 Found 199 hits with Last Name = 'valentine' and Initial = 'a'
Found 199 hits with Last Name = 'valentine' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85530
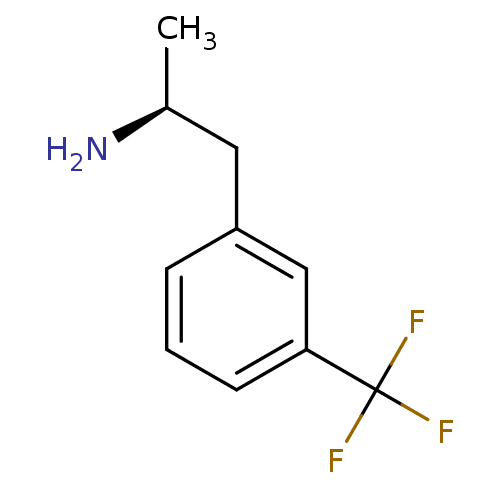
(Nor-d-fenfluramine | Nor-dexfenfluramine | Norfenf...)Show InChI InChI=1S/C10H12F3N/c1-7(14)5-8-3-2-4-9(6-8)10(11,12)13/h2-4,6-7H,5,14H2,1H3/t7-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85530

(Nor-d-fenfluramine | Nor-dexfenfluramine | Norfenf...)Show InChI InChI=1S/C10H12F3N/c1-7(14)5-8-3-2-4-9(6-8)10(11,12)13/h2-4,6-7H,5,14H2,1H3/t7-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85598
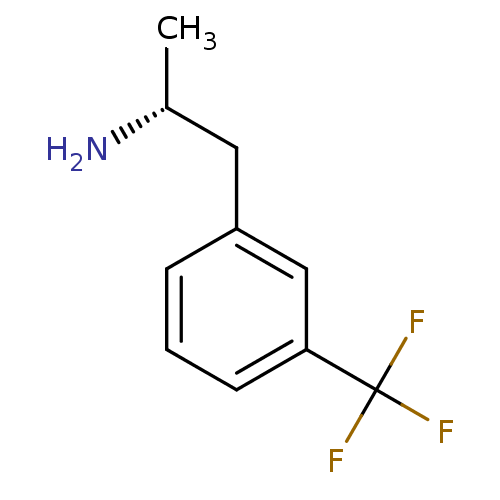
(l-norfenfluramine)Show InChI InChI=1S/C10H12F3N/c1-7(14)5-8-3-2-4-9(6-8)10(11,12)13/h2-4,6-7H,5,14H2,1H3/t7-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85598

(l-norfenfluramine)Show InChI InChI=1S/C10H12F3N/c1-7(14)5-8-3-2-4-9(6-8)10(11,12)13/h2-4,6-7H,5,14H2,1H3/t7-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85530

(Nor-d-fenfluramine | Nor-dexfenfluramine | Norfenf...)Show InChI InChI=1S/C10H12F3N/c1-7(14)5-8-3-2-4-9(6-8)10(11,12)13/h2-4,6-7H,5,14H2,1H3/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 187 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85598

(l-norfenfluramine)Show InChI InChI=1S/C10H12F3N/c1-7(14)5-8-3-2-4-9(6-8)10(11,12)13/h2-4,6-7H,5,14H2,1H3/t7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 267 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85597
(CAS_37577-24-5 | NSC_65801 | l-Fenfluramine)Show InChI InChI=1S/C12H16F3N/c1-3-16-9(2)7-10-5-4-6-11(8-10)12(13,14)15/h4-6,8-9,16H,3,7H2,1-2H3/t9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85597

(CAS_37577-24-5 | NSC_65801 | l-Fenfluramine)Show InChI InChI=1S/C12H16F3N/c1-3-16-9(2)7-10-5-4-6-11(8-10)12(13,14)15/h4-6,8-9,16H,3,7H2,1-2H3/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85597

(CAS_37577-24-5 | NSC_65801 | l-Fenfluramine)Show InChI InChI=1S/C12H16F3N/c1-3-16-9(2)7-10-5-4-6-11(8-10)12(13,14)15/h4-6,8-9,16H,3,7H2,1-2H3/t9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85596

(CAS_3239-45-0 | NSC_65801 | d-Fenfluramine)Show InChI InChI=1S/C12H16F3N/c1-3-16-9(2)7-10-5-4-6-11(8-10)12(13,14)15/h4-6,8-9,16H,3,7H2,1-2H3/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| 2.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85596

(CAS_3239-45-0 | NSC_65801 | d-Fenfluramine)Show InChI InChI=1S/C12H16F3N/c1-3-16-9(2)7-10-5-4-6-11(8-10)12(13,14)15/h4-6,8-9,16H,3,7H2,1-2H3/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 75-81 (2000)
BindingDB Entry DOI: 10.7270/Q2P26WPH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325177
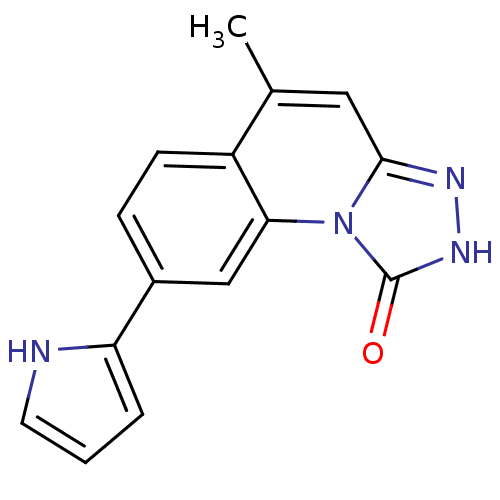
(5-methyl-8-(1H-pyrrol-2-yl)-[1,2,4]triazolo[4,3-a]...)Show InChI InChI=1S/C15H12N4O/c1-9-7-14-17-18-15(20)19(14)13-8-10(4-5-11(9)13)12-3-2-6-16-12/h2-8,16H,1H3,(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of His6-CHK1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 20: 5133-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.015
BindingDB Entry DOI: 10.7270/Q2FF3SJZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM7667

