 Found 108 hits with Last Name = 'uttamsingh' and Initial = 'v'
Found 108 hits with Last Name = 'uttamsingh' and Initial = 'v' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50185909
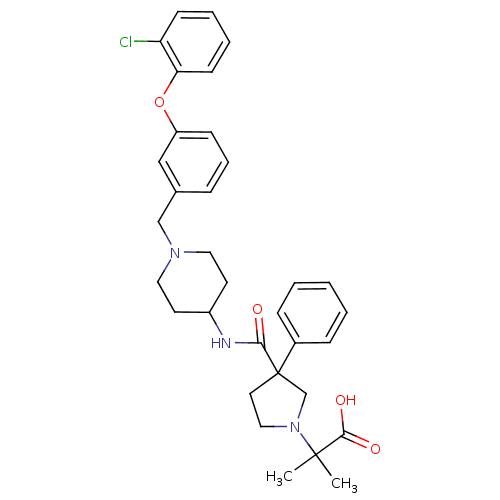
(2-(3-((1-(3-(2-chlorophenoxy)benzyl)piperidin-4-yl...)Show SMILES CC(C)(N1CCC(C1)(C(=O)NC1CCN(Cc2cccc(Oc3ccccc3Cl)c2)CC1)c1ccccc1)C(O)=O Show InChI InChI=1S/C33H38ClN3O4/c1-32(2,31(39)40)37-20-17-33(23-37,25-10-4-3-5-11-25)30(38)35-26-15-18-36(19-16-26)22-24-9-8-12-27(21-24)41-29-14-7-6-13-28(29)34/h3-14,21,26H,15-20,22-23H2,1-2H3,(H,35,38)(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells |
J Med Chem 49: 2669-72 (2006)
Article DOI: 10.1021/jm050965z
BindingDB Entry DOI: 10.7270/Q2GH9HJX |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50185912

(2-(4-{1-[3-(2-chloro-phenoxy)-benzyl]-piperidin-4-...)Show SMILES CC(C)(N1CCC(CC1)(C(=O)NC1CCN(Cc2cccc(Oc3ccccc3Cl)c2)CC1)c1ccccc1)C(O)=O Show InChI InChI=1S/C34H40ClN3O4/c1-33(2,32(40)41)38-21-17-34(18-22-38,26-10-4-3-5-11-26)31(39)36-27-15-19-37(20-16-27)24-25-9-8-12-28(23-25)42-30-14-7-6-13-29(30)35/h3-14,23,27H,15-22,24H2,1-2H3,(H,36,39)(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells |
J Med Chem 49: 2669-72 (2006)
Article DOI: 10.1021/jm050965z
BindingDB Entry DOI: 10.7270/Q2GH9HJX |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50185908
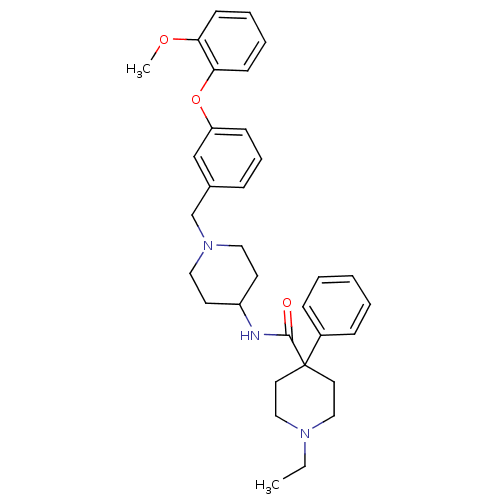
(1-ethyl-4-phenyl-piperidine-4-carboxylic acid {1-[...)Show SMILES CCN1CCC(CC1)(C(=O)NC1CCN(Cc2cccc(Oc3ccccc3OC)c2)CC1)c1ccccc1 Show InChI InChI=1S/C33H41N3O3/c1-3-35-22-18-33(19-23-35,27-11-5-4-6-12-27)32(37)34-28-16-20-36(21-17-28)25-26-10-9-13-29(24-26)39-31-15-8-7-14-30(31)38-2/h4-15,24,28H,3,16-23,25H2,1-2H3,(H,34,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells |
J Med Chem 49: 2669-72 (2006)
Article DOI: 10.1021/jm050965z
BindingDB Entry DOI: 10.7270/Q2GH9HJX |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50185910

