

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001888 ((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against the binding of [3H]-spiperone to Dopamine receptor D2 in rat striatal membranes | J Med Chem 30: 1631-5 (1987) BindingDB Entry DOI: 10.7270/Q2FB51ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50016778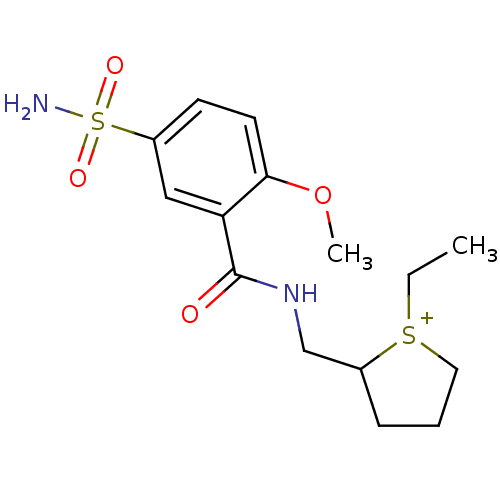 (1-Ethyl-2-[(2-methoxy-5-sulfamoyl-benzoylamino)-me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [3H]- spiperone from D2 binding site in rat striatal membranes | J Med Chem 32: 874-80 (1989) BindingDB Entry DOI: 10.7270/Q23B5Z48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM11638 (CHEMBL26 | Compound 7 | N-[(1-ethylpyrrolidin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [3H]- spiperone from Dopamine receptor D2 in rat striatal membranes | J Med Chem 32: 874-80 (1989) BindingDB Entry DOI: 10.7270/Q23B5Z48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Staphylococcus aureus) | BDBM161472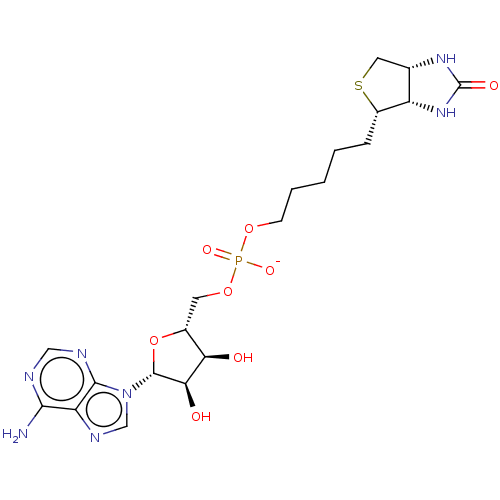 (US9108978, 2.02) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 30 | -44.7 | 203 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001888 ((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for Competitive binding inhibition of [3H]-Spiperone to Dopamine receptor D2 in rat striatal membrane. | Bioorg Med Chem Lett 3: 1241-1244 (1993) Article DOI: 10.1016/S0960-894X(00)80323-6 BindingDB Entry DOI: 10.7270/Q26W9B00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Staphylococcus aureus) | BDBM161468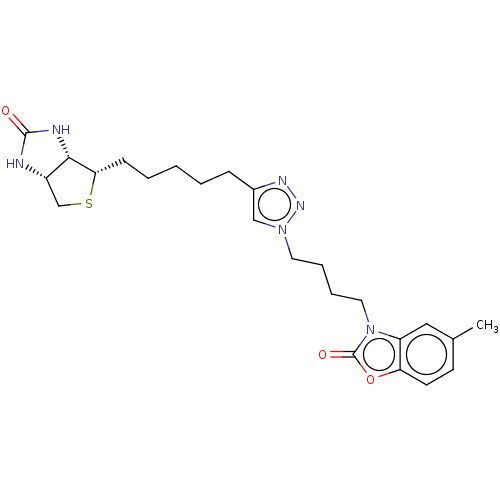 (US9108978, 6.09) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | 90 | -41.8 | 530 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional ligase/repressor BirA (Staphylococcus