(4-[(4-{6-bromoimidazo[1,2-a]pyridin-3-yl}pyrimidin...)Show SMILES COCCNS(=O)(=O)c1ccc(Nc2nccc(n2)-c2cnc3ccc(Br)cn23)cc1 Show InChI InChI=1S/C20H19BrN6O3S/c1-30-11-10-24-31(28,29)16-5-3-15(4-6-16)25-20-22-9-8-17(26-20)18-12-23-19-7-2-14(21)13-27(18)19/h2-9,12-13,24H,10-11H2,1H3,(H,22,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
AstraZeneca
| Assay Description
The kinase activity was assayed using an in vitro scintillation proximity assay (SPA) for measuring incorporation of [gamma-33P] ATP into GST-Rb. |
Bioorg Med Chem Lett 14: 2249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.008
BindingDB Entry DOI: 10.7270/Q2XG9PCF |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM7668
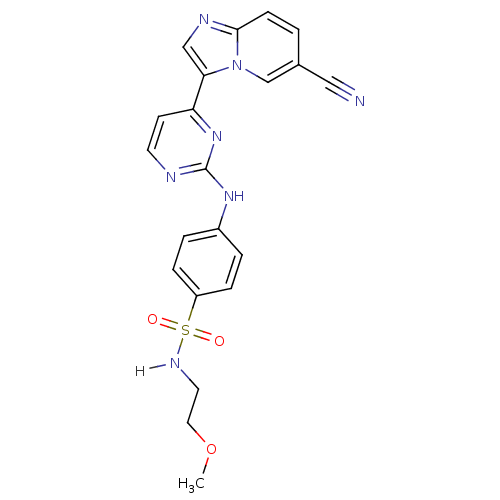
(4-[(4-{6-cyanoimidazo[1,2-a]pyridin-3-yl}pyrimidin...)Show SMILES COCCNS(=O)(=O)c1ccc(Nc2nccc(n2)-c2cnc3ccc(cn23)C#N)cc1 Show InChI InChI=1S/C21H19N7O3S/c1-31-11-10-25-32(29,30)17-5-3-16(4-6-17)26-21-23-9-8-18(27-21)19-13-24-20-7-2-15(12-22)14-28(19)20/h2-9,13-14,25H,10-11H2,1H3,(H,23,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
AstraZeneca
| Assay Description
The kinase activity was assayed using an in vitro scintillation proximity assay (SPA) for measuring incorporation of [gamma-33P] ATP into GST-Rb. |
Bioorg Med Chem Lett 14: 2249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.008
BindingDB Entry DOI: 10.7270/Q2XG9PCF |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM7671
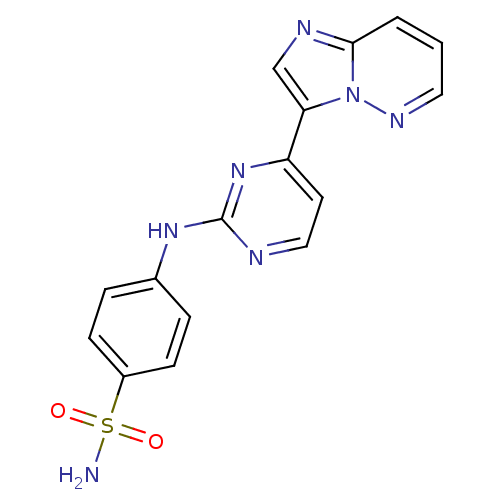
(4-[(4-{imidazo[1,2-a]pyridazin-3-yl}pyrimidin-2-yl...)Show SMILES NS(=O)(=O)c1ccc(Nc2nccc(n2)-c2cnc3cccnn23)cc1 Show InChI InChI=1S/C16H13N7O2S/c17-26(24,25)12-5-3-11(4-6-12)21-16-18-9-7-13(22-16)14-10-19-15-2-1-8-20-23(14)15/h1-10H,(H2,17,24,25)(H,18,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
AstraZeneca
| Assay Description
The kinase activity was assayed using an in vitro scintillation proximity assay (SPA) for measuring incorporation of [gamma-33P] ATP into GST-Rb. |
Bioorg Med Chem Lett 14: 2249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.008
BindingDB Entry DOI: 10.7270/Q2XG9PCF |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM7675
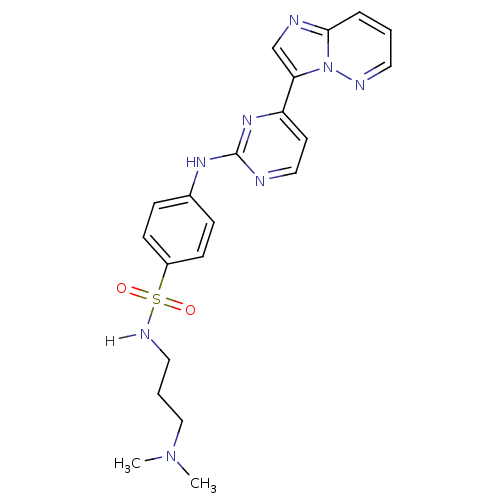
(Imidazo[1,2-b]pyridazine deriv. 2e | N-[3-(dimethy...)Show SMILES CN(C)CCCNS(=O)(=O)c1ccc(Nc2nccc(n2)-c2cnc3cccnn23)cc1 Show InChI InChI=1S/C21H24N8O2S/c1-28(2)14-4-12-25-32(30,31)17-8-6-16(7-9-17)26-21-22-13-10-18(27-21)19-15-23-20-5-3-11-24-29(19)20/h3,5-11,13,15,25H,4,12,14H2,1-2H3,(H,22,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
AstraZeneca
| Assay Description
The kinase activity was assayed using an in vitro scintillation proximity assay (SPA) for measuring incorporation of [gamma-33P] ATP into GST-Rb. |
Bioorg Med Chem Lett 14: 2249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.008
BindingDB Entry DOI: 10.7270/Q2XG9PCF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM7669
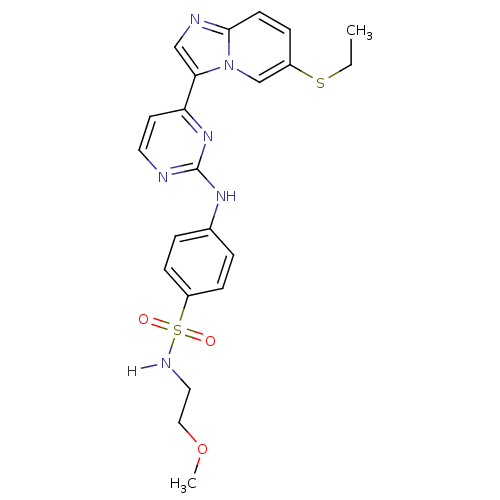
(4-({4-[6-(ethylsulfanyl)imidazo[1,2-a]pyridin-3-yl...)Show SMILES CCSc1ccc2ncc(-c3ccnc(Nc4ccc(cc4)S(=O)(=O)NCCOC)n3)n2c1 Show InChI InChI=1S/C22H24N6O3S2/c1-3-32-17-6-9-21-24-14-20(28(21)15-17)19-10-11-23-22(27-19)26-16-4-7-18(8-5-16)33(29,30)25-12-13-31-2/h4-11,14-15,25H,3,12-13H2,1-2H3,(H,23,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
AstraZeneca
| Assay Description
The kinase activity was assayed using an in vitro scintillation proximity assay (SPA) for measuring incorporation of [gamma-33P] ATP into GST-Rb. |
Bioorg Med Chem Lett 14: 2249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.008
BindingDB Entry DOI: 10.7270/Q2XG9PCF |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM7679
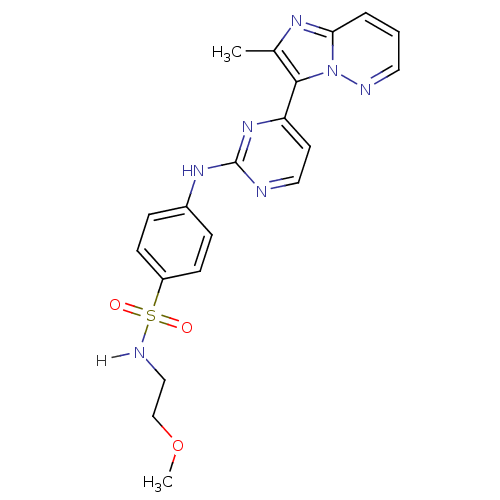
(Imidazo[1,2-b]pyridazine deriv. 4b | N-(2-methoxye...)Show SMILES COCCNS(=O)(=O)c1ccc(Nc2nccc(n2)-c2c(C)nc3cccnn23)cc1 Show InChI InChI=1S/C20H21N7O3S/c1-14-19(27-18(24-14)4-3-10-22-27)17-9-11-21-20(26-17)25-15-5-7-16(8-6-15)31(28,29)23-12-13-30-2/h3-11,23H,12-13H2,1-2H3,(H,21,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
AstraZeneca
| Assay Description
The kinase activity was assayed using an in vitro scintillation proximity assay (SPA) for measuring incorporation of [gamma-33P] ATP into GST-Rb. |
Bioorg Med Chem Lett 14: 2249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.008
BindingDB Entry DOI: 10.7270/Q2XG9PCF |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM7672
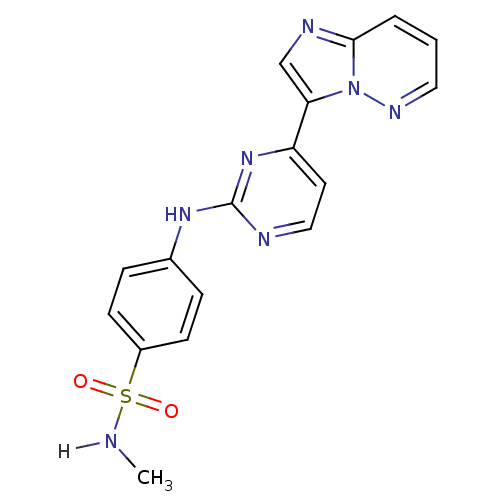
(4-[(4-{imidazo[1,2-a]pyridazin-3-yl}pyrimidin-2-yl...)Show SMILES CNS(=O)(=O)c1ccc(Nc2nccc(n2)-c2cnc3cccnn23)cc1 Show InChI InChI=1S/C17H15N7O2S/c1-18-27(25,26)13-6-4-12(5-7-13)22-17-19-10-8-14(23-17)15-11-20-16-3-2-9-21-24(15)16/h2-11,18H,1H3,(H,19,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
