
Bioorg Med Chem Lett 18: 5809-14 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.045
BindingDB Entry DOI: 10.7270/Q2N29WZX |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17418
(1,2,4-Triazole Compound, 76 | methyl 4-[(5-{[(5-me...)Show InChI InChI=1S/C15H15N5O3S/c1-9-7-12(20-23-9)8-24-15-17-14(18-19-15)16-11-5-3-10(4-6-11)13(21)22-2/h3-7H,8H2,1-2H3,(H2,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50342963
((2R,3R)-N-((R)-1-(4-(1H-pyrazol-1-yl)phenyl)ethyl)...)Show SMILES C[C@@H](NC(=O)[C@H](O)[C@@H](O)C(=O)N1CCC[C@@H]1c1cccc(Cl)c1)c1ccc(cc1)-n1cccn1 |r| Show InChI InChI=1S/C25H27ClN4O4/c1-16(17-8-10-20(11-9-17)30-14-4-12-27-30)28-24(33)22(31)23(32)25(34)29-13-3-7-21(29)18-5-2-6-19(26)15-18/h2,4-6,8-12,14-16,21-23,31-32H,3,7,13H2,1H3,(H,28,33)/t16-,21-,22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 4812-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.104
BindingDB Entry DOI: 10.7270/Q2GX4BWK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data