aureus) | BDBM92383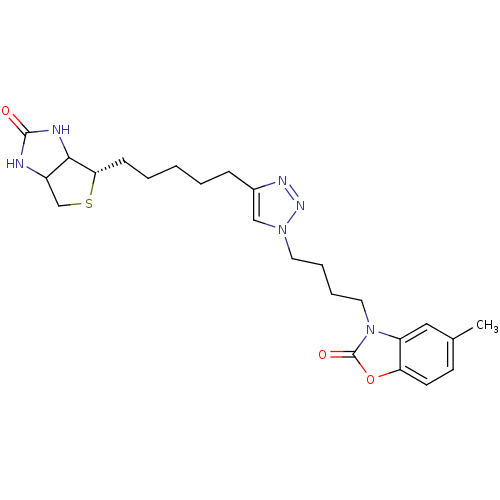 (Biotin triazole, 14) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide | Assay Description In vitro enzyme inhibition using biotin protein ligase. | J Biol Chem 287: 17823-32 (2012) Article DOI: 10.1074/jbc.M112.356576 BindingDB Entry DOI: 10.7270/Q2DJ5D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Biotin--protein ligase (Homo sapiens (Human)) | BDBM50388931 (CHEMBL2063402) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-biotin from human BPL after 20 mins by scintillation counting | ACS Med Chem Lett 3: 509-514 (2012) Article DOI: 10.1021/ml300106p BindingDB Entry DOI: 10.7270/Q2DZ09CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Biotin--protein ligase (Homo sapiens (Human)) | BDBM50388933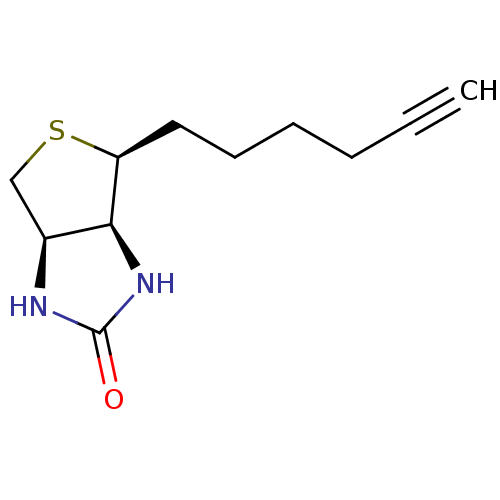 (CHEMBL2063403) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-biotin from human BPL after 20 mins by scintillation counting | ACS Med Chem Lett 3: 509-514 (2012) Article DOI: 10.1021/ml300106p BindingDB Entry DOI: 10.7270/Q2DZ09CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Escherichia coli) | BDBM161472 (US9108978, 2.02) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 225 | -39.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50019878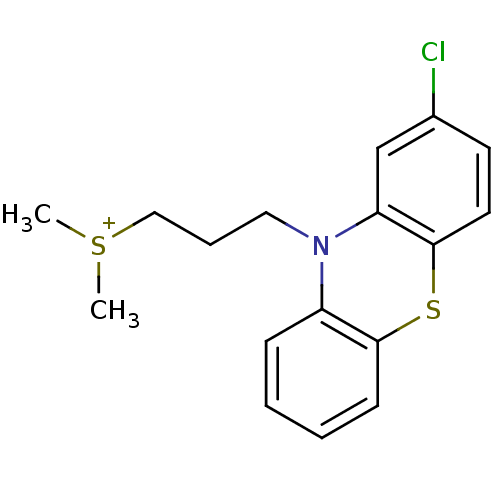 (CHEMBL44394 | [3-(2-Chloro-phenothiazin-10-yl)-pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against the binding of [3H]-spiperone toDopamine receptor D2 in rat striatal membranes | J Med Chem 30: 1631-5 (1987) BindingDB Entry DOI: 10.7270/Q2FB51ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Escherichia coli) | BDBM161472 (US9108978, 2.02) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 420 | -37.