AstraZeneca
| Assay Description
The kinase activity was assayed using an in vitro scintillation proximity assay (SPA) for measuring incorporation of [gamma-33P] ATP into GST-Rb. |
Bioorg Med Chem Lett 14: 2249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.008
BindingDB Entry DOI: 10.7270/Q2XG9PCF |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM7673
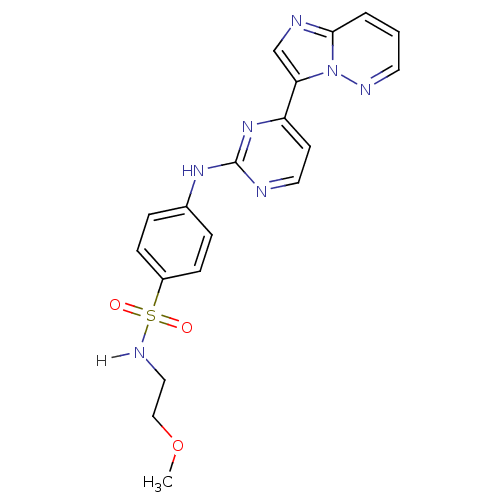
(4-[(4-{imidazo[1,2-a]pyridazin-3-yl}pyrimidin-2-yl...)Show SMILES COCCNS(=O)(=O)c1ccc(Nc2nccc(n2)-c2cnc3cccnn23)cc1 Show InChI InChI=1S/C19H19N7O3S/c1-29-12-11-23-30(27,28)15-6-4-14(5-7-15)24-19-20-10-8-16(25-19)17-13-21-18-3-2-9-22-26(17)18/h2-10,13,23H,11-12H2,1H3,(H,20,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
AstraZeneca
| Assay Description
The kinase activity was assayed using an in vitro scintillation proximity assay (SPA) for measuring incorporation of [gamma-33P] ATP into GST-Rb. |
Bioorg Med Chem Lett 14: 2249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.008
BindingDB Entry DOI: 10.7270/Q2XG9PCF |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325145
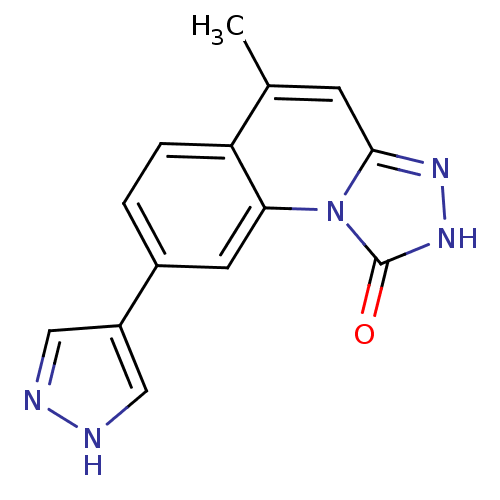
(5-methyl-8-(1H-pyrazol-4-yl)-[1,2,4]triazolo[4,3-a...)Show InChI InChI=1S/C14H11N5O/c1-8-4-13-17-18-14(20)19(13)12-5-9(2-3-11(8)12)10-6-15-16-7-10/h2-7H,1H3,(H,15,16)(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of His6-CHK1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 20: 5133-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.015
BindingDB Entry DOI: 10.7270/Q2FF3SJZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325161
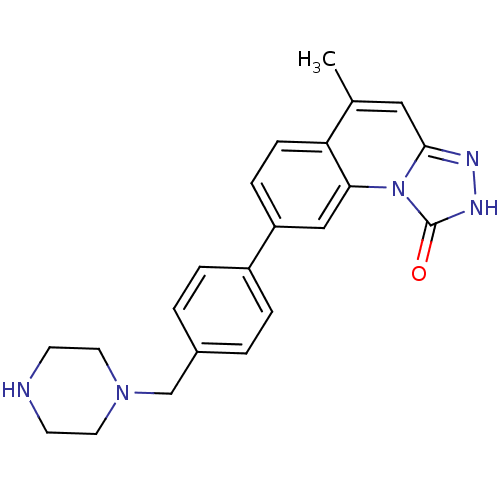
(5-methyl-8-(4-(piperazin-1-ylmethyl)phenyl)-[1,2,4...)Show SMILES Cc1cc2n[nH]c(=O)n2c2cc(ccc12)-c1ccc(CN2CCNCC2)cc1 Show InChI InChI=1S/C22H23N5O/c1-15-12-21-24-25-22(28)27(21)20-13-18(6-7-19(15)20)17-4-2-16(3-5-17)14-26-10-8-23-9-11-26/h2-7,12-13,23H,8-11,14H2,1H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of His6-CHK1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 20: 5133-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.015
BindingDB Entry DOI: 10.7270/Q2FF3SJZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325162
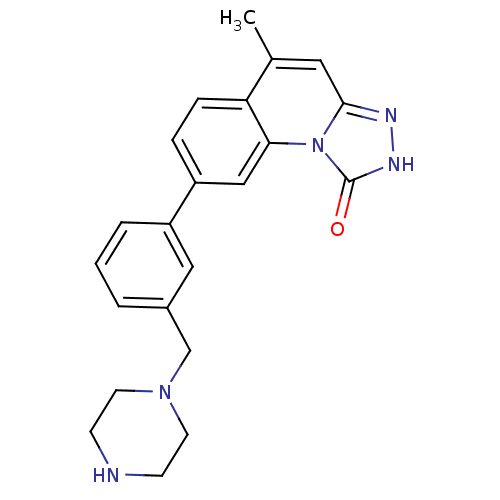
(5-methyl-8-(3-(piperazin-1-ylmethyl)phenyl)-[1,2,4...)Show SMILES Cc1cc2n[nH]c(=O)n2c2cc(ccc12)-c1cccc(CN2CCNCC2)c1 Show InChI InChI=1S/C22H23N5O/c1-15-11-21-24-25-22(28)27(21)20-13-18(5-6-19(15)20)17-4-2-3-16(12-17)14-26-9-7-23-8-10-26/h2-6,11-13,23H,7-10,14H2,1H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of His6-CHK1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 20: 5133-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.015
BindingDB Entry DOI: 10.7270/Q2FF3SJZ |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50605601
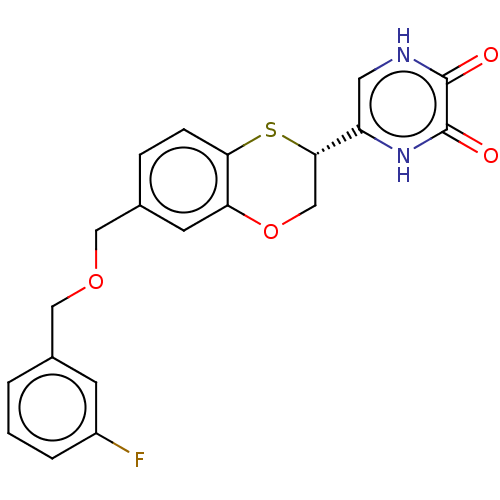
(CHEMBL5184138)Show SMILES Fc1cccc(COCc2ccc3S[C@@H](COc3c2)c2c[nH]c(=O)c(=O)[nH]2)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00118
BindingDB Entry DOI: 10.7270/Q2MG7TK7 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM7674
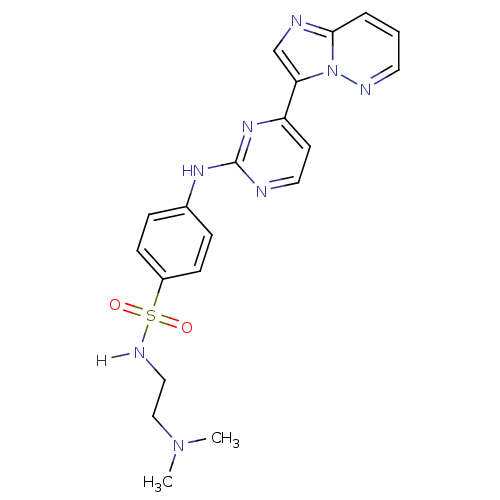
(CHEMBL484571 | Imidazo[1,2-b]pyridazine deriv. 2d ...)Show SMILES CN(C)CCNS(=O)(=O)c1ccc(Nc2nccc(n2)-c2cnc3cccnn23)cc1 Show InChI InChI=1S/C20H22N8O2S/c1-27(2)13-12-24-31(29,30)16-7-5-15(6-8-16)25-20-21-11-9-17(26-20)18-14-22-19-4-3-10-23-28(18)19/h3-11,14,24H,12-13H2,1-2H3,(H,21,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.0 | 22 |
AstraZeneca
| Assay Description
The kinase activity was assayed using an in vitro scintillation proximity assay (SPA) for measuring incorporation of [gamma-33P] ATP into GST-Rb. |
Bioorg Med Chem Lett 14: 2249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.008
BindingDB Entry DOI: 10.7270/Q2XG9PCF |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50605597
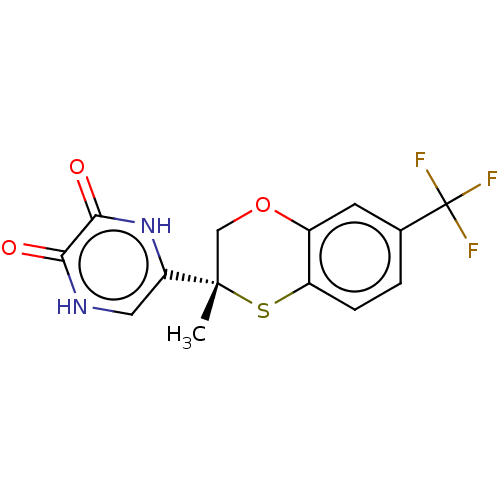
(CHEMBL5204161)Show SMILES C[C@]1(COc2cc(ccc2S1)C(F)(F)F)c1c[nH]c(=O)c(=O)[nH]1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00118
BindingDB Entry DOI: 10.7270/Q2MG7TK7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325163
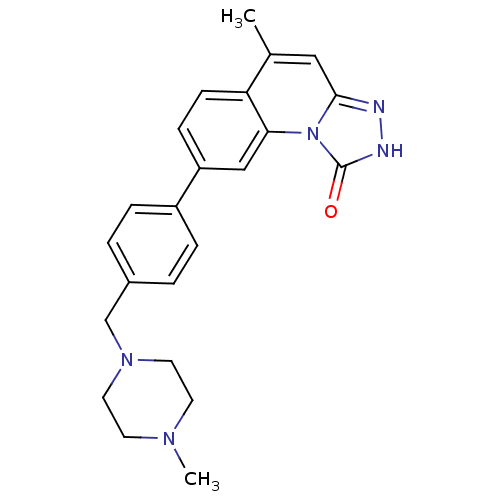
(5-methyl-8-(4-((4-methylpiperazin-1-yl)methyl)phen...)Show SMILES CN1CCN(Cc2ccc(cc2)-c2ccc3c(C)cc4n[nH]c(=O)n4c3c2)CC1 Show InChI InChI=1S/C23H25N5O/c1-16-13-22-24-25-23(29)28(22)21-14-19(7-8-20(16)21)18-5-3-17(4-6-18)15-27-11-9-26(2)10-12-27/h3-8,13-14H,9-12,15H2,1-2H3,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of His6-CHK1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 20: 5133-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.015
BindingDB Entry DOI: 10.7270/Q2FF3SJZ |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50605598

