 Found 1407 hits with Last Name = 'wang' and Initial = 'th'
Found 1407 hits with Last Name = 'wang' and Initial = 'th' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50427810
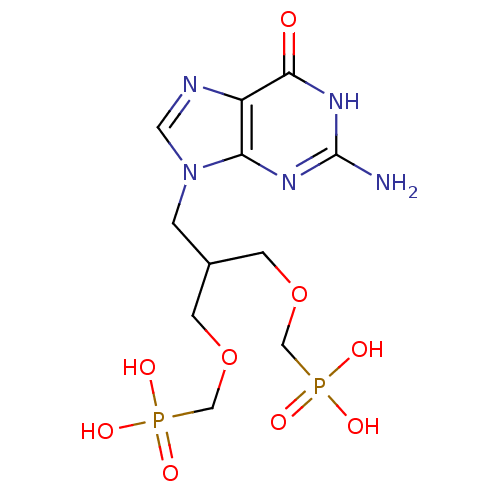
(CHEMBL2325752)Show SMILES Nc1nc2n(CC(COCP(O)(O)=O)COCP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C11H19N5O9P2/c12-11-14-9-8(10(17)15-11)13-4-16(9)1-7(2-24-5-26(18,19)20)3-25-6-27(21,22)23/h4,7H,1-3,5-6H2,(H2,18,19,20)(H2,21,22,23)(H3,12,14,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal hexahistidine-tagged HGPRT |
J Med Chem 56: 2513-26 (2013)
Article DOI: 10.1021/jm301893b
BindingDB Entry DOI: 10.7270/Q2MW2JGT |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50059910
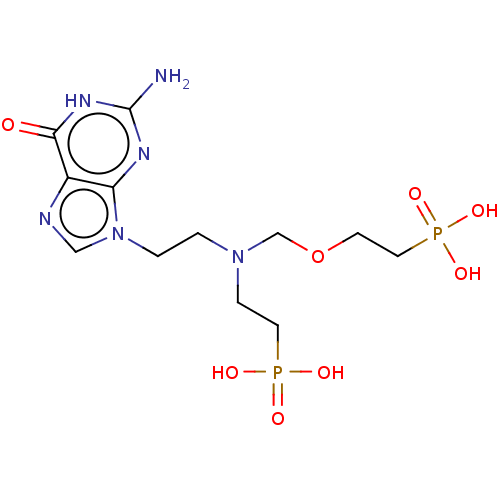
(CHEMBL3394315)Show SMILES Nc1nc2n(CCN(CCP(O)(O)=O)COCCP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C12H22N6O8P2/c13-12-15-10-9(11(19)16-12)14-7-18(10)2-1-17(3-5-27(20,21)22)8-26-4-6-28(23,24)25/h7H,1-6,8H2,(H2,20,21,22)(H2,23,24,25)(H3,13,15,16,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
J Med Chem 58: 827-46 (2015)
Article DOI: 10.1021/jm501416t
BindingDB Entry DOI: 10.7270/Q2HM5B3V |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50059908

(CHEMBL3394327)Show SMILES Nc1nc2n(CCN(CCOCCP(O)(O)=O)CCP(O)(O)=O)c(Br)nc2c(=O)[nH]1 Show InChI InChI=1S/C13H23BrN6O8P2/c14-12-16-9-10(17-13(15)18-11(9)21)20(12)2-1-19(4-7-29(22,23)24)3-5-28-6-8-30(25,26)27/h1-8H2,(H2,22,23,24)(H2,25,26,27)(H3,15,17,18,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
J Med Chem 58: 827-46 (2015)
Article DOI: 10.1021/jm501416t
BindingDB Entry DOI: 10.7270/Q2HM5B3V |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50392263