(1-ethyl-4-phenyl-piperidine-4-carboxylic acid {1-[...)Show SMILES CCN1CCC(CC1)(C(=O)NC1CCN(Cc2cccc(Oc3ccccc3Cl)c2)CC1)c1ccccc1 Show InChI InChI=1S/C32H38ClN3O2/c1-2-35-21-17-32(18-22-35,26-10-4-3-5-11-26)31(37)34-27-15-19-36(20-16-27)24-25-9-8-12-28(23-25)38-30-14-7-6-13-29(30)33/h3-14,23,27H,2,15-22,24H2,1H3,(H,34,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells |
J Med Chem 49: 2669-72 (2006)
Article DOI: 10.1021/jm050965z
BindingDB Entry DOI: 10.7270/Q2GH9HJX |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50185906
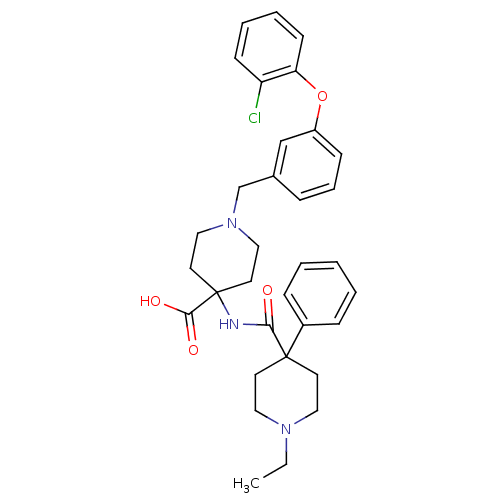
(1-[3-(2-chlorophenoxy)benzyl]-4-{[(1-ethyl-4-pheny...)Show SMILES CCN1CCC(CC1)(C(=O)NC1(CCN(Cc2cccc(Oc3ccccc3Cl)c2)CC1)C(O)=O)c1ccccc1 Show InChI InChI=1S/C33H38ClN3O4/c1-2-36-19-15-32(16-20-36,26-10-4-3-5-11-26)30(38)35-33(31(39)40)17-21-37(22-18-33)24-25-9-8-12-27(23-25)41-29-14-7-6-13-28(29)34/h3-14,23H,2,15-22,24H2,1H3,(H,35,38)(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells |
J Med Chem 49: 2669-72 (2006)
Article DOI: 10.1021/jm050965z
BindingDB Entry DOI: 10.7270/Q2GH9HJX |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50185904
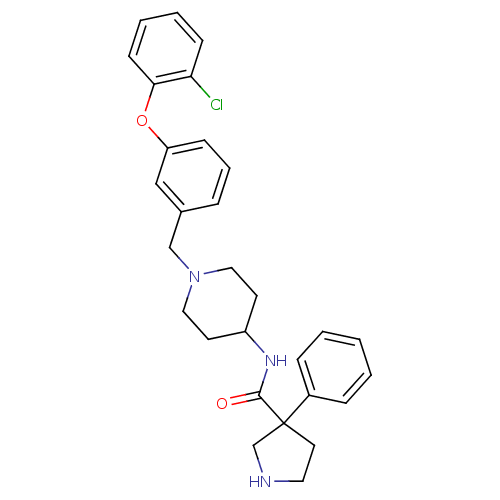
((+/-) 3-phenyl-pyrrolidine-3-carboxylic acid {1-[3...)Show SMILES Clc1ccccc1Oc1cccc(CN2CCC(CC2)NC(=O)C2(CCNC2)c2ccccc2)c1 Show InChI InChI=1S/C29H32ClN3O2/c30-26-11-4-5-12-27(26)35-25-10-6-7-22(19-25)20-33-17-13-24(14-18-33)32-28(34)29(15-16-31-21-29)23-8-2-1-3-9-23/h1-12,19,24,31H,13-18,20-21H2,(H,32,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells |
J Med Chem 49: 2669-72 (2006)
Article DOI: 10.1021/jm050965z
BindingDB Entry DOI: 10.7270/Q2GH9HJX |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50185902
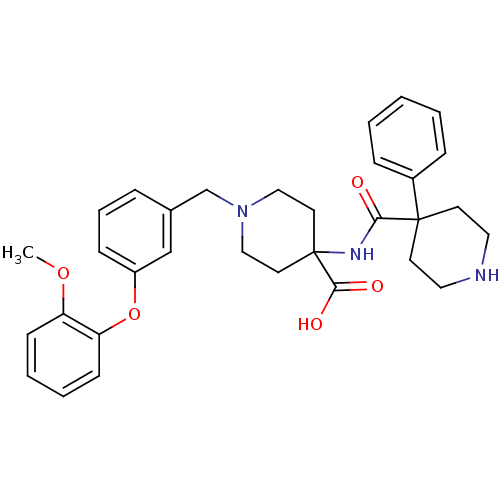
(1-[3-(2-methoxy-phenoxy)-benzyl]-4-[(4-phenyl-pipe...)Show SMILES COc1ccccc1Oc1cccc(CN2CCC(CC2)(NC(=O)C2(CCNCC2)c2ccccc2)C(O)=O)c1 Show InChI InChI=1S/C32H37N3O5/c1-39-27-12-5-6-13-28(27)40-26-11-7-8-24(22-26)23-35-20-16-32(17-21-35,30(37)38)34-29(36)31(14-18-33-19-15-31)25-9-3-2-4-10-25/h2-13,22,33H,14-21,23H2,1H3,(H,34,36)(H,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells |
J Med Chem 49: 2669-72 (2006)
Article DOI: 10.1021/jm050965z
BindingDB Entry DOI: 10.7270/Q2GH9HJX |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50185905
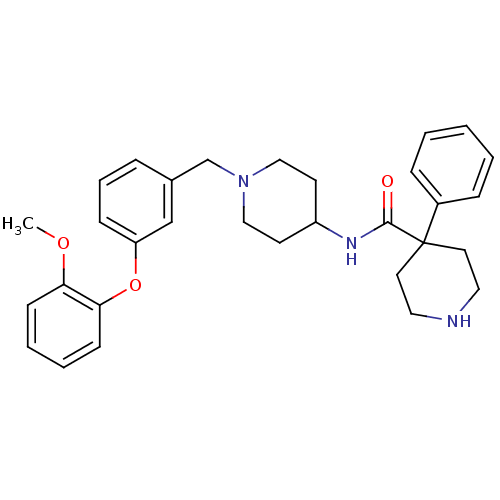
(4-phenyl-piperidine-4-carboxylic acid {1-[3-(2-met...)Show SMILES COc1ccccc1Oc1cccc(CN2CCC(CC2)NC(=O)C2(CCNCC2)c2ccccc2)c1 Show InChI InChI=1S/C31H37N3O3/c1-36-28-12-5-6-13-29(28)37-27-11-7-8-24(22-27)23-34-20-14-26(15-21-34)33-30(35)31(16-18-32-19-17-31)25-9-3-2-4-10-25/h2-13,22,26,32H,14-21,23H2,1H3,(H,33,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells |
J Med Chem 49: 2669-72 (2006)
Article DOI: 10.1021/jm050965z
BindingDB Entry DOI: 10.7270/Q2GH9HJX |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50185915

(1-(4-chloro-phenyl)-cyclohexanecarboxylic acid {1-...)Show SMILES COc1ccccc1Oc1cccc(CN2CCC(CC2)NC(=O)C2(CCCCC2)c2ccc(Cl)cc2)c1 Show InChI InChI=1S/C32H37ClN2O3/c1-37-29-10-3-4-11-30(29)38-28-9-7-8-24(22-28)23-35-20-16-27(17-21-35)34-31(36)32(18-5-2-6-19-32)25-12-14-26(33)15-13-25/h3-4,7-15,22,27H,2,5-6,16-21,23H2,1H3,(H,34,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells |
J Med Chem 49: 2669-72 (2006)
Article DOI: 10.1021/jm050965z
BindingDB Entry DOI: 10.7270/Q2GH9HJX |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50185907
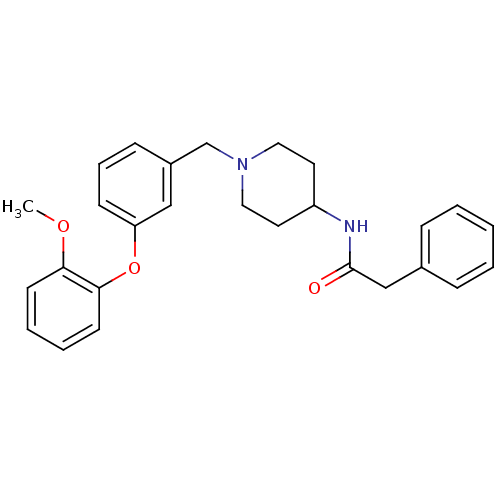
(CHEMBL210322 | N-{1-[3-(2-methoxy-phenoxy)-benzyl]...)Show SMILES COc1ccccc1Oc1cccc(CN2CCC(CC2)NC(=O)Cc2ccccc2)c1 Show InChI InChI=1S/C27H30N2O3/c1-31-25-12-5-6-13-26(25)32-24-11-7-10-22(18-24)20-29-16-14-23(15-17-29)28-27(30)19-21-8-3-2-4-9-21/h2-13,18,23H,14-17,19-20H2,1H3,(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells |
J Med Chem 49: 2669-72 (2006)
Article DOI: 10.1021/jm050965z
BindingDB Entry DOI: 10.7270/Q2GH9HJX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50185905