(CHEMBL5188193)Show SMILES C[C@]1(COc2cc(Cl)ccc2S1)c1c[nH]c(=O)c(=O)[nH]1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00118
BindingDB Entry DOI: 10.7270/Q2MG7TK7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325164
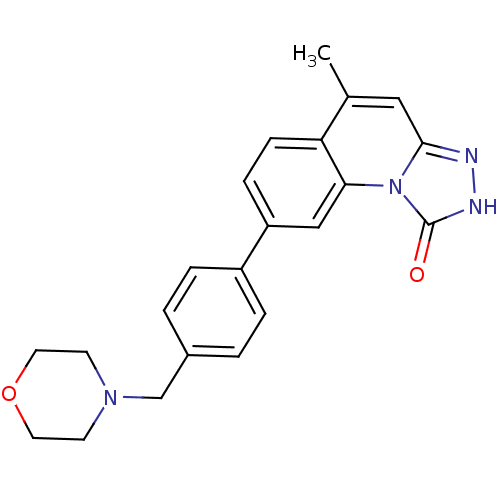
(5-methyl-8-(4-(morpholinomethyl)phenyl)-[1,2,4]tri...)Show SMILES Cc1cc2n[nH]c(=O)n2c2cc(ccc12)-c1ccc(CN2CCOCC2)cc1 Show InChI InChI=1S/C22H22N4O2/c1-15-12-21-23-24-22(27)26(21)20-13-18(6-7-19(15)20)17-4-2-16(3-5-17)14-25-8-10-28-11-9-25/h2-7,12-13H,8-11,14H2,1H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of His6-CHK1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 20: 5133-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.015
BindingDB Entry DOI: 10.7270/Q2FF3SJZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50280450
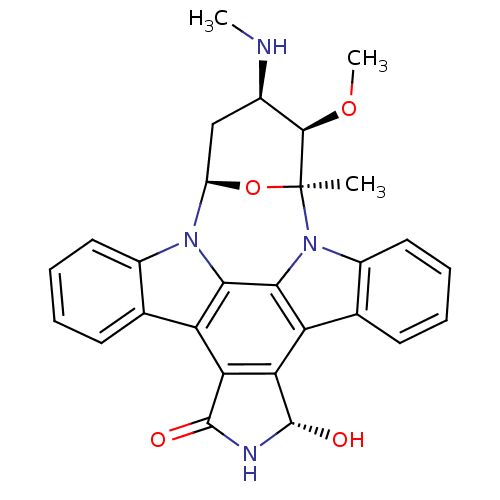
(18-hydroxy-3-methoxy-2-methyl-4-methylamino-(2R,3S...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4[C@H](O)NC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O4/c1-28-25(35-3)15(29-2)12-18(36-28)31-16-10-6-4-8-13(16)19-21-22(27(34)30-26(21)33)20-14-9-5-7-11-17(14)32(28)24(20)23(19)31/h4-11,15,18,25,27,29,34H,12H2,1-3H3,(H,30,33)/t15-,18-,25-,27+,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of CHK2 |
Bioorg Med Chem Lett 20: 5133-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.015
BindingDB Entry DOI: 10.7270/Q2FF3SJZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50280450

