

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM159251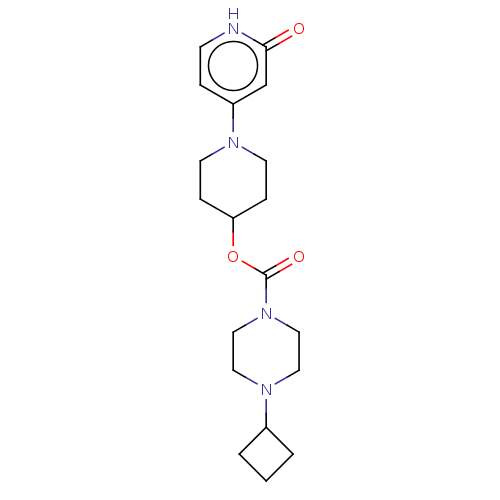 (US9034874, 2.2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ... | US Patent US9034874 (2015) BindingDB Entry DOI: 10.7270/Q2C82819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM159252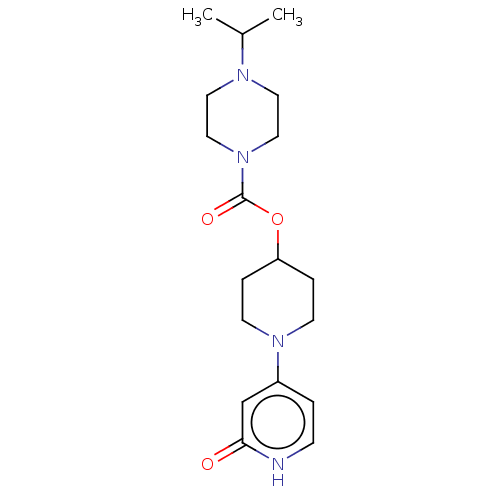 (US9034874, 2.3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ... | US Patent US9034874 (2015) BindingDB Entry DOI: 10.7270/Q2C82819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM159248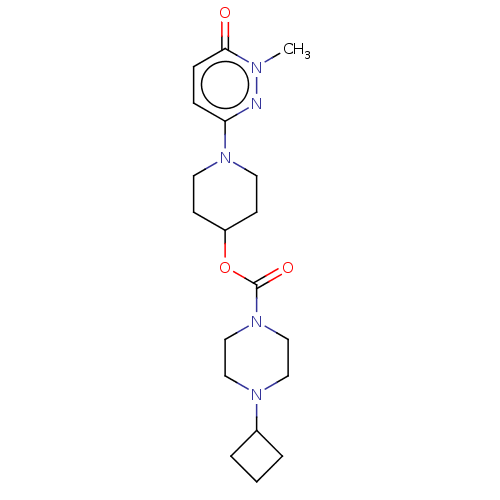 (US9034874, 1.5) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ... | US Patent US9034874 (2015) BindingDB Entry DOI: 10.7270/Q2C82819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM159253 (US9034874, 3.1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M... | US Patent US9034874 (2015) BindingDB Entry DOI: 10.7270/Q2C82819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM159246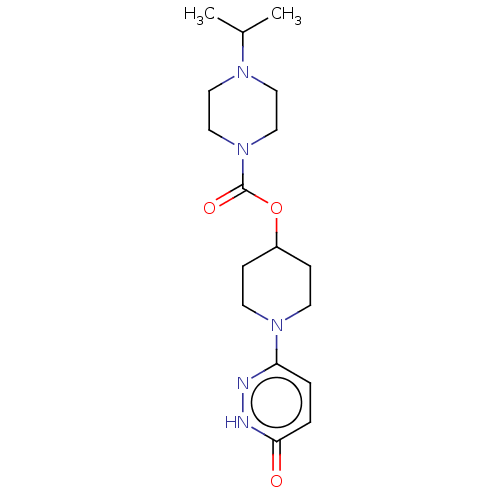 (US9034874, 1.3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ... | US Patent US9034874 (2015) BindingDB Entry DOI: 10.7270/Q2C82819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM159244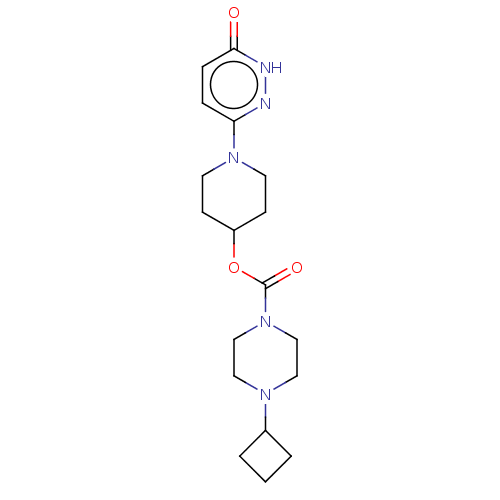 (US9034874, 1.1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ... | US Patent US9034874 (2015) BindingDB Entry DOI: 10.7270/Q2C82819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM159253 (US9034874, 3.1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ... | US Patent US9034874 (2015) BindingDB Entry DOI: 10.7270/Q2C82819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM159247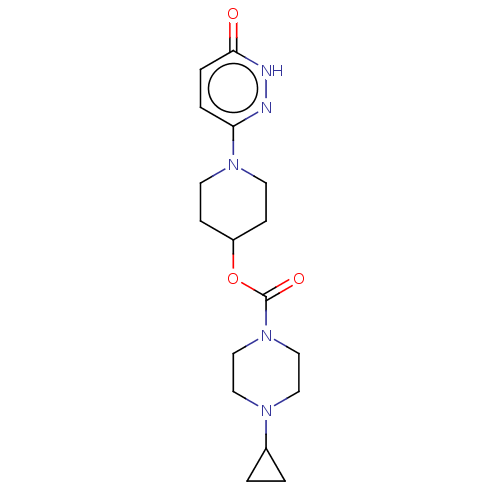 (US9034874, 1.4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ... | US Patent US9034874 (2015) BindingDB Entry DOI: 10.7270/Q2C82819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM159245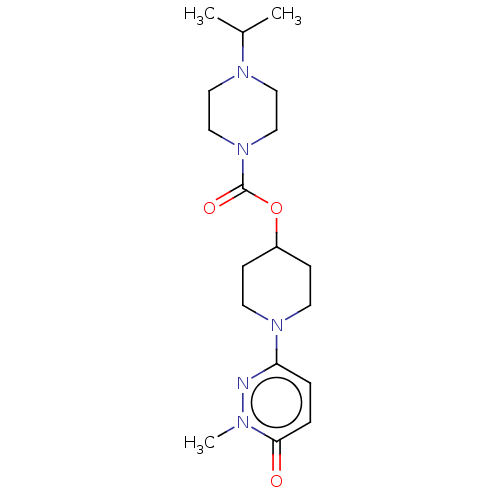 (US9034874, 1.2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ... | US Patent US9034874 (2015) BindingDB Entry DOI: 10.7270/Q2C82819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM159249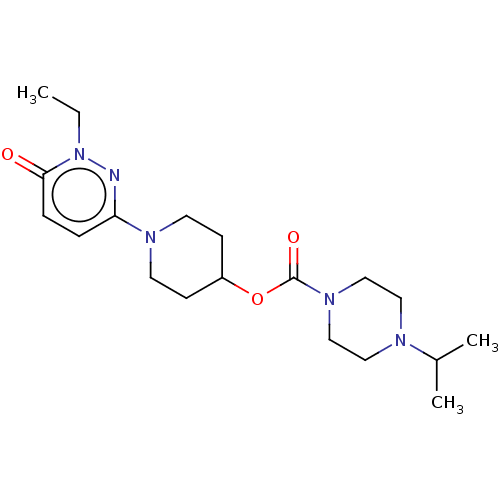 (US9034874, 1.6) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ... | US Patent US9034874 (2015) BindingDB Entry DOI: 10.7270/Q2C82819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM159250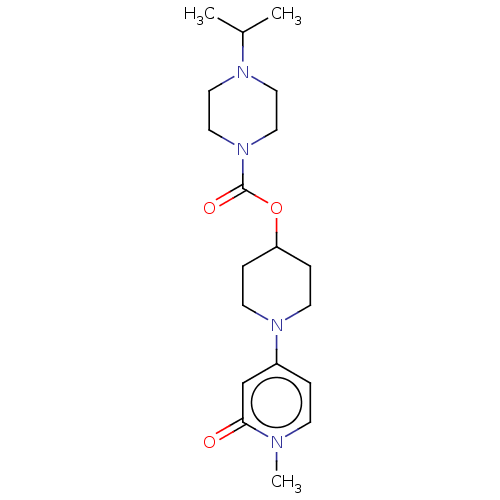 (US9034874, 2.1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ... | US Patent US9034874 (2015) BindingDB Entry DOI: 10.7270/Q2C82819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM159246 (US9034874, 1.3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 