(4-phenyl-piperidine-4-carboxylic acid {1-[3-(2-met...)Show SMILES COc1ccccc1Oc1cccc(CN2CCC(CC2)NC(=O)C2(CCNCC2)c2ccccc2)c1 Show InChI InChI=1S/C31H37N3O3/c1-36-28-12-5-6-13-29(28)37-27-11-7-8-24(22-27)23-34-20-14-26(15-21-34)33-30(35)31(16-18-32-19-17-31)25-9-3-2-4-10-25/h2-13,22,26,32H,14-21,23H2,1H3,(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells |
J Med Chem 49: 2669-72 (2006)
Article DOI: 10.1021/jm050965z
BindingDB Entry DOI: 10.7270/Q2GH9HJX |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50185911
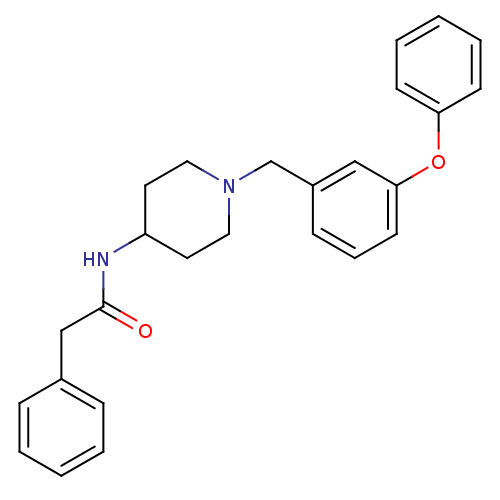
(CHEMBL205692 | N-[1-(3-phenoxy-benzyl)-piperidin-4...)Show SMILES O=C(Cc1ccccc1)NC1CCN(Cc2cccc(Oc3ccccc3)c2)CC1 Show InChI InChI=1S/C26H28N2O2/c29-26(19-21-8-3-1-4-9-21)27-23-14-16-28(17-15-23)20-22-10-7-13-25(18-22)30-24-11-5-2-6-12-24/h1-13,18,23H,14-17,19-20H2,(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells |
J Med Chem 49: 2669-72 (2006)
Article DOI: 10.1021/jm050965z
BindingDB Entry DOI: 10.7270/Q2GH9HJX |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50185903
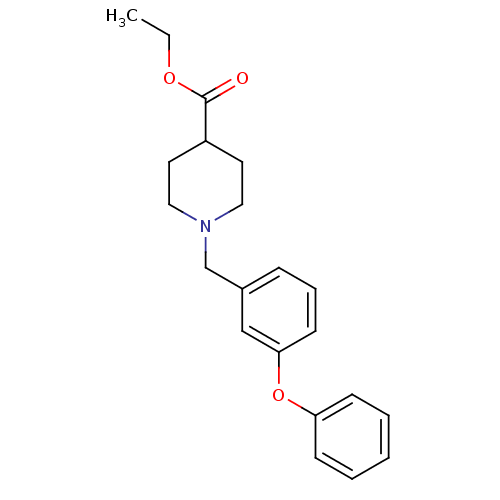
(1-(3-phenoxy-benzyl)-piperidine-4-carboxylic acid ...)Show InChI InChI=1S/C21H25NO3/c1-2-24-21(23)18-11-13-22(14-12-18)16-17-7-6-10-20(15-17)25-19-8-4-3-5-9-19/h3-10,15,18H,2,11-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells |
J Med Chem 49: 2669-72 (2006)
Article DOI: 10.1021/jm050965z
BindingDB Entry DOI: 10.7270/Q2GH9HJX |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50185916

(1-(3-phenoxy-benzyl)-piperidine-4-carboxylic acid ...)Show InChI InChI=1S/C21H26N2O2/c1-2-22-21(24)18-11-13-23(14-12-18)16-17-7-6-10-20(15-17)25-19-8-4-3-5-9-19/h3-10,15,18H,2,11-14,16H2,1H3,(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells |
J Med Chem 49: 2669-72 (2006)
Article DOI: 10.1021/jm050965z
BindingDB Entry DOI: 10.7270/Q2GH9HJX |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50185913
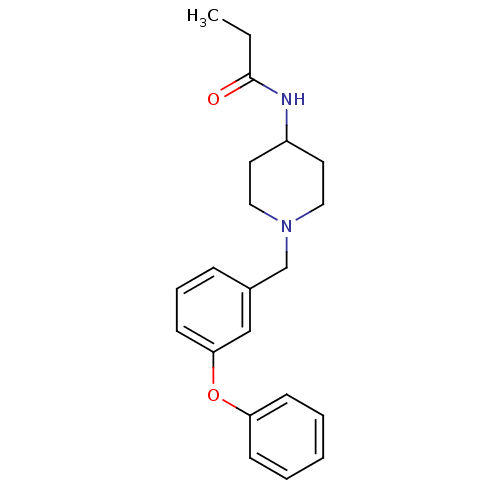
(CHEMBL381354 | N-[1-(3-phenoxy-benzyl)-piperidin-4...)Show InChI InChI=1S/C21H26N2O2/c1-2-21(24)22-18-11-13-23(14-12-18)16-17-7-6-10-20(15-17)25-19-8-4-3-5-9-19/h3-10,15,18H,2,11-14,16H2,1H3,(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells |
J Med Chem 49: 2669-72 (2006)
Article DOI: 10.1021/jm050965z
BindingDB Entry DOI: 10.7270/Q2GH9HJX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50185906

(1-[3-(2-chlorophenoxy)benzyl]-4-{[(1-ethyl-4-pheny...)Show SMILES CCN1CCC(CC1)(C(=O)NC1(CCN(Cc2cccc(Oc3ccccc3Cl)c2)CC1)C(O)=O)c1ccccc1 Show InChI InChI=1S/C33H38ClN3O4/c1-2-36-19-15-32(16-20-36,26-10-4-3-5-11-26)30(38)35-33(31(39)40)17-21-37(22-18-33)24-25-9-8-12-27(23-25)41-29-14-7-6-13-28(29)34/h3-14,23H,2,15-22,24H2,1H3,(H,35,38)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells |
J Med Chem 49: 2669-72 (2006)
Article DOI: 10.1021/jm050965z
BindingDB Entry DOI: 10.7270/Q2GH9HJX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50185902

(1-[3-(2-methoxy-phenoxy)-benzyl]-4-[(4-phenyl-pipe...)Show SMILES COc1ccccc1Oc1cccc(CN2CCC(CC2)(NC(=O)C2(CCNCC2)c2ccccc2)C(O)=O)c1 Show InChI InChI=1S/C32H37N3O5/c1-39-27-12-5-6-13-28(27)40-26-11-7-8-24(22-26)23-35-20-16-32(17-21-35,30(37)38)34-29(36)31(14-18-33-19-15-31)25-9-3-2-4-10-25/h2-13,22,33H,14-21,23H2,1H3,(H,34,36)(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells |
J Med Chem 49: 2669-72 (2006)
Article DOI: 10.1021/jm050965z
BindingDB Entry DOI: 10.7270/Q2GH9HJX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50185909

(2-(3-((1-(3-(2-chlorophenoxy)benzyl)piperidin-4-yl...)Show SMILES CC(C)(N1CCC(C1)(C(=O)NC1CCN(Cc2cccc(Oc3ccccc3Cl)c2)CC1)c1ccccc1)C(O)=O Show InChI InChI=1S/C33H38ClN3O4/c1-32(2,31(39)40)37-20-17-33(23-37,25-10-4-3-5-11-25)30(38)35-26-15-18-36(19-16-26)22-24-9-8-12-27(21-24)41-29-14-7-6-13-28(29)34/h3-14,21,26H,15-20,22-23H2,1-2H3,(H,35,38)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells |
J Med Chem 49: 2669-72 (2006)
Article DOI: 10.1021/jm050965z
BindingDB Entry DOI: 10.7270/Q2GH9HJX |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50185914