(18-hydroxy-3-methoxy-2-methyl-4-methylamino-(2R,3S...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4[C@H](O)NC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O4/c1-28-25(35-3)15(29-2)12-18(36-28)31-16-10-6-4-8-13(16)19-21-22(27(34)30-26(21)33)20-14-9-5-7-11-17(14)32(28)24(20)23(19)31/h4-11,15,18,25,27,29,34H,12H2,1-3H3,(H,30,33)/t15-,18-,25-,27+,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 |
Bioorg Med Chem Lett 20: 5133-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.015
BindingDB Entry DOI: 10.7270/Q2FF3SJZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325169
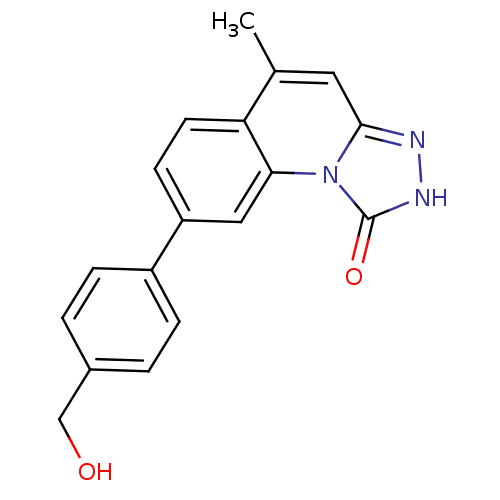
(8-(4-(hydroxymethyl)phenyl)-5-methyl-[1,2,4]triazo...)Show InChI InChI=1S/C18H15N3O2/c1-11-8-17-19-20-18(23)21(17)16-9-14(6-7-15(11)16)13-4-2-12(10-22)3-5-13/h2-9,22H,10H2,1H3,(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of His6-CHK1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 20: 5133-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.015
BindingDB Entry DOI: 10.7270/Q2FF3SJZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325150