10 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M... | US Patent US9034874 (2015) BindingDB Entry DOI: 10.7270/Q2C82819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM159252 (US9034874, 2.3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 12 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M... | US Patent US9034874 (2015) BindingDB Entry DOI: 10.7270/Q2C82819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM159250 (US9034874, 2.1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 20 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M... | US Patent US9034874 (2015) BindingDB Entry DOI: 10.7270/Q2C82819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM159247 (US9034874, 1.4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 25 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M... | US Patent US9034874 (2015) BindingDB Entry DOI: 10.7270/Q2C82819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM159248 (US9034874, 1.5) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | 25 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M... | US Patent US9034874 (2015) BindingDB Entry DOI: 10.7270/Q2C82819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM159251 (US9034874, 2.2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 25 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M... | US Patent US9034874 (2015) BindingDB Entry DOI: 10.7270/Q2C82819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM159244 (US9034874, 1.1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 26 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M... | US Patent US9034874 (2015) BindingDB Entry DOI: 10.7270/Q2C82819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM159245 (US9034874, 1.2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 31 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M... | US Patent US9034874 (2015) BindingDB Entry DOI: 10.7270/Q2C82819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM159249 (US9034874, 1.6) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 44 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description The affinity of compounds of the invention to the H3 receptor can be assessed by measuring displacement of binding of the radioligand [3H]-N-alpha -M... | US Patent US9034874 (2015) BindingDB Entry DOI: 10.7270/Q2C82819 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 [420-757] (Homo sapiens (Human)) | BDBM239101 (US10195181, Example 2.2 | US9403810, 2.2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ... | US Patent US9403810 (2016) BindingDB Entry DOI: 10.7270/Q22V2F1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 (Homo sapiens (Human)) | BDBM239101 (US10195181, Example 2.2 | US9403810, 2.2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1... | US Patent US10195181 (2019) BindingDB Entry DOI: 10.7270/Q28054Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 (Homo sapiens (Human)) | BDBM239117 (US10195181, Example 22c | US9403810, 22) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1... | US Patent US10195181 (2019) BindingDB Entry DOI: 10.7270/Q28054Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 [420-757] (Homo sapiens (Human)) | BDBM239132 (US9403810, 22c) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ... | US Patent US9403810 (2016) BindingDB Entry DOI: 10.7270/Q22V2F1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 (Homo sapiens (Human)) | BDBM239094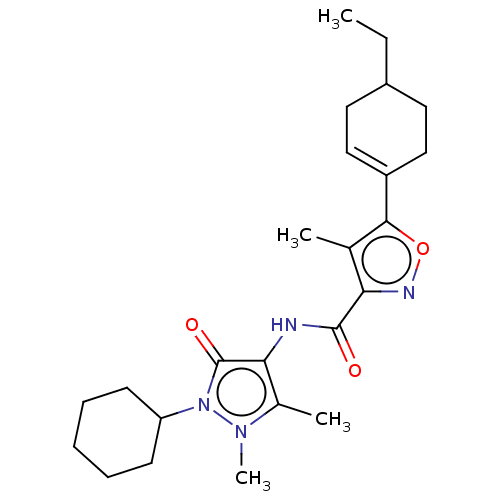 (US10195181, Example 1.3 | US9403810, 1.3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1... | US Patent US10195181 (2019) BindingDB Entry DOI: 10.7270/Q28054Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 [420-757] (Homo sapiens (Human)) | BDBM239094 (US10195181, Example 1.3 | US9403810, 1.3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ... | US Patent US9403810 (2016) BindingDB Entry DOI: 10.7270/Q22V2F1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 [420-757] (Homo sapiens (Human)) | BDBM239092 (US10195181, Example 1.1 | US9403810, 1.1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ... | US Patent US9403810 (2016) BindingDB Entry DOI: 10.7270/Q22V2F1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 (Homo sapiens (Human)) | BDBM239092 (US10195181, Example 1.1 | US9403810, 1.1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1... | US Patent US10195181 (2019) BindingDB Entry DOI: 10.7270/Q28054Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 [420-757] (Homo sapiens (Human)) | BDBM239099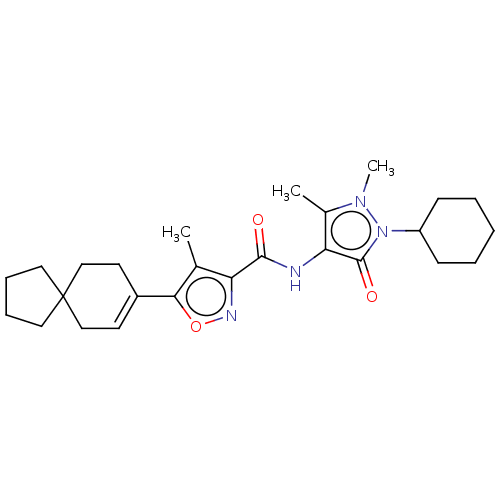 (US10195181, Example 2 | US9403810, 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ... | US Patent US9403810 (2016) BindingDB Entry DOI: 10.7270/Q22V2F1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 (Homo sapiens (Human)) | BDBM239099 (US10195181, Example 2 | US9403810, 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1... | US Patent US10195181 (2019) BindingDB Entry DOI: 10.7270/Q28054Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 [420-757] (Homo sapiens (Human)) | BDBM239091 (US10195181, Example 1 | US9403810, 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ... | US Patent US9403810 (2016) BindingDB Entry DOI: 10.7270/Q22V2F1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 (Homo sapiens (Human)) | BDBM239091 (US10195181, Example 1 | US9403810, 