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Staphylococcus aureus) | BDBM161470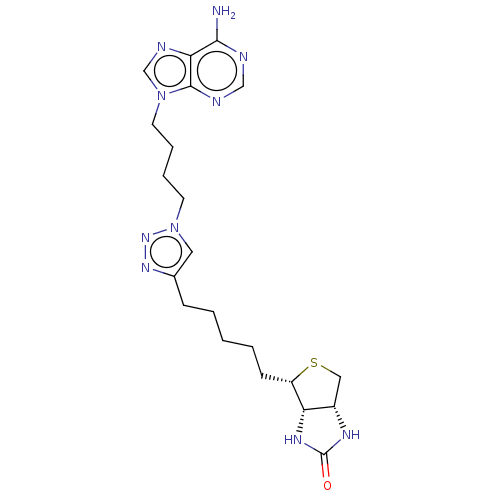 (US9108978, 4.01) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 660 | -36.7 | 4.00E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50281901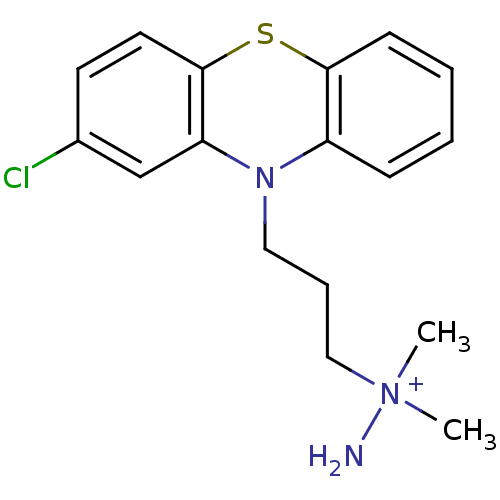 (CHEMBL21564 | MethanesulfonateN-[3-(2-chloro-pheno...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for Competitive binding inhibition of [3H]-Spiperone to Dopamine receptor D2 in rat striatal membrane. | Bioorg Med Chem Lett 3: 1241-1244 (1993) Article DOI: 10.1016/S0960-894X(00)80323-6 BindingDB Entry DOI: 10.7270/Q26W9B00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Staphylococcus aureus) | BDBM161469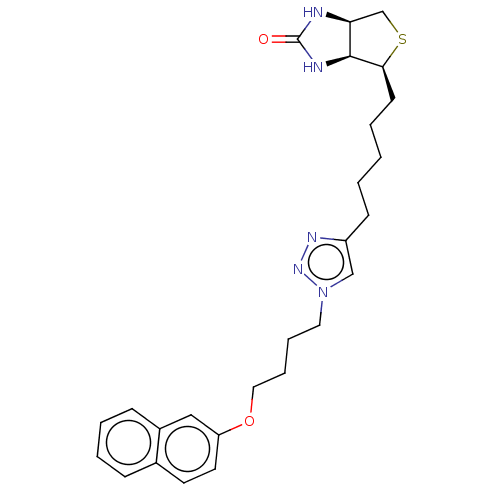 (US9108978, 6.07) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.17E+3 | -35.2 | 7.00E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Staphylococcus aureus) | BDBM92382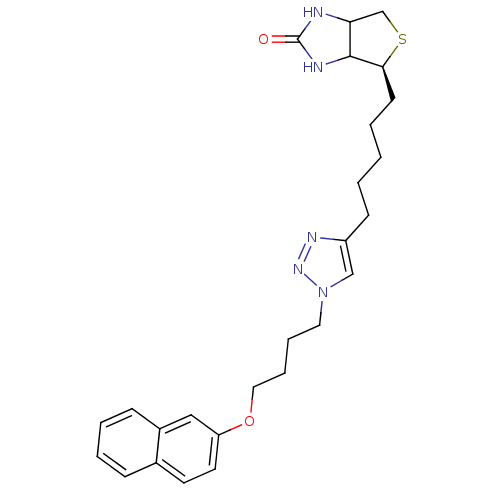 (Biotin triazole, 13) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide | Assay Description In vitro enzyme inhibition using biotin protein ligase. | J Biol Chem 287: 17823-32 (2012) Article DOI: 10.1074/jbc.M112.356576 BindingDB Entry DOI: 10.7270/Q2DJ5D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Staphylococcus aureus) | BDBM92378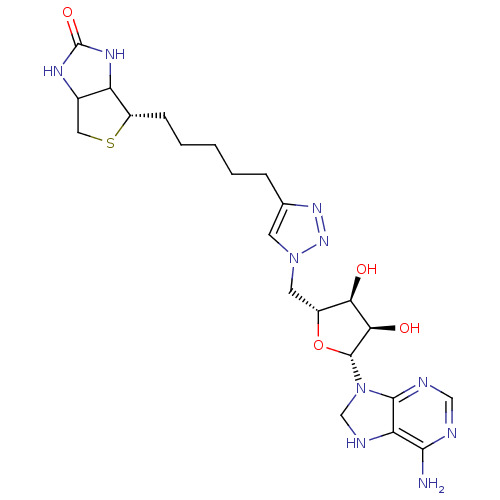 (Biotin triazole, 5) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide | Assay Description In vitro enzyme inhibition using biotin protein ligase. | J Biol Chem 287: 17823-32 (2012) Article DOI: 10.1074/jbc.M112.356576 BindingDB Entry DOI: 10.7270/Q2DJ5D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Staphylococcus aureus) | BDBM161467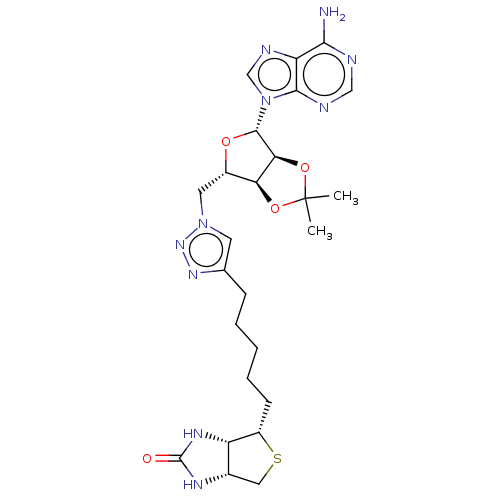 (US9108978, 3.25) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.83E+3 | -34.1 | 1.16E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50019879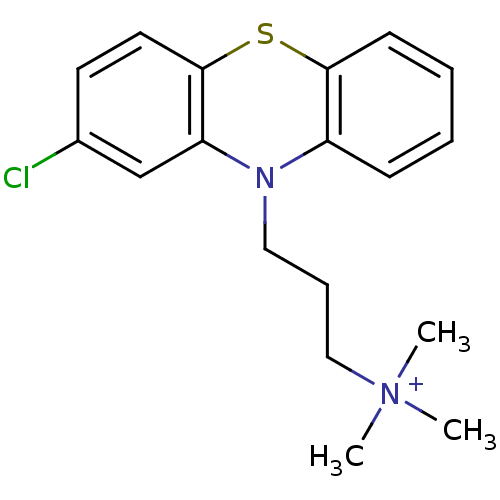 (CHEMBL279905 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against the binding of [3H]-spiperone to Dopamine receptor D2 in rat striatal membranes | J Med Chem 30: 1631-5 (1987) BindingDB Entry DOI: 10.7270/Q2FB51ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Biotin--protein ligase (Homo sapiens (Human)) | BDBM50388934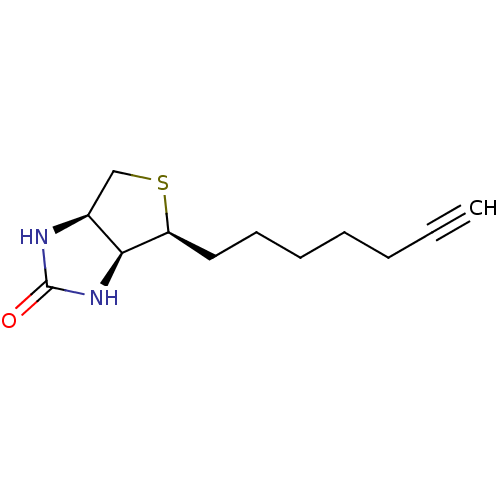 (CHEMBL2063404) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-biotin from human BPL after 20 mins by scintillation counting | ACS Med Chem Lett 3: 509-514 (2012) Article DOI: 10.1021/ml300106p BindingDB Entry DOI: 10.7270/Q2DZ09CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase VIM-2 (Pseudomonas aeruginosa (g-Proteobacteria)) | BDBM153698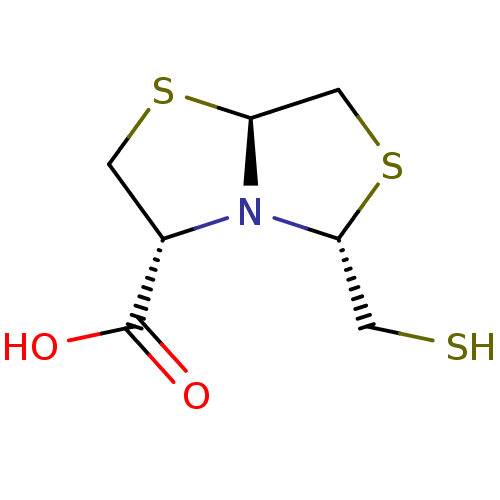 (L-CS319) | PDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metallo-beta-lactamase VIM-2 (Pseudomonas aeruginosa (g-Proteobacteria)) | BDBM153700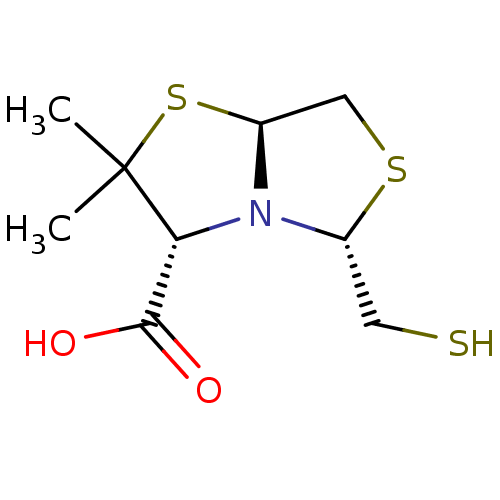 (L-VC26) | PDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Biotin--protein ligase (Homo sapiens (Human)) | BDBM50388927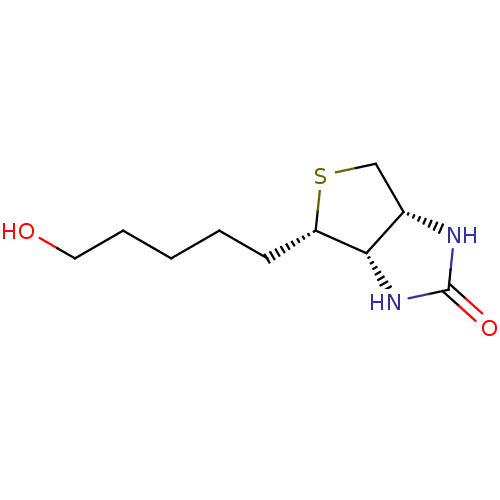 (CHEMBL2063398) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-biotin from human BPL after 20 mins by scintillation counting | ACS Med Chem Lett 3: 509-514 (2012) Article DOI: 10.1021/ml300106p BindingDB Entry DOI: 10.7270/Q2DZ09CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50281900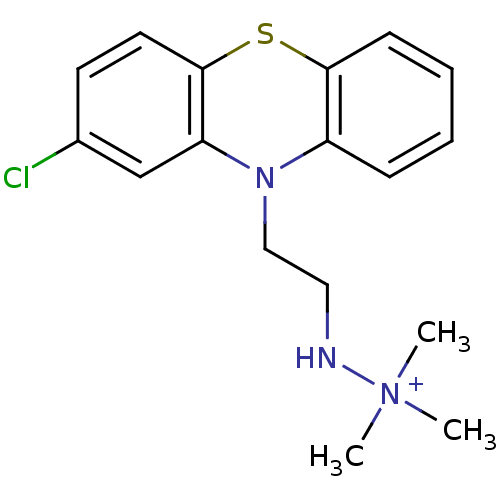 (CHEMBL21398 | N'-[2-(2-Chloro-phenothiazin-10-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for Competitive binding inhibition of [3H]-Spiperone to Dopamine receptor D2 in rat striatal membrane. | Bioorg