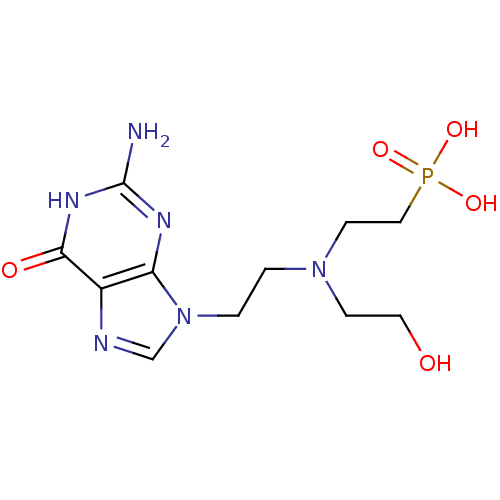
(CHEMBL2153497)Show InChI InChI=1S/C11H19N6O5P/c12-11-14-9-8(10(19)15-11)13-7-17(9)2-1-16(3-5-18)4-6-23(20,21)22/h7,18H,1-6H2,(H2,20,21,22)(H3,12,14,15,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine |
J Med Chem 55: 6209-23 (2012)
Article DOI: 10.1021/jm300662d
BindingDB Entry DOI: 10.7270/Q2VX0HND |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50392261
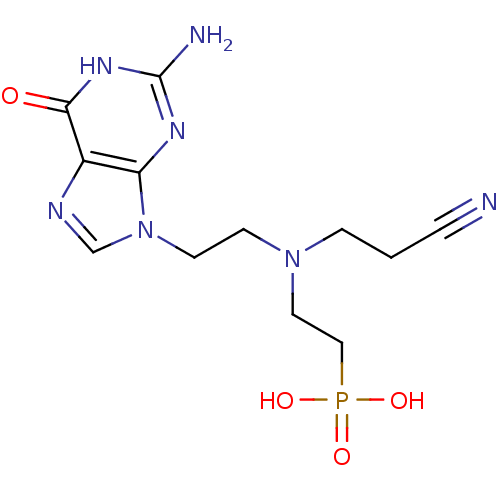
(CHEMBL2153480)Show SMILES Nc1nc2n(CCN(CCC#N)CCP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C12H18N7O4P/c13-2-1-3-18(6-7-24(21,22)23)4-5-19-8-15-9-10(19)16-12(14)17-11(9)20/h8H,1,3-7H2,(H2,21,22,23)(H3,14,16,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine |
J Med Chem 55: 6209-23 (2012)
Article DOI: 10.1021/jm300662d
BindingDB Entry DOI: 10.7270/Q2VX0HND |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50392261

(CHEMBL2153480)Show SMILES Nc1nc2n(CCN(CCC#N)CCP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C12H18N7O4P/c13-2-1-3-18(6-7-24(21,22)23)4-5-19-8-15-9-10(19)16-12(14)17-11(9)20/h8H,1,3-7H2,(H2,21,22,23)(H3,14,16,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
J Med Chem 58: 827-46 (2015)
Article DOI: 10.1021/jm501416t
BindingDB Entry DOI: 10.7270/Q2HM5B3V |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50059909
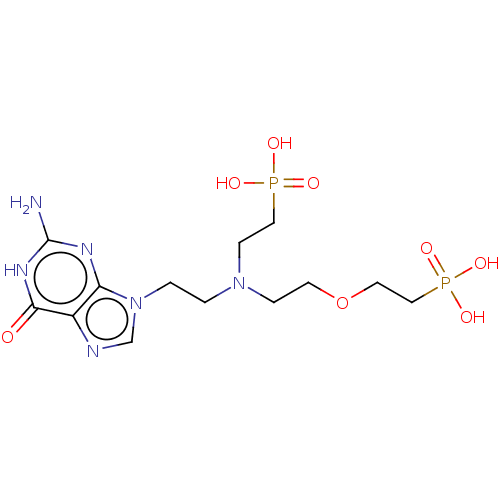
(CHEMBL3394316)Show SMILES Nc1nc2n(CCN(CCOCCP(O)(O)=O)CCP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C13H24N6O8P2/c14-13-16-11-10(12(20)17-13)15-9-19(11)2-1-18(4-7-28(21,22)23)3-5-27-6-8-29(24,25)26/h9H,1-8H2,(H2,21,22,23)(H2,24,25,26)(H3,14,16,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