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1... | US Patent US10195181 (2019) BindingDB Entry DOI: 10.7270/Q28054Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 [420-757] (Homo sapiens (Human)) | BDBM239095 (US10195181, Example 1.4 | US9403810, 1.4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ... | US Patent US9403810 (2016) BindingDB Entry DOI: 10.7270/Q22V2F1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 (Homo sapiens (Human)) | BDBM239095 (US10195181, Example 1.4 | US9403810, 1.4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1... | US Patent US10195181 (2019) BindingDB Entry DOI: 10.7270/Q28054Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 [420-757] (Homo sapiens (Human)) | BDBM239105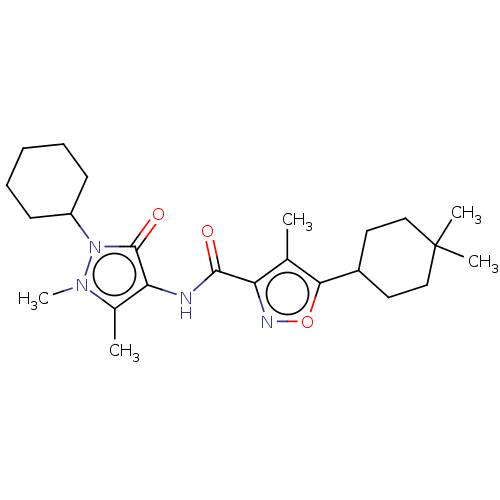 (US10195181, Example 6 | US9403810, 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ... | US Patent US9403810 (2016) BindingDB Entry DOI: 10.7270/Q22V2F1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 (Homo sapiens (Human)) | BDBM239105 (US10195181, Example 6 | US9403810, 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1... | US Patent US10195181 (2019) BindingDB Entry DOI: 10.7270/Q28054Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 [420-757] (Homo sapiens (Human)) | BDBM239096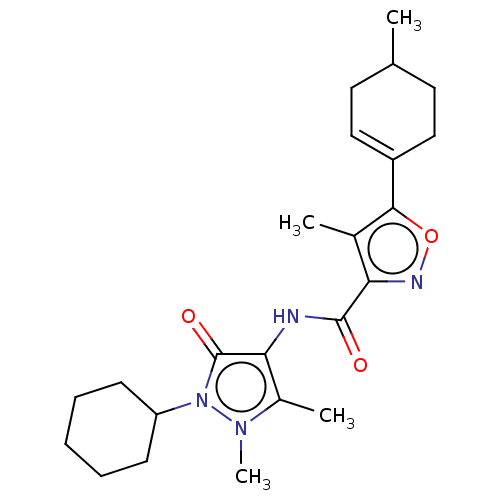 (US10195181, Example 1.5 | US9403810, 1.5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ... | US Patent US9403810 (2016) BindingDB Entry DOI: 10.7270/Q22V2F1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 (Homo sapiens (Human)) | BDBM239096 (US10195181, Example 1.5 | US9403810, 1.5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1... | US Patent US10195181 (2019) BindingDB Entry DOI: 10.7270/Q28054Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 (Homo sapiens (Human)) | BDBM239102 (US10195181, Example 3 | US9403810, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1... | US Patent US10195181 (2019) BindingDB Entry DOI: 10.7270/Q28054Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 [420-757] (Homo sapiens (Human)) | BDBM239102 (US10195181, Example 3 | US9403810, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ... | US Patent US9403810 (2016) BindingDB Entry DOI: 10.7270/Q22V2F1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 (Homo sapiens (Human)) | BDBM239097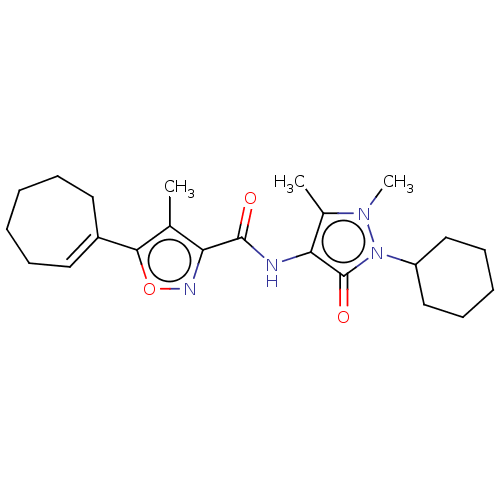 (US10195181, Example 1.6 | US9403810, 1.6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1... | US Patent US10195181 (2019) BindingDB Entry DOI: 10.7270/Q28054Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 [420-757] (Homo sapiens (Human)) | BDBM239097 (US10195181, Example 1.6 | US9403810, 