Med Chem Lett 3: 1241-1244 (1993) Article DOI: 10.1016/S0960-894X(00)80323-6 BindingDB Entry DOI: 10.7270/Q26W9B00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-24 (Klebsiella pneumoniae (Enterobacteria)) | BDBM153700 (L-VC26) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase VIM-2 (Pseudomonas aeruginosa (g-Proteobacteria)) | BDBM153699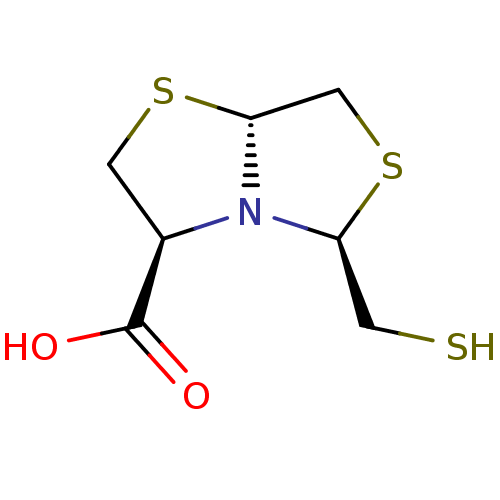 (D-CS319) | PDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-24 (Klebsiella pneumoniae (Enterobacteria)) | BDBM153698 (L-CS319) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Biotin--protein ligase (Homo sapiens (Human)) | BDBM50388932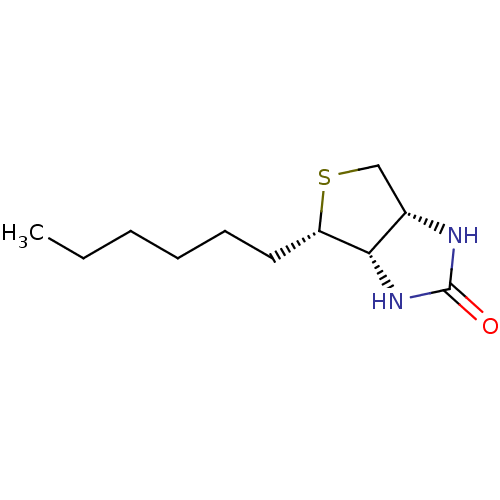 (CHEMBL2062535) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-biotin from human BPL after 20 mins by scintillation counting | ACS Med Chem Lett 3: 509-514 (2012) Article DOI: 10.1021/ml300106p BindingDB Entry DOI: 10.7270/Q2DZ09CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50019879 (CHEMBL279905 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | 8.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for Competitive binding inhibition of [3H]-Spiperone to Dopamine receptor D2 in rat striatal membrane. | Bioorg Med Chem Lett 3: 1241-1244 (1993) Article DOI: 10.1016/S0960-894X(00)80323-6 BindingDB Entry DOI: 10.7270/Q26W9B00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Staphylococcus aureus) | BDBM161471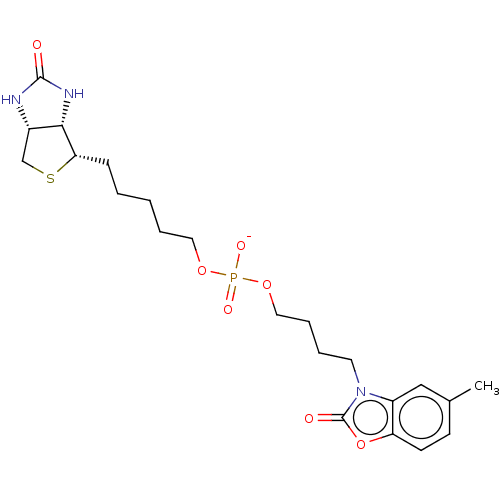 (US9108978, 6.34) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+4 | >-29.7 | >5.