J Med Chem 58: 827-46 (2015)
Article DOI: 10.1021/jm501416t
BindingDB Entry DOI: 10.7270/Q2HM5B3V |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50392258

(CHEMBL2153476)Show SMILES CCOC(=O)CN(CCn1cnc2c1nc(N)[nH]c2=O)CCP(O)(O)=O Show InChI InChI=1S/C13H21N6O6P/c1-2-25-9(20)7-18(5-6-26(22,23)24)3-4-19-8-15-10-11(19)16-13(14)17-12(10)21/h8H,2-7H2,1H3,(H2,22,23,24)(H3,14,16,17,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine |
J Med Chem 55: 6209-23 (2012)
Article DOI: 10.1021/jm300662d
BindingDB Entry DOI: 10.7270/Q2VX0HND |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50392260
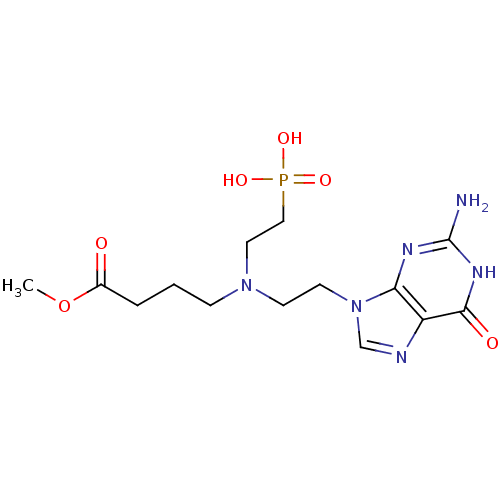
(CHEMBL2153478)Show SMILES COC(=O)CCCN(CCn1cnc2c1nc(N)[nH]c2=O)CCP(O)(O)=O Show InChI InChI=1S/C14H23N6O6P/c1-26-10(21)3-2-4-19(7-8-27(23,24)25)5-6-20-9-16-11-12(20)17-14(15)18-13(11)22/h9H,2-8H2,1H3,(H2,23,24,25)(H3,15,17,18,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine |
J Med Chem 55: 6209-23 (2012)
Article DOI: 10.1021/jm300662d
BindingDB Entry DOI: 10.7270/Q2VX0HND |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50392266

(CHEMBL2153484)Show SMILES Nc1nc2n(CCN(CCCC(O)=O)CCP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C13H21N6O6P/c14-13-16-11-10(12(22)17-13)15-8-19(11)5-4-18(3-1-2-9(20)21)6-7-26(23,24)25/h8H,1-7H2,(H,20,21)(H2,23,24,25)(H3,14,16,17,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine |
J Med Chem 55: 6209-23 (2012)
Article DOI: 10.1021/jm300662d
BindingDB Entry DOI: 10.7270/Q2VX0HND |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50392265
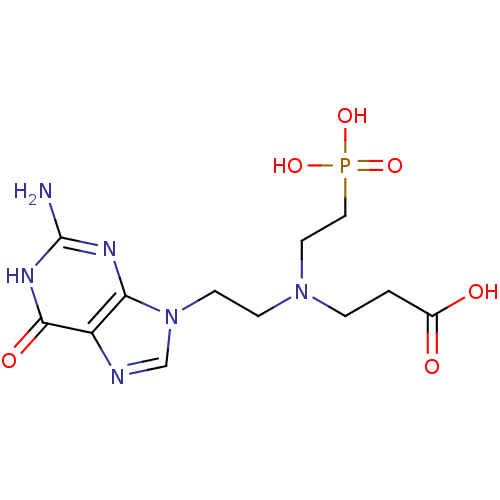