(1-(3-phenoxybenzyl)piperidine-4-carboxylic acid | ...)Show InChI InChI=1S/C19H21NO3/c21-19(22)16-9-11-20(12-10-16)14-15-5-4-8-18(13-15)23-17-6-2-1-3-7-17/h1-8,13,16H,9-12,14H2,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells |
J Med Chem 49: 2669-72 (2006)
Article DOI: 10.1021/jm050965z
BindingDB Entry DOI: 10.7270/Q2GH9HJX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350832
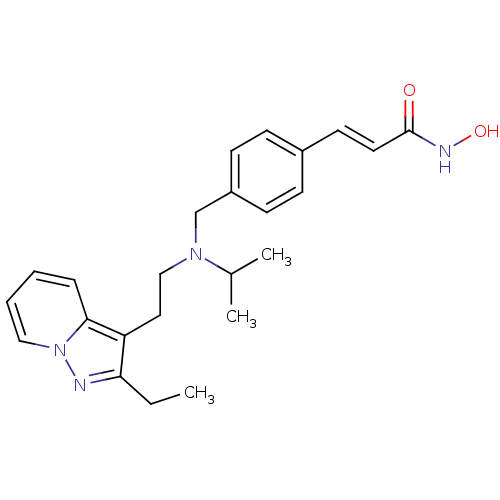
(CHEMBL1819274)Show SMILES CCc1nn2ccccc2c1CCN(Cc1ccc(\C=C\C(=O)NO)cc1)C(C)C Show InChI InChI=1S/C24H30N4O2/c1-4-22-21(23-7-5-6-15-28(23)25-22)14-16-27(18(2)3)17-20-10-8-19(9-11-20)12-13-24(29)26-30/h5-13,15,18,30H,4,14,16-17H2,1-3H3,(H,26,29)/b13-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50185909