(8-(4-(2-(diethylamino)ethoxy)phenyl)-5-methyl-[1,2...)Show SMILES CCN(CC)CCOc1ccc(cc1)-c1ccc2c(C)cc3n[nH]c(=O)n3c2c1 Show InChI InChI=1S/C23H26N4O2/c1-4-26(5-2)12-13-29-19-9-6-17(7-10-19)18-8-11-20-16(3)14-22-24-25-23(28)27(22)21(20)15-18/h6-11,14-15H,4-5,12-13H2,1-3H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of His6-CHK1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 20: 5133-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.015
BindingDB Entry DOI: 10.7270/Q2FF3SJZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325147
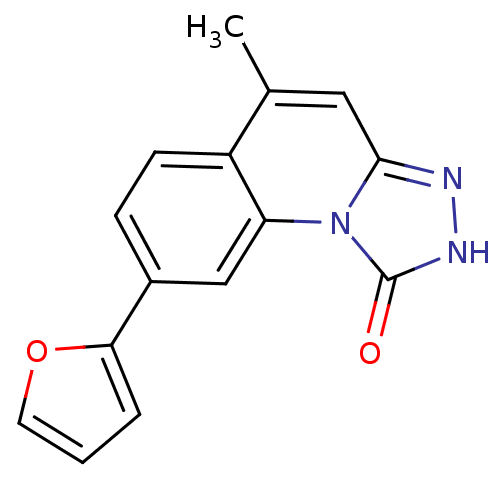
(8-(furan-2-yl)-5-methyl-[1,2,4]triazolo[4,3-a]quin...)Show InChI InChI=1S/C15H11N3O2/c1-9-7-14-16-17-15(19)18(14)12-8-10(4-5-11(9)12)13-3-2-6-20-13/h2-8H,1H3,(H,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of His6-CHK1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 20: 5133-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.015
BindingDB Entry DOI: 10.7270/Q2FF3SJZ |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50605555
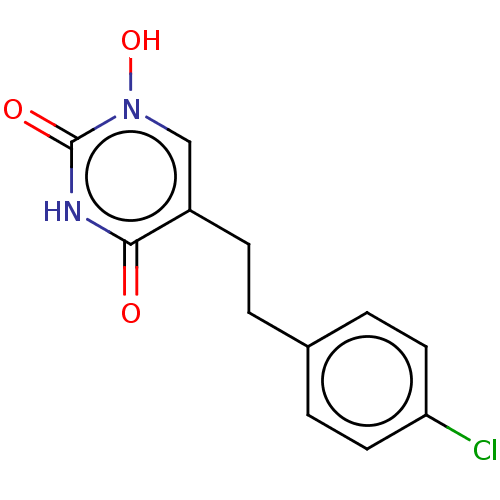
(CHEMBL5173250) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00118
BindingDB Entry DOI: 10.7270/Q2MG7TK7 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50605593
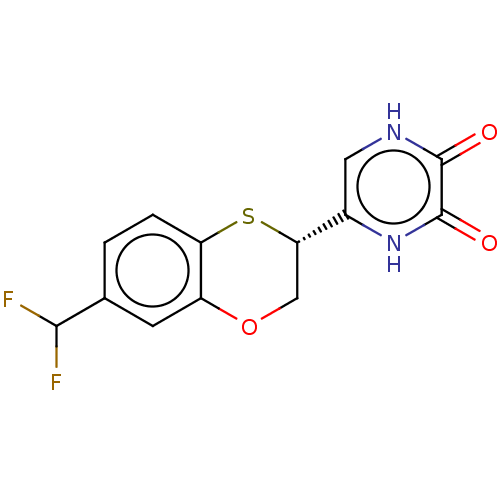
(CHEMBL5179642)Show SMILES FC(F)c1ccc2S[C@@H](COc2c1)c1c[nH]c(=O)c(=O)[nH]1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00118
BindingDB Entry DOI: 10.7270/Q2MG7TK7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325171
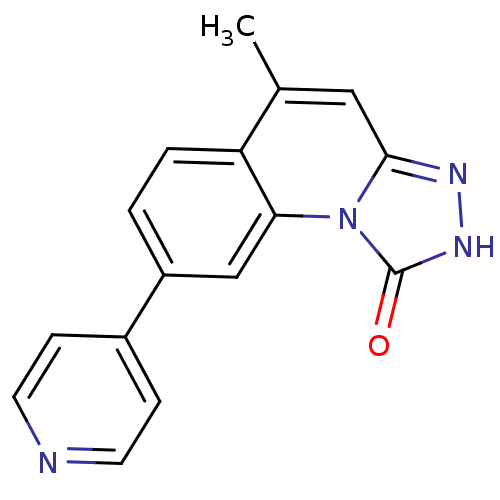
(5-METHYL-8-PYRIDIN-4-YL[1,2,4]TRIAZOLO[4,3-A]QUINO...)Show InChI InChI=1S/C16H12N4O/c1-10-8-15-18-19-16(21)20(15)14-9-12(2-3-13(10)14)11-4-6-17-7-5-11/h2-9H,1H3,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of His6-CHK1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 20: 5133-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.015
BindingDB Entry DOI: 10.7270/Q2FF3SJZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM7670
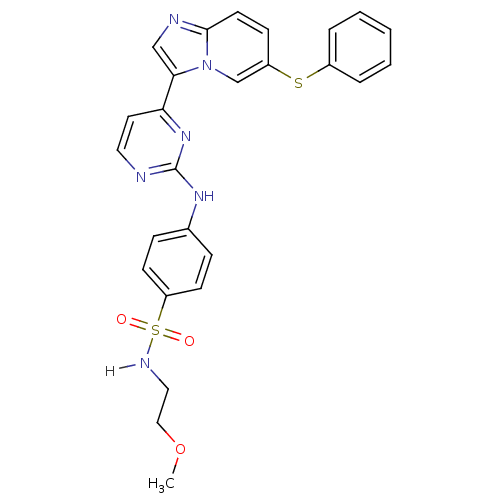
(Imidazo[1,2-a]pyridine deriv. 4g | N-(2-methoxyeth...)Show SMILES COCCNS(=O)(=O)c1ccc(Nc2nccc(n2)-c2cnc3ccc(Sc4ccccc4)cn23)cc1 Show InChI InChI=1S/C26H24N6O3S2/c1-35-16-15-29-37(33,34)22-10-7-19(8-11-22)30-26-27-14-13-23(31-26)24-17-28-25-12-9-21(18-32(24)25)36-20-5-3-2-4-6-20/h2-14,17-18,29H,15-16H2,1H3,(H,27,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.0 | 22 |
AstraZeneca
| Assay Description
The kinase activity was assayed using an in vitro scintillation proximity assay (SPA) for measuring incorporation of [gamma-33P] ATP into GST-Rb. |
Bioorg Med Chem Lett 14: 2249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.008
BindingDB Entry DOI: 10.7270/Q2XG9PCF |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM210802
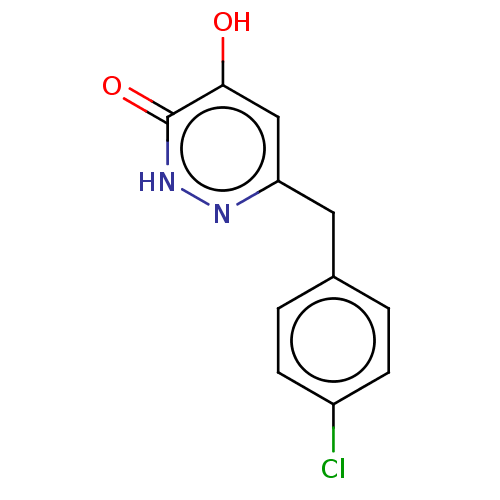
(US10463663, Example 40 | US11129828, Example 40 | ...)Show InChI InChI=1S/C11H9ClN2O2/c12-8-3-1-7(2-4-8)5-9-6-10(15)11(16)14-13-9/h1-4,6H,5H2,(H,13,15)(H,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00118
BindingDB Entry DOI: 10.7270/Q2MG7TK7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325175
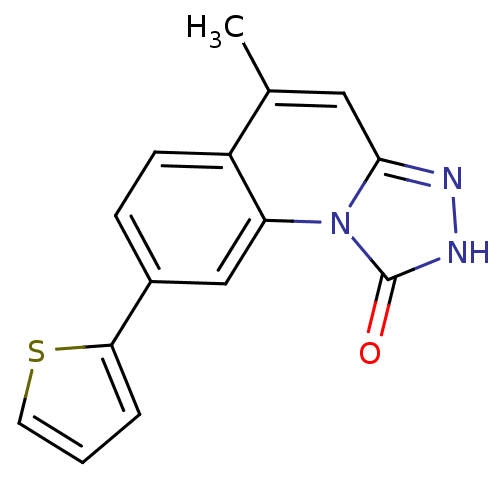
(5-methyl-8-(thiophen-2-yl)-[1,2,4]triazolo[4,3-a]q...)Show InChI InChI=1S/C15H11N3OS/c1-9-7-14-16-17-15(19)18(14)12-8-10(4-5-11(9)12)13-3-2-6-20-13/h2-8H,1H3,(H,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of His6-CHK1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 20: 5133-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.015
BindingDB Entry DOI: 10.7270/Q2FF3SJZ |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50605594