(CHEMBL2153483)Show SMILES Nc1nc2n(CCN(CCC(O)=O)CCP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C12H19N6O6P/c13-12-15-10-9(11(21)16-12)14-7-18(10)4-3-17(2-1-8(19)20)5-6-25(22,23)24/h7H,1-6H2,(H,19,20)(H2,22,23,24)(H3,13,15,16,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine |
J Med Chem 55: 6209-23 (2012)
Article DOI: 10.1021/jm300662d
BindingDB Entry DOI: 10.7270/Q2VX0HND |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50059913
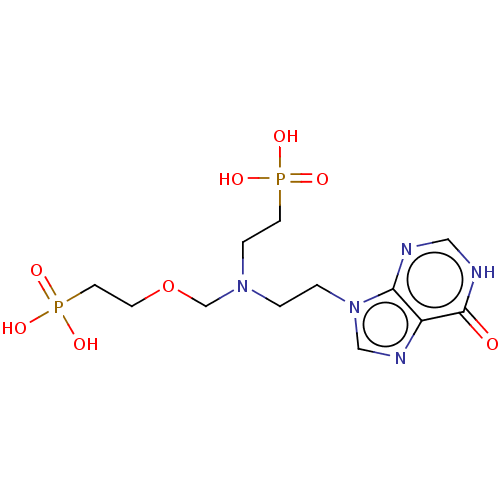
(CHEMBL3394312)Show SMILES OP(O)(=O)CCOCN(CCn1cnc2c1nc[nH]c2=O)CCP(O)(O)=O Show InChI InChI=1S/C12H21N5O8P2/c18-12-10-11(13-7-14-12)17(8-15-10)2-1-16(3-5-26(19,20)21)9-25-4-6-27(22,23)24/h7-8H,1-6,9H2,(H,13,14,18)(H2,19,20,21)(H2,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
J Med Chem 58: 827-46 (2015)
Article DOI: 10.1021/jm501416t
BindingDB Entry DOI: 10.7270/Q2HM5B3V |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50392267
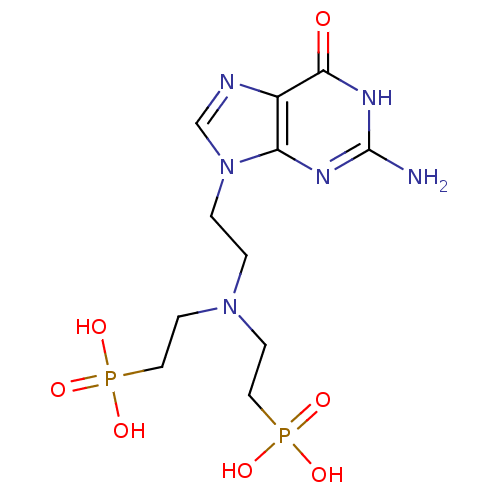
(CHEMBL2153485)Show SMILES Nc1nc2n(CCN(CCP(O)(O)=O)CCP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C11H20N6O7P2/c12-11-14-9-8(10(18)15-11)13-7-17(9)2-1-16(3-5-25(19,20)21)4-6-26(22,23)24/h7H,1-6H2,(H2,19,20,21)(H2,22,23,24)(H3,12,14,15,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine |
J Med Chem 55: 6209-23 (2012)
Article DOI: 10.1021/jm300662d
BindingDB Entry DOI: 10.7270/Q2VX0HND |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50059911

(CHEMBL3394314)Show SMILES Nc1nc2n(CCN(CCCCP(O)(O)=O)CCP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C13H24N6O7P2/c14-13-16-11-10(12(20)17-13)15-9-19(11)5-4-18(6-8-28(24,25)26)3-1-2-7-27(21,22)23/h9H,1-8H2,(H2,21,22,23)(H2,24,25,26)(H3,14,16,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