(2-(3-((1-(3-(2-chlorophenoxy)benzyl)piperidin-4-yl...)Show SMILES CC(C)(N1CCC(C1)(C(=O)NC1CCN(Cc2cccc(Oc3ccccc3Cl)c2)CC1)c1ccccc1)C(O)=O Show InChI InChI=1S/C33H38ClN3O4/c1-32(2,31(39)40)37-20-17-33(23-37,25-10-4-3-5-11-25)30(38)35-26-15-18-36(19-16-26)22-24-9-8-12-27(21-24)41-29-14-7-6-13-28(29)34/h3-14,21,26H,15-20,22-23H2,1-2H3,(H,35,38)(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of I309-induced chemotaxis in L1.2 cells expressing CCR8 |
J Med Chem 49: 2669-72 (2006)
Article DOI: 10.1021/jm050965z
BindingDB Entry DOI: 10.7270/Q2GH9HJX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350831
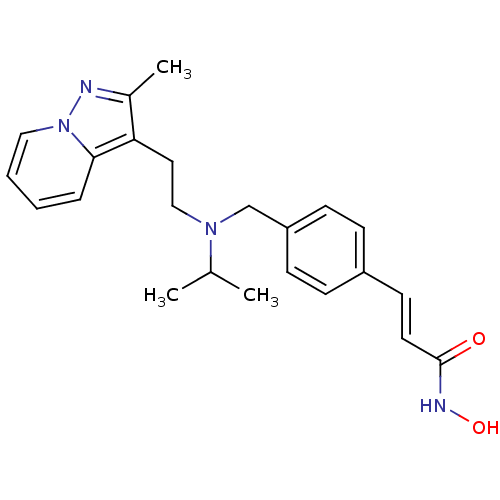
(CHEMBL1819273)Show SMILES CC(C)N(CCc1c(C)nn2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C23H28N4O2/c1-17(2)26(15-13-21-18(3)24-27-14-5-4-6-22(21)27)16-20-9-7-19(8-10-20)11-12-23(28)25-29/h4-12,14,17,29H,13,15-16H2,1-3H3,(H,25,28)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350818
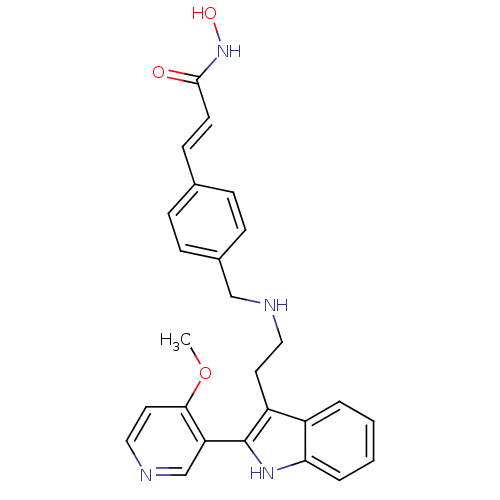
(CHEMBL1819257)Show SMILES COc1ccncc1-c1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C26H26N4O3/c1-33-24-13-15-28-17-22(24)26-21(20-4-2-3-5-23(20)29-26)12-14-27-16-19-8-6-18(7-9-19)10-11-25(31)30-32/h2-11,13,15,17,27,29,32H,12,14,16H2,1H3,(H,30,31)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350827
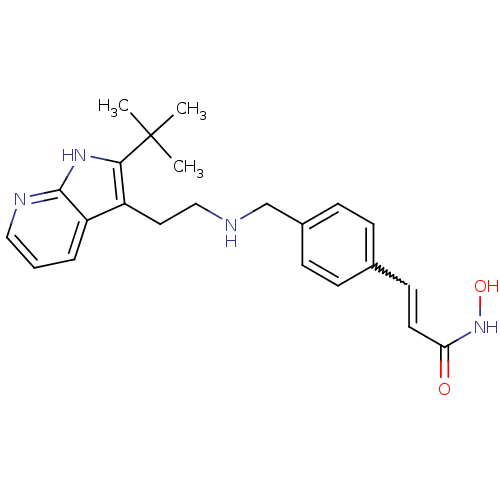
(CHEMBL1819267)Show SMILES CC(C)(C)c1[nH]c2ncccc2c1CCNCc1ccc(C=CC(=O)NO)cc1 |w:21.22| Show InChI InChI=1S/C23H28N4O2/c1-23(2,3)21-18(19-5-4-13-25-22(19)26-21)12-14-24-15-17-8-6-16(7-9-17)10-11-20(28)27-29/h4-11,13,24,29H,12,14-15H2,1-3H3,(H,25,26)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
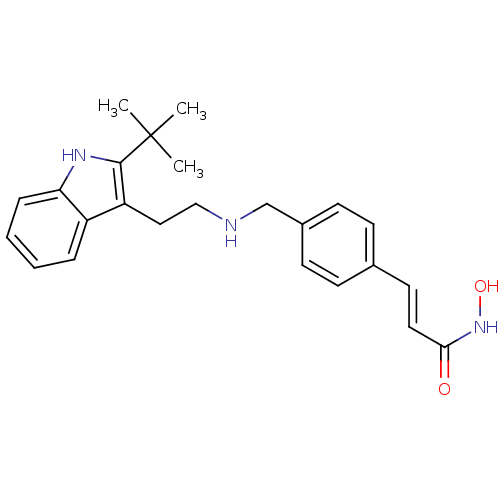
(Homo sapiens (Human)) | BDBM50350820
(CHEMBL1819260)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C24H29N3O2/c1-24(2,3)23-20(19-6-4-5-7-21(19)26-23)14-15-25-16-18-10-8-17(9-11-18)12-13-22(28)27-29/h4-13,25-26,29H,14-16H2,1-3H3,(H,27,28)/b13-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350835
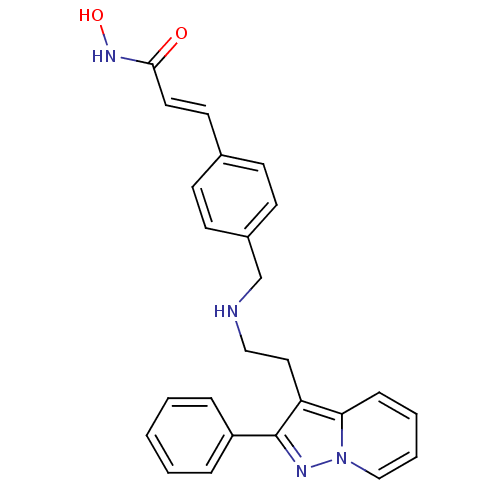
(CHEMBL1819272)Show SMILES ONC(=O)\C=C\c1ccc(CNCCc2c(nn3ccccc23)-c2ccccc2)cc1 Show InChI InChI=1S/C25H24N4O2/c30-24(28-31)14-13-19-9-11-20(12-10-19)18-26-16-15-22-23-8-4-5-17-29(23)27-25(22)21-6-2-1-3-7-21/h1-14,17,26,31H,15-16,18H2,(H,28,30)/b14-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350833
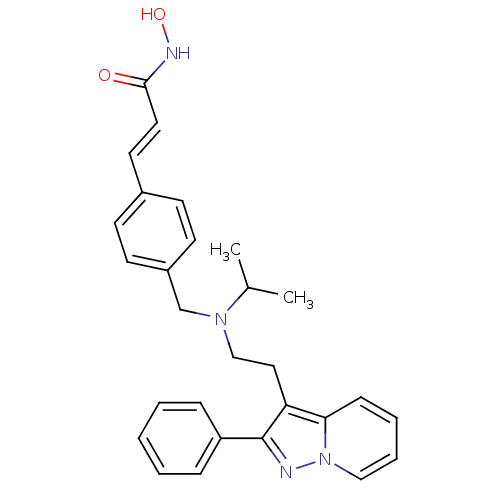
(CHEMBL1819275)Show SMILES CC(C)N(CCc1c(nn2ccccc12)-c1ccccc1)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C28H30N4O2/c1-21(2)31(20-23-13-11-22(12-14-23)15-16-27(33)30-34)19-17-25-26-10-6-7-18-32(26)29-28(25)24-8-4-3-5-9-24/h3-16,18,21,34H,17,19-20H2,1-2H3,(H,30,33)/b16-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350830
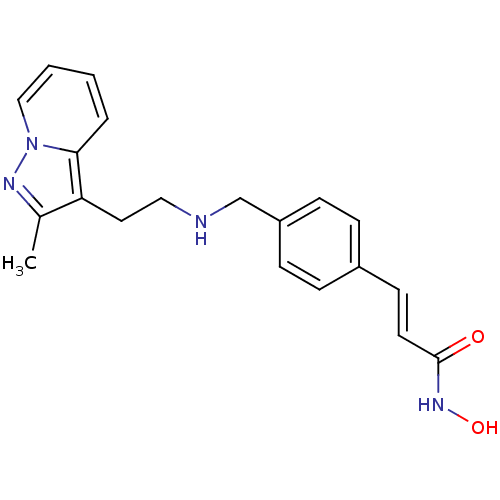
(CHEMBL1819270)Show InChI InChI=1S/C20H22N4O2/c1-15-18(19-4-2-3-13-24(19)22-15)11-12-21-14-17-7-5-16(6-8-17)9-10-20(25)23-26/h2-10,13,21,26H,11-12,14H2,1H3,(H,23,25)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350821
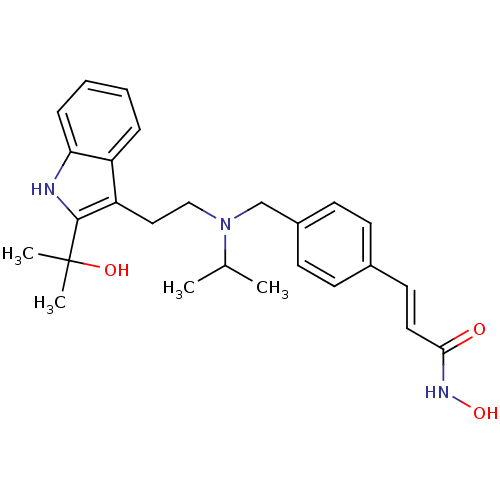
(CHEMBL1819261)Show SMILES CC(C)N(CCc1c([nH]c2ccccc12)C(C)(C)O)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C26H33N3O3/c1-18(2)29(17-20-11-9-19(10-12-20)13-14-24(30)28-32)16-15-22-21-7-5-6-8-23(21)27-25(22)26(3,4)31/h5-14,18,27,31-32H,15-17H2,1-4H3,(H,28,30)/b14-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50185912