(CHEMBL5186374)Show SMILES O=c1[nH]cc([nH]c1=O)[C@@H]1COc2cc(ccc2S1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00118
BindingDB Entry DOI: 10.7270/Q2MG7TK7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50605599

(CHEMBL5178413)Show SMILES C[C@]1(COc2cc(ccc2S1)C#N)c1c[nH]c(=O)c(=O)[nH]1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00118
BindingDB Entry DOI: 10.7270/Q2MG7TK7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325158
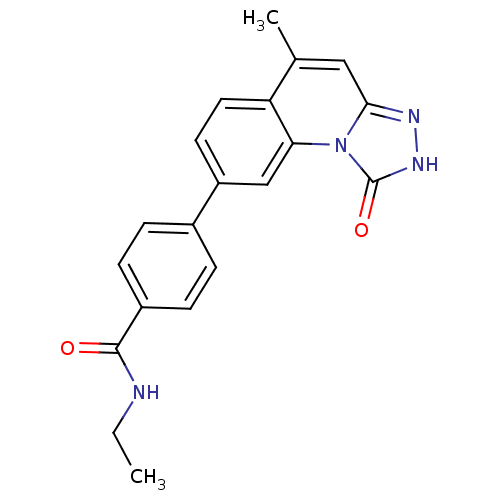
(CHEMBL1223317 | N-ethyl-4-(5-methyl-1-oxo-1,2-dihy...)Show SMILES CCNC(=O)c1ccc(cc1)-c1ccc2c(C)cc3n[nH]c(=O)n3c2c1 Show InChI InChI=1S/C20H18N4O2/c1-3-21-19(25)14-6-4-13(5-7-14)15-8-9-16-12(2)10-18-22-23-20(26)24(18)17(16)11-15/h4-11H,3H2,1-2H3,(H,21,25)(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of His6-CHK1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 20: 5133-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.015
BindingDB Entry DOI: 10.7270/Q2FF3SJZ |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50605592

(CHEMBL5179643)Show SMILES Clc1ccc2S[C@@H](COc2c1)c1c[nH]c(=O)c(=O)[nH]1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00118
BindingDB Entry DOI: 10.7270/Q2MG7TK7 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50605595