J Med Chem 58: 827-46 (2015)
Article DOI: 10.1021/jm501416t
BindingDB Entry DOI: 10.7270/Q2HM5B3V |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50392259
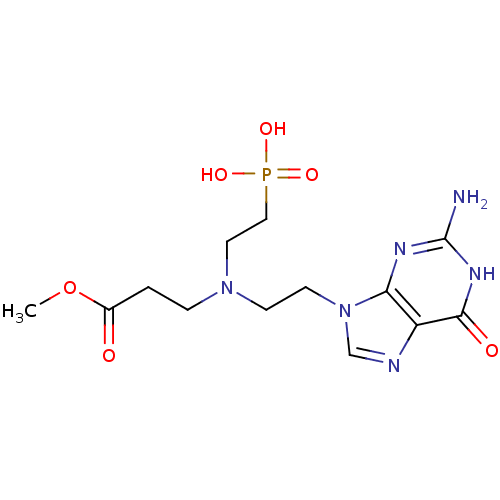
(CHEMBL2153477)Show SMILES COC(=O)CCN(CCn1cnc2c1nc(N)[nH]c2=O)CCP(O)(O)=O Show InChI InChI=1S/C13H21N6O6P/c1-25-9(20)2-3-18(6-7-26(22,23)24)4-5-19-8-15-10-11(19)16-13(14)17-12(10)21/h8H,2-7H2,1H3,(H2,22,23,24)(H3,14,16,17,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine |
J Med Chem 55: 6209-23 (2012)
Article DOI: 10.1021/jm300662d
BindingDB Entry DOI: 10.7270/Q2VX0HND |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50392262
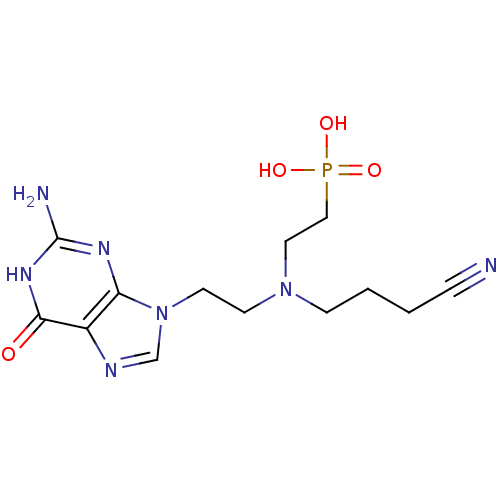
(CHEMBL2153481)Show SMILES Nc1nc2n(CCN(CCCC#N)CCP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C13H20N7O4P/c14-3-1-2-4-19(7-8-25(22,23)24)5-6-20-9-16-10-11(20)17-13(15)18-12(10)21/h9H,1-2,4-8H2,(H2,22,23,24)(H3,15,17,18,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine |
J Med Chem 55: 6209-23 (2012)
Article DOI: 10.1021/jm300662d
BindingDB Entry DOI: 10.7270/Q2VX0HND |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50427808
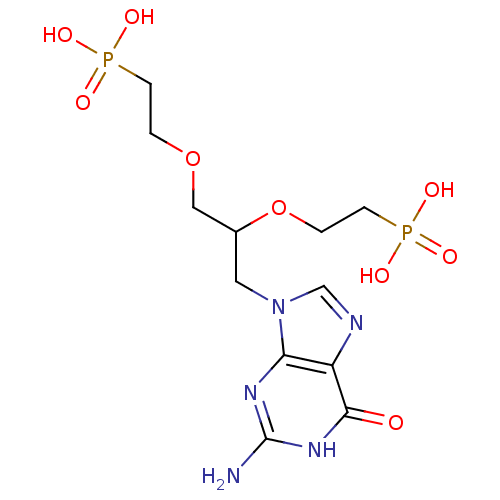
(CHEMBL2325754)Show SMILES Nc1nc2n(CC(COCCP(O)(O)=O)OCCP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C12H21N5O9P2/c13-12-15-10-9(11(18)16-12)14-7-17(10)5-8(26-2-4-28(22,23)24)6-25-1-3-27(19,20)21/h7-8H,1-6H2,(H2,19,20,21)(H2,22,23,24)(H3,13,15,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal hexahistidine-tagged HGPRT |
J Med Chem 56: 2513-26 (2013)
Article DOI: 10.1021/jm301893b