(2-(4-{1-[3-(2-chloro-phenoxy)-benzyl]-piperidin-4-...)Show SMILES CC(C)(N1CCC(CC1)(C(=O)NC1CCN(Cc2cccc(Oc3ccccc3Cl)c2)CC1)c1ccccc1)C(O)=O Show InChI InChI=1S/C34H40ClN3O4/c1-33(2,32(40)41)38-21-17-34(18-22-38,26-10-4-3-5-11-26)31(39)36-27-15-19-37(20-16-27)24-25-9-8-12-28(23-25)42-30-14-7-6-13-29(30)35/h3-14,23,27H,15-22,24H2,1-2H3,(H,36,39)(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of I309-induced chemotaxis in L1.2 cells expressing CCR8 |
J Med Chem 49: 2669-72 (2006)
Article DOI: 10.1021/jm050965z
BindingDB Entry DOI: 10.7270/Q2GH9HJX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350826
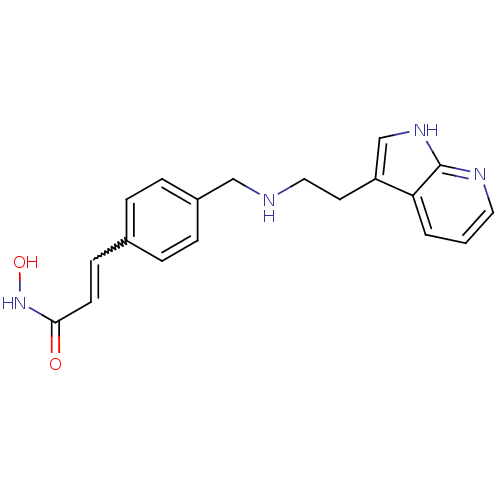
(CHEMBL1819266)Show SMILES ONC(=O)C=Cc1ccc(CNCCc2c[nH]c3ncccc23)cc1 |w:5.5| Show InChI InChI=1S/C19H20N4O2/c24-18(23-25)8-7-14-3-5-15(6-4-14)12-20-11-9-16-13-22-19-17(16)2-1-10-21-19/h1-8,10,13,20,25H,9,11-12H2,(H,21,22)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350838
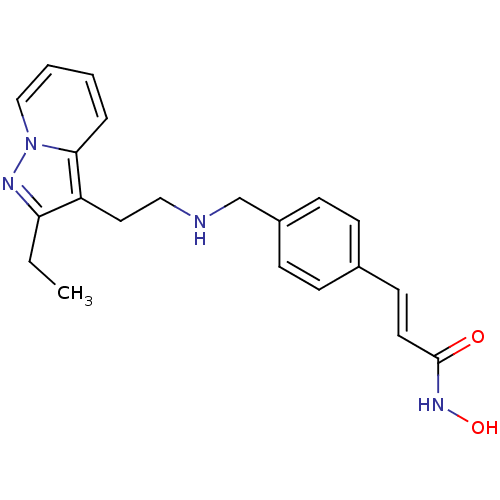
(CHEMBL1819271)Show InChI InChI=1S/C21H24N4O2/c1-2-19-18(20-5-3-4-14-25(20)23-19)12-13-22-15-17-8-6-16(7-9-17)10-11-21(26)24-27/h3-11,14,22,27H,2,12-13,15H2,1H3,(H,24,26)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350829
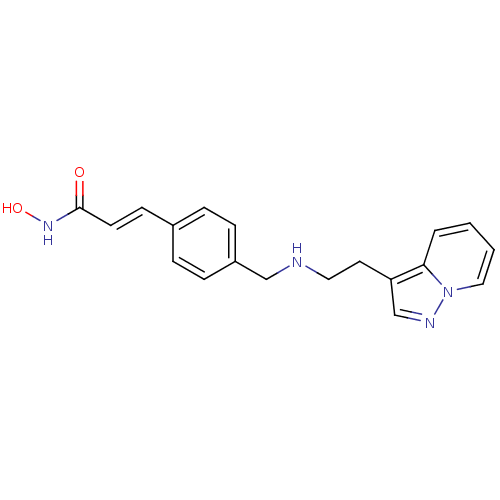
(CHEMBL1819269)Show InChI InChI=1S/C19H20N4O2/c24-19(22-25)9-8-15-4-6-16(7-5-15)13-20-11-10-17-14-21-23-12-2-1-3-18(17)23/h1-9,12,14,20,25H,10-11,13H2,(H,22,24)/b9-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350824
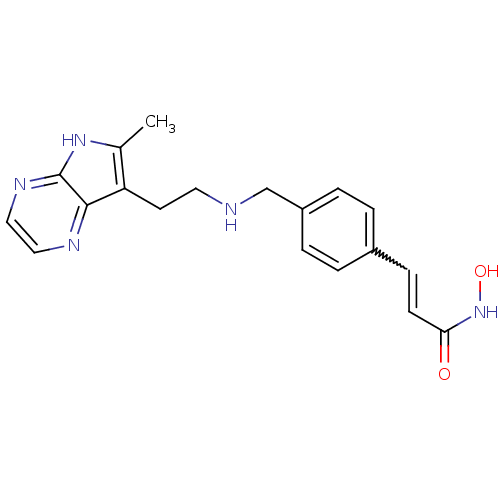
(CHEMBL1819264)Show SMILES Cc1[nH]c2nccnc2c1CCNCc1ccc(C=CC(=O)NO)cc1 |w:18.19| Show InChI InChI=1S/C19H21N5O2/c1-13-16(18-19(23-13)22-11-10-21-18)8-9-20-12-15-4-2-14(3-5-15)6-7-17(25)24-26/h2-7,10-11,20,26H,8-9,12H2,1H3,(H,22,23)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350828
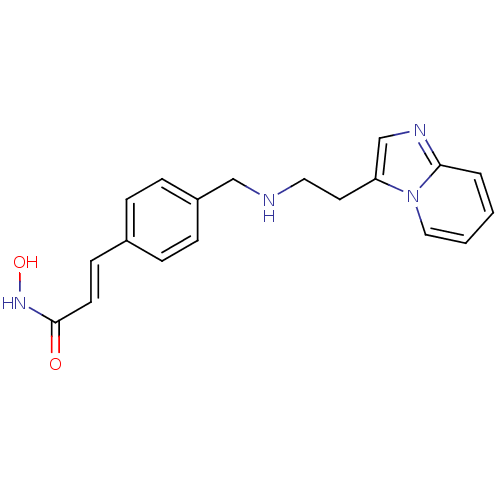
(CHEMBL1819268)Show InChI InChI=1S/C19H20N4O2/c24-19(22-25)9-8-15-4-6-16(7-5-15)13-20-11-10-17-14-21-18-3-1-2-12-23(17)18/h1-9,12,14,20,25H,10-11,13H2,(H,22,24)/b9-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350822
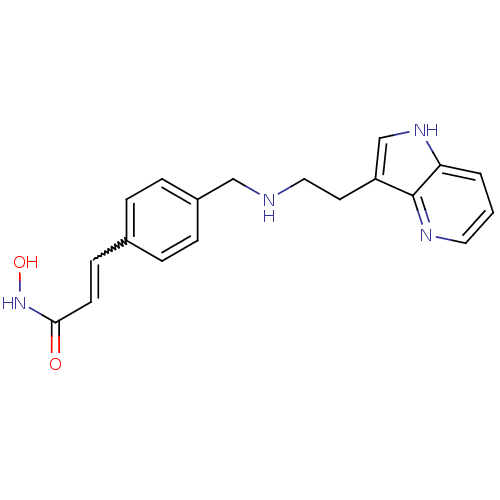
(CHEMBL1819262)Show SMILES ONC(=O)C=Cc1ccc(CNCCc2c[nH]c3cccnc23)cc1 |w:5.5| Show InChI InChI=1S/C19H20N4O2/c24-18(23-25)8-7-14-3-5-15(6-4-14)12-20-11-9-16-13-22-17-2-1-10-21-19(16)17/h1-8,10,13,20,22,25H,9,11-12H2,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50185905