1.6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ... | US Patent US9403810 (2016) BindingDB Entry DOI: 10.7270/Q22V2F1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 (Homo sapiens (Human)) | BDBM239093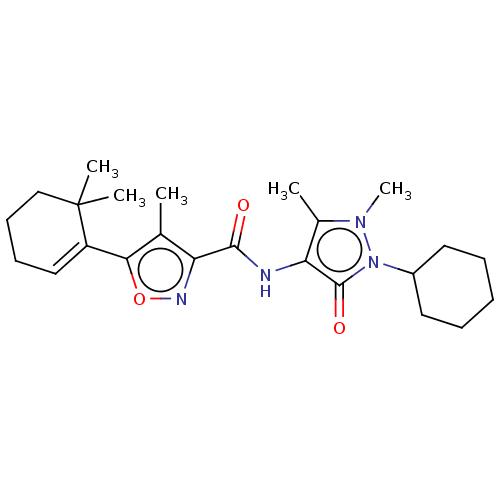 (US10195181, Example 1.2 | US9403810, 1.2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1... | US Patent US10195181 (2019) BindingDB Entry DOI: 10.7270/Q28054Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 [420-757] (Homo sapiens (Human)) | BDBM239093 (US10195181, Example 1.2 | US9403810, 1.2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ... | US Patent US9403810 (2016) BindingDB Entry DOI: 10.7270/Q22V2F1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 [420-757] (Homo sapiens (Human)) | BDBM239107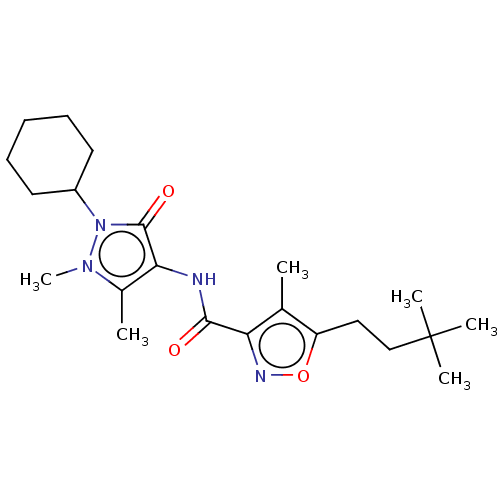 (US10195181, Example 7 | US9403810, 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ... | US Patent US9403810 (2016) BindingDB Entry DOI: 10.7270/Q22V2F1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 (Homo sapiens (Human)) | BDBM239107 (US10195181, Example 7 | US9403810, 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1... | US Patent US10195181 (2019) BindingDB Entry DOI: 10.7270/Q28054Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 [420-757] (Homo sapiens (Human)) | BDBM239106 (US10195181, Example 6.1 | US9403810, 6.1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ... | US Patent US9403810 (2016) BindingDB Entry DOI: 10.7270/Q22V2F1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 (Homo sapiens (Human)) | BDBM239106 (US10195181, Example 6.1 | US9403810, 6.1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1... | US Patent US10195181 (2019) BindingDB Entry DOI: 10.7270/Q28054Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 [420-757] (Homo sapiens (Human)) | BDBM239098 (US10195181, Example 1.7 | US9403810, 1.7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description For the biochemical assay panel, 50 nl of the test compounds, reference compounds and buffer/DMSO control are transferred to the respective wells of ... | US Patent US9403810 (2016) BindingDB Entry DOI: 10.7270/Q22V2F1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase SMURF1 (Homo sapiens (Human)) | BDBM239098 (US10195181, Example 1.7 | US9403810, 1.7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description To determine the HECT E3 ligase selectivity of the compounds, a panel of biochemical HECT E3 ligase autoubiquitinylation assays was employed (Smurf-1... | US Patent US10195181 (2019) BindingDB Entry DOI: 10.7270/Q28054Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 133 total ) | Next | Last >> |