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Staphylococcus aureus) | BDBM92384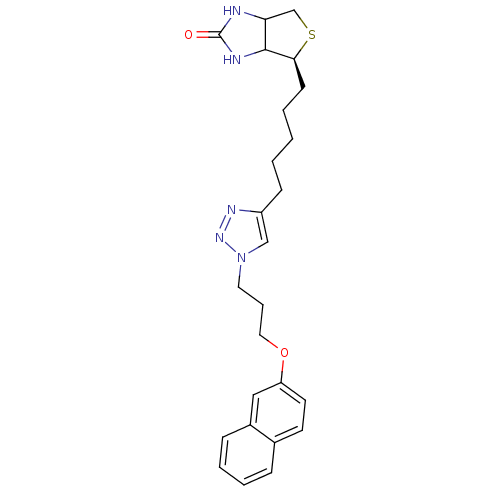 (Biotin triazole, 15) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide | Assay Description In vitro enzyme inhibition using biotin protein ligase. | J Biol Chem 287: 17823-32 (2012) Article DOI: 10.1074/jbc.M112.356576 BindingDB Entry DOI: 10.7270/Q2DJ5D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Staphylococcus aureus) | BDBM92385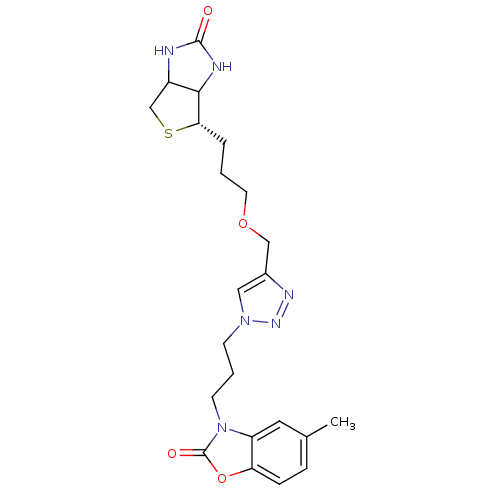 (Biotin triazole, 16) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide | Assay Description In vitro enzyme inhibition using biotin protein ligase. | J Biol Chem 287: 17823-32 (2012) Article DOI: 10.1074/jbc.M112.356576 BindingDB Entry DOI: 10.7270/Q2DJ5D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Staphylococcus aureus) | BDBM92379 (Biotin triazole, 10) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide | Assay Description In vitro enzyme inhibition using biotin protein ligase. | J Biol Chem 287: 17823-32 (2012) Article DOI: 10.1074/jbc.M112.356576 BindingDB Entry DOI: 10.7270/Q2DJ5D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Staphylococcus aureus) | BDBM92381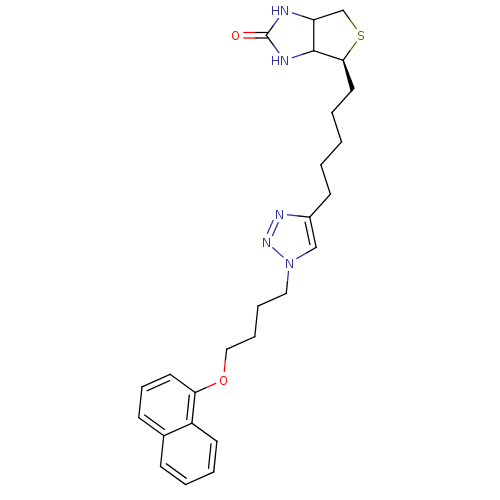 (Biotin triazole, 12) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide | Assay Description In vitro enzyme inhibition using biotin protein ligase. | J Biol Chem 287: 17823-32 (2012) Article DOI: 10.1074/jbc.M112.356576 BindingDB Entry DOI: 10.7270/Q2DJ5D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Staphylococcus aureus) | BDBM92380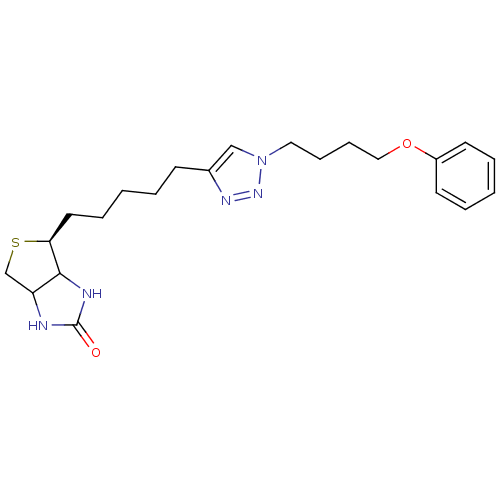 (Biotin triazole, 11) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Adelaide | Assay Description In vitro enzyme inhibition using biotin protein ligase. | J Biol Chem 287: 17823-32 (2012) Article DOI: 10.1074/jbc.M112.356576 BindingDB Entry DOI: 10.7270/Q2DJ5D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-24 (Klebsiella pneumoniae (Enterobacteria)) | BDBM153699 (D-CS319) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-24 (Klebsiella pneumoniae (Enterobacteria)) | BDBM153701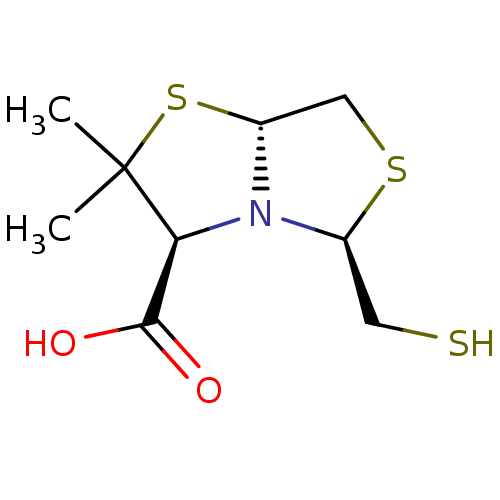 (D-VC26) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Biotin--protein ligase (Homo sapiens (Human)) | BDBM50388935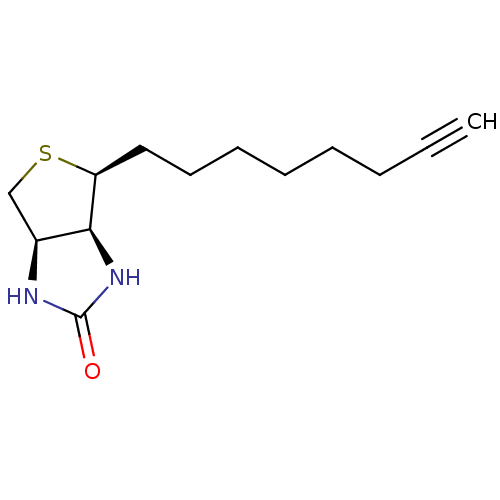 (CHEMBL2063405) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-biotin from human BPL after 20 mins by scintillation counting | ACS Med Chem Lett 3: 509-514 (2012) Article DOI: 10.1021/ml300106p BindingDB Entry DOI: 10.7270/Q2DZ09CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase VIM-2 (Pseudomonas aeruginosa (g-Proteobacteria)) | BDBM153701 (D-VC26) | PDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Biotin--protein ligase (Homo sapiens (Human)) | BDBM50388928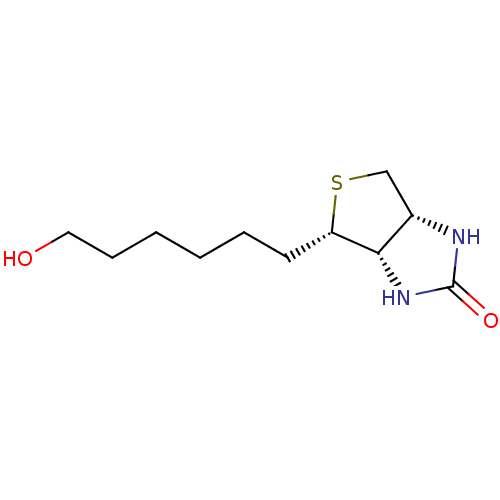 (CHEMBL2063399) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-biotin from human BPL after 20 mins by scintillation counting | ACS Med Chem Lett 3: 509-514 (2012) Article DOI: 10.1021/ml300106p BindingDB Entry DOI: 10.7270/Q2DZ09CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Biotin--protein ligase (Homo sapiens (Human)) | BDBM50388929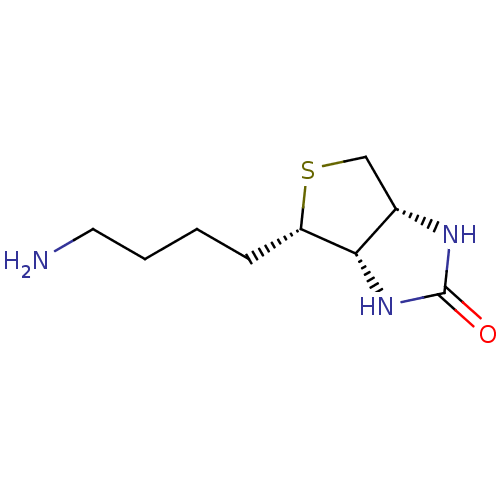 (CHEMBL2063400) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-biotin from human BPL after 20 mins by scintillation counting | ACS Med Chem Lett 3: 509-514 (2012) Article DOI: 10.1021/ml300106p BindingDB Entry DOI: 10.7270/Q2DZ09CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Biotin--protein ligase (Homo sapiens (Human)) | BDBM50388930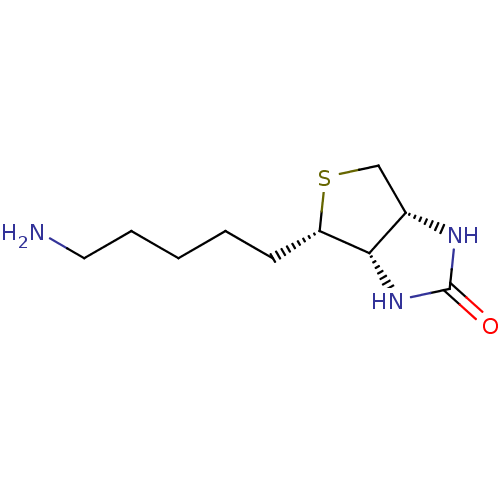 (CHEMBL2063401) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-biotin from human BPL after 20 mins by scintillation counting | ACS Med Chem Lett 3: 509-514 (2012) Article DOI: 10.1021/ml300106p BindingDB Entry DOI: 10.7270/Q2DZ09CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Escherichia coli) | BDBM161468 (US9108978, 6.09) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | >3.00E+4 | >-26.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Escherichia coli) | BDBM161469 (US9108978, 6.07) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >3.00E+4 | >-26.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Escherichia coli) | BDBM161470 (US9108978, 4.01) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | >3.00E+4 | >-26.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Escherichia coli) | BDBM161467 (US9108978, 3.25) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >3.00E+4 | >-26.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Escherichia coli) | BDBM161470 (US9108978, 4.01) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | >3.00E+4 | >-26.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Escherichia coli) | BDBM161469 (US9108978, 6.07) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >3.00E+4 | >-26.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Escherichia coli) | BDBM161468 (US9108978, 6.09) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | >3.00E+4 | >-26.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional ligase/repressor BirA (Escherichia coli) | BDBM161467 (US9108978, 3.25) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >3.00E+4 | >-26.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
Monash University; Adelaide Research & Innovation Pty Ltd US Patent | Assay Description Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol... | US Patent US9108978 (2015) BindingDB Entry DOI: 10.7270/Q2T43RVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 80 total ) | Next | Last >> |