(4-phenyl-piperidine-4-carboxylic acid {1-[3-(2-met...)Show SMILES COc1ccccc1Oc1cccc(CN2CCC(CC2)NC(=O)C2(CCNCC2)c2ccccc2)c1 Show InChI InChI=1S/C31H37N3O3/c1-36-28-12-5-6-13-29(28)37-27-11-7-8-24(22-27)23-34-20-14-26(15-21-34)33-30(35)31(16-18-32-19-17-31)25-9-3-2-4-10-25/h2-13,22,26,32H,14-21,23H2,1H3,(H,33,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of I309-induced chemotaxis in L1.2 cells expressing CCR8 |
J Med Chem 49: 2669-72 (2006)
Article DOI: 10.1021/jm050965z
BindingDB Entry DOI: 10.7270/Q2GH9HJX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350817
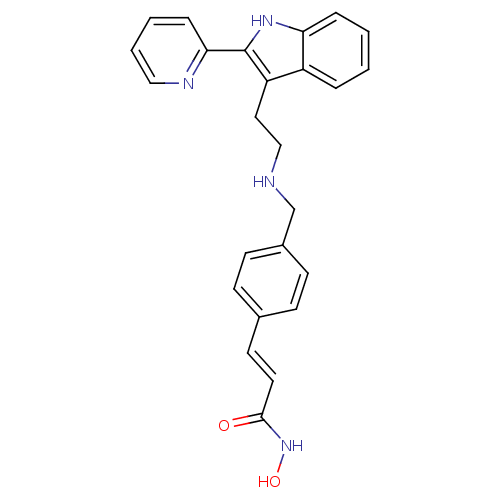
(CHEMBL1819256)Show SMILES ONC(=O)\C=C\c1ccc(CNCCc2c([nH]c3ccccc23)-c2ccccn2)cc1 Show InChI InChI=1S/C25H24N4O2/c30-24(29-31)13-12-18-8-10-19(11-9-18)17-26-16-14-21-20-5-1-2-6-22(20)28-25(21)23-7-3-4-15-27-23/h1-13,15,26,28,31H,14,16-17H2,(H,29,30)/b13-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350813
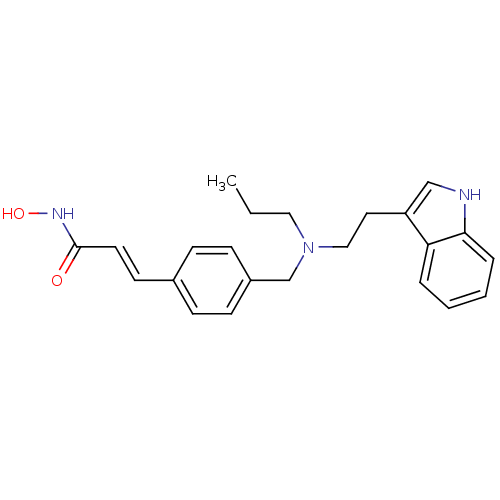
(CHEMBL1819141)Show SMILES CCCN(CCc1c[nH]c2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C23H27N3O2/c1-2-14-26(15-13-20-16-24-22-6-4-3-5-21(20)22)17-19-9-7-18(8-10-19)11-12-23(27)25-28/h3-12,16,24,28H,2,13-15,17H2,1H3,(H,25,27)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350816
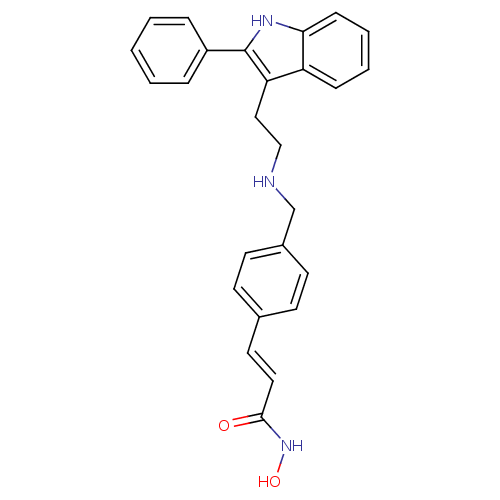
(CHEMBL1819255)Show SMILES ONC(=O)\C=C\c1ccc(CNCCc2c([nH]c3ccccc23)-c2ccccc2)cc1 Show InChI InChI=1S/C26H25N3O2/c30-25(29-31)15-14-19-10-12-20(13-11-19)18-27-17-16-23-22-8-4-5-9-24(22)28-26(23)21-6-2-1-3-7-21/h1-15,27-28,31H,16-18H2,(H,29,30)/b15-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350810
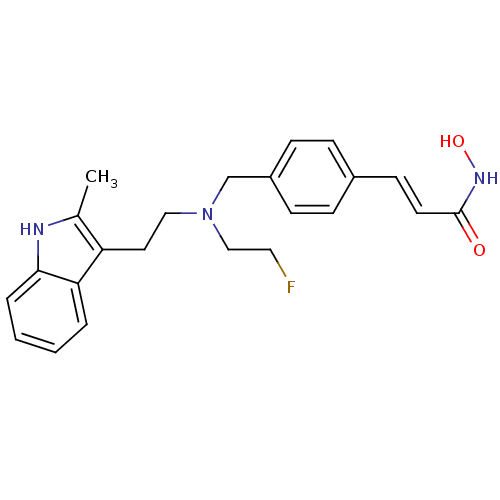
(CHEMBL1819138)Show SMILES Cc1[nH]c2ccccc2c1CCN(CCF)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C23H26FN3O2/c1-17-20(21-4-2-3-5-22(21)25-17)12-14-27(15-13-24)16-19-8-6-18(7-9-19)10-11-23(28)26-29/h2-11,25,29H,12-16H2,1H3,(H,26,28)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50134232
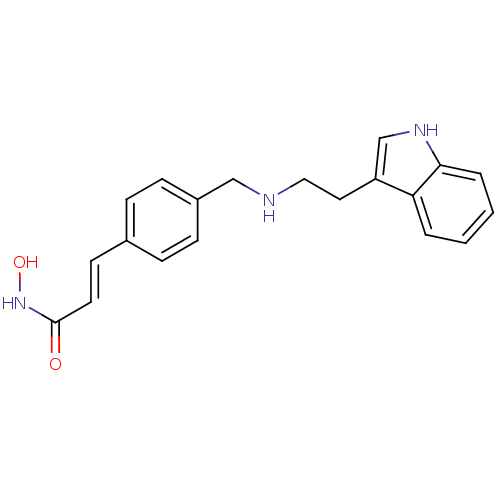
((E)-N-Hydroxy-3-(4-{[2-(1H-indol-3-yl)-ethylamino]...)Show InChI InChI=1S/C20H21N3O2/c24-20(23-25)10-9-15-5-7-16(8-6-15)13-21-12-11-17-14-22-19-4-2-1-3-18(17)19/h1-10,14,21-22,25H,11-13H2,(H,23,24)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19428

((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...)Show SMILES OCCN(CCc1c[nH]c2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C22H25N3O3/c26-14-13-25(12-11-19-15-23-21-4-2-1-3-20(19)21)16-18-7-5-17(6-8-18)9-10-22(27)24-28/h1-10,15,23,26,28H,11-14,16H2,(H,24,27)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350834
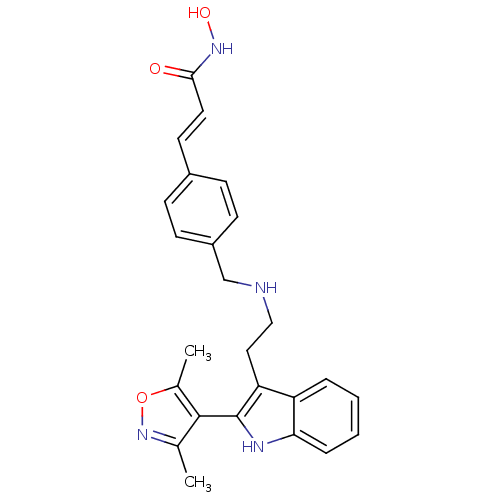
(CHEMBL1819259)Show SMILES Cc1noc(C)c1-c1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 |(-6.03,-13.03,;-4.7,-12.25,;-3.29,-12.87,;-2.26,-11.72,;-3.04,-10.39,;-2.42,-8.98,;-4.56,-10.72,;-5.71,-9.69,;-7.22,-10.01,;-8,-8.67,;-9.51,-8.34,;-9.98,-6.87,;-8.94,-5.73,;-7.44,-6.05,;-6.96,-7.52,;-5.55,-8.15,;-4.21,-7.37,;-2.88,-8.14,;-1.54,-7.37,;-.21,-8.14,;1.12,-7.37,;1.12,-5.82,;2.45,-5.05,;3.79,-5.82,;5.12,-5.04,;6.46,-5.81,;7.79,-5.03,;7.78,-3.49,;9.12,-5.8,;10.45,-5.02,;3.79,-7.37,;2.46,-8.14,)| Show InChI InChI=1S/C25H26N4O3/c1-16-24(17(2)32-29-16)25-21(20-5-3-4-6-22(20)27-25)13-14-26-15-19-9-7-18(8-10-19)11-12-23(30)28-31/h3-12,26-27,31H,13-15H2,1-2H3,(H,28,30)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350825