(CHEMBL5174518)Show SMILES CS(=O)(=O)c1ccc2S[C@@H](COc2c1)c1c[nH]c(=O)c(=O)[nH]1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00118
BindingDB Entry DOI: 10.7270/Q2MG7TK7 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50605589
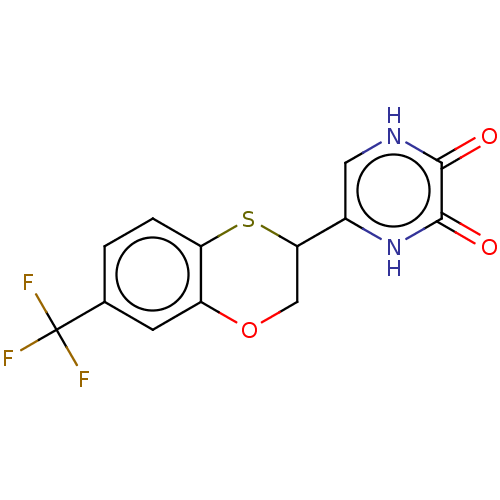
(CHEMBL5196542)Show SMILES FC(F)(F)c1ccc2SC(COc2c1)c1c[nH]c(=O)c(=O)[nH]1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00118
BindingDB Entry DOI: 10.7270/Q2MG7TK7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325144
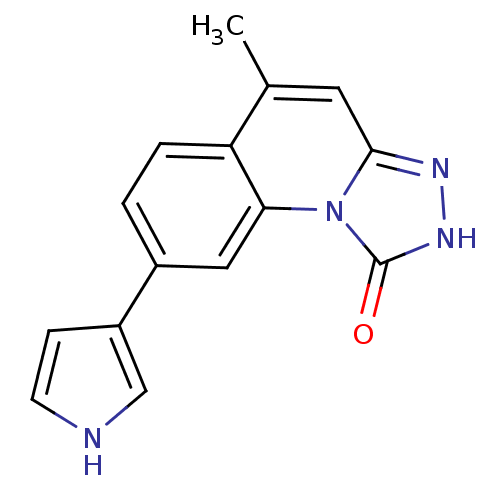
(5-methyl-8-(1H-pyrrol-3-yl)-[1,2,4]triazolo[4,3-a]...)Show InChI InChI=1S/C15H12N4O/c1-9-6-14-17-18-15(20)19(14)13-7-10(2-3-12(9)13)11-4-5-16-8-11/h2-8,16H,1H3,(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of His6-CHK1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 20: 5133-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.015
BindingDB Entry DOI: 10.7270/Q2FF3SJZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50605552
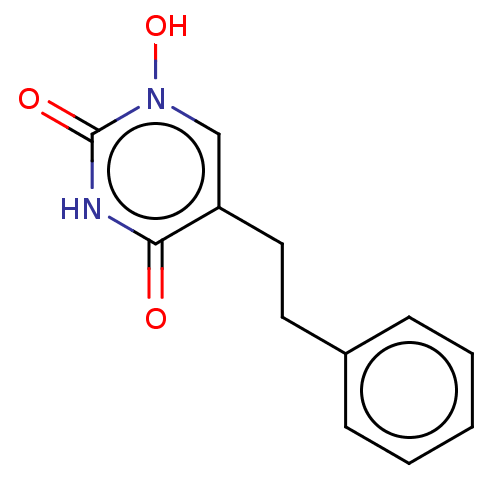
(CHEMBL5204839) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00118
BindingDB Entry DOI: 10.7270/Q2MG7TK7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325151
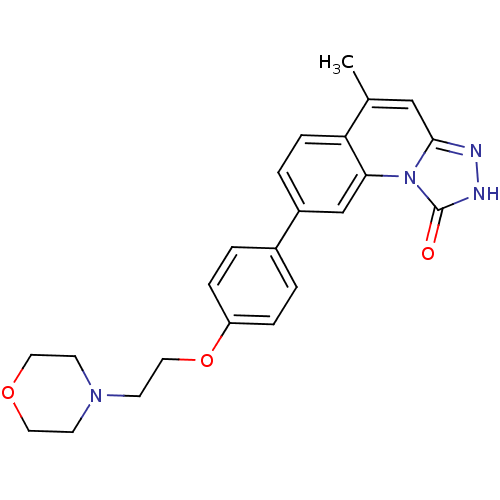
(5-methyl-8-(4-(2-morpholinoethoxy)phenyl)-[1,2,4]t...)Show SMILES Cc1cc2n[nH]c(=O)n2c2cc(ccc12)-c1ccc(OCCN2CCOCC2)cc1 Show InChI InChI=1S/C23H24N4O3/c1-16-14-22-24-25-23(28)27(22)21-15-18(4-7-20(16)21)17-2-5-19(6-3-17)30-13-10-26-8-11-29-12-9-26/h2-7,14-15H,8-13H2,1H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of His6-CHK1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 20: 5133-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.015
BindingDB Entry DOI: 10.7270/Q2FF3SJZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325155
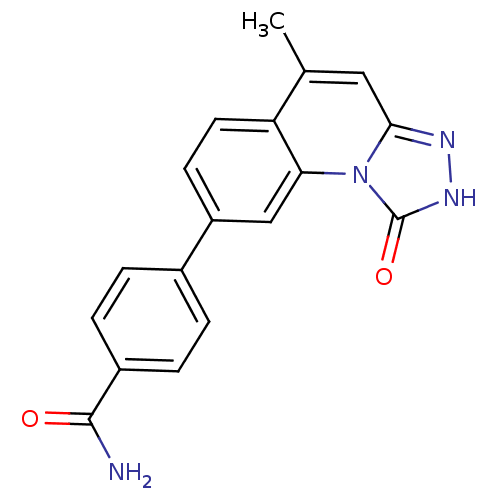
(4-(5-methyl-1-oxo-1,2-dihydro-[1,2,4]triazolo[4,3-...)Show SMILES Cc1cc2n[nH]c(=O)n2c2cc(ccc12)-c1ccc(cc1)C(N)=O Show InChI InChI=1S/C18H14N4O2/c1-10-8-16-20-21-18(24)22(16)15-9-13(6-7-14(10)15)11-2-4-12(5-3-11)17(19)23/h2-9H,1H3,(H2,19,23)(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of His6-CHK1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 20: 5133-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.015
BindingDB Entry DOI: 10.7270/Q2FF3SJZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data