(CHEMBL1819265)Show SMILES ONC(=O)C=Cc1ccc(CNCCc2c[nH]c3ccncc23)cc1 |w:5.5| Show InChI InChI=1S/C19H20N4O2/c24-19(23-25)6-5-14-1-3-15(4-2-14)11-20-9-7-16-12-22-18-8-10-21-13-17(16)18/h1-6,8,10,12-13,20,22,25H,7,9,11H2,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350809
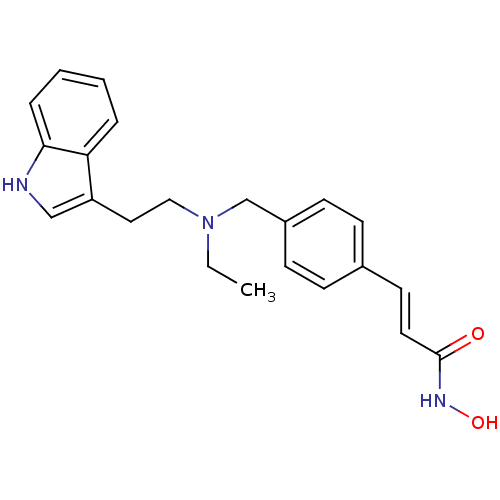
(CHEMBL1819137)Show SMILES CCN(CCc1c[nH]c2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C22H25N3O2/c1-2-25(14-13-19-15-23-21-6-4-3-5-20(19)21)16-18-9-7-17(8-10-18)11-12-22(26)24-27/h3-12,15,23,27H,2,13-14,16H2,1H3,(H,24,26)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50185904

((+/-) 3-phenyl-pyrrolidine-3-carboxylic acid {1-[3...)Show SMILES Clc1ccccc1Oc1cccc(CN2CCC(CC2)NC(=O)C2(CCNC2)c2ccccc2)c1 Show InChI InChI=1S/C29H32ClN3O2/c30-26-11-4-5-12-27(26)35-25-10-6-7-22(19-25)20-33-17-13-24(14-18-33)32-28(34)29(15-16-31-21-29)23-8-2-1-3-9-23/h1-12,19,24,31H,13-18,20-21H2,(H,32,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of I309-induced chemotaxis in L1.2 cells expressing CCR8 |
J Med Chem 49: 2669-72 (2006)
Article DOI: 10.1021/jm050965z
BindingDB Entry DOI: 10.7270/Q2GH9HJX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50134227
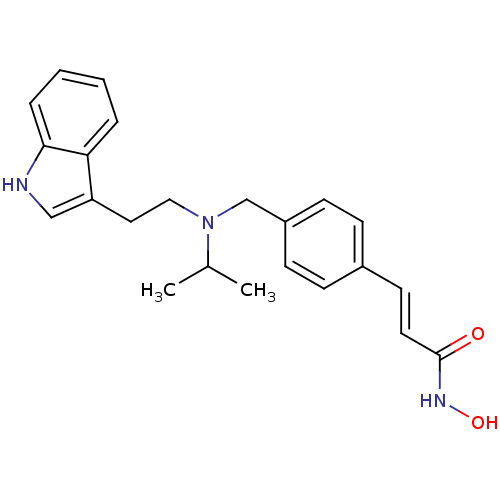
((E)-N-Hydroxy-3-[4-({[2-(1H-indol-3-yl)-ethyl]-iso...)Show SMILES CC(C)N(CCc1c[nH]c2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C23H27N3O2/c1-17(2)26(14-13-20-15-24-22-6-4-3-5-21(20)22)16-19-9-7-18(8-10-19)11-12-23(27)25-28/h3-12,15,17,24,28H,13-14,16H2,1-2H3,(H,25,27)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350815
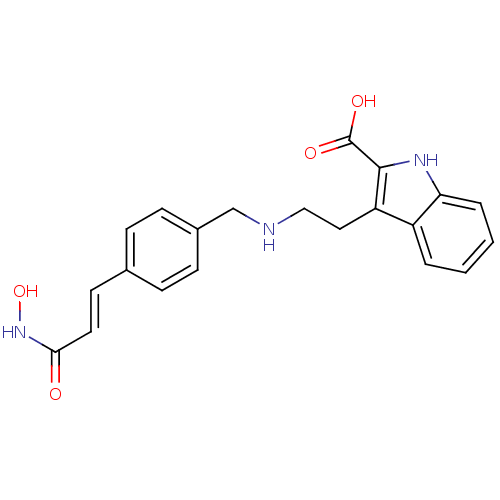
(CHEMBL1819254)Show SMILES ONC(=O)\C=C\c1ccc(CNCCc2c([nH]c3ccccc23)C(O)=O)cc1 Show InChI InChI=1S/C21H21N3O4/c25-19(24-28)10-9-14-5-7-15(8-6-14)13-22-12-11-17-16-3-1-2-4-18(16)23-20(17)21(26)27/h1-10,22-23,28H,11-13H2,(H,24,25)(H,26,27)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350823
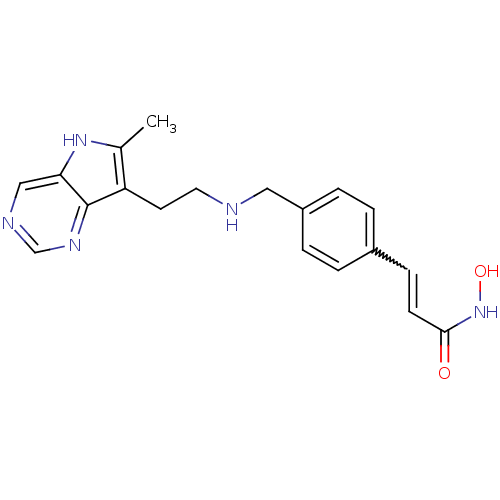
(CHEMBL1819263)Show SMILES Cc1[nH]c2cncnc2c1CCNCc1ccc(C=CC(=O)NO)cc1 |w:18.19| Show InChI InChI=1S/C19H21N5O2/c1-13-16(19-17(23-13)11-21-12-22-19)8-9-20-10-15-4-2-14(3-5-15)6-7-18(25)24-26/h2-7,11-12,20,23,26H,8-10H2,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data