BindingDB Entry DOI: 10.7270/Q2MW2JGT |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50427809

(CHEMBL2325753)Show SMILES OP(O)(=O)COCC(COCP(O)(O)=O)Cn1cnc2c1nc[nH]c2=O Show InChI InChI=1S/C11H18N4O9P2/c16-11-9-10(12-4-13-11)15(5-14-9)1-8(2-23-6-25(17,18)19)3-24-7-26(20,21)22/h4-5,8H,1-3,6-7H2,(H,12,13,16)(H2,17,18,19)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal hexahistidine-tagged HGPRT |
J Med Chem 56: 2513-26 (2013)
Article DOI: 10.1021/jm301893b
BindingDB Entry DOI: 10.7270/Q2MW2JGT |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50059912

(CHEMBL3394313)Show SMILES OP(O)(=O)CCOCCN(CCn1cnc2c1nc[nH]c2=O)CCP(O)(O)=O Show InChI InChI=1S/C13H23N5O8P2/c19-13-11-12(14-9-15-13)18(10-16-11)2-1-17(4-7-27(20,21)22)3-5-26-6-8-28(23,24)25/h9-10H,1-8H2,(H,14,15,19)(H2,20,21,22)(H2,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
J Med Chem 58: 827-46 (2015)
Article DOI: 10.1021/jm501416t
BindingDB Entry DOI: 10.7270/Q2HM5B3V |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50392264
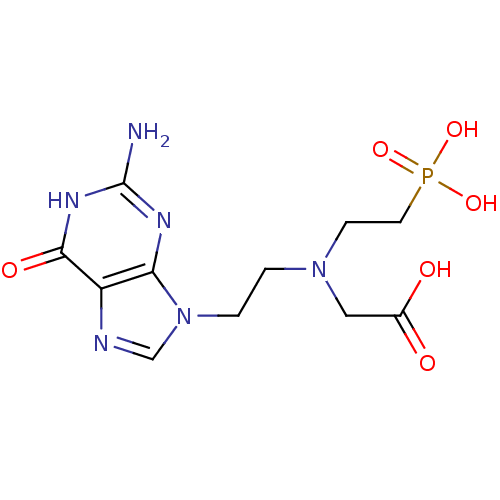
(CHEMBL2153482)Show SMILES Nc1nc2n(CCN(CCP(O)(O)=O)CC(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C11H17N6O6P/c12-11-14-9-8(10(20)15-11)13-6-17(9)2-1-16(5-7(18)19)3-4-24(21,22)23/h6H,1-5H2,(H,18,19)(H2,21,22,23)(H3,12,14,15,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine |
J Med Chem 55: 6209-23 (2012)
Article DOI: 10.1021/jm300662d
BindingDB Entry DOI: 10.7270/Q2VX0HND |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
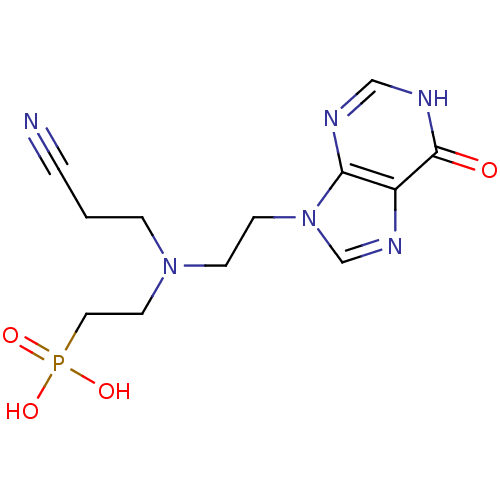
(Homo sapiens (Human)) | BDBM50392251
(CHEMBL2153490)Show InChI InChI=1S/C12H17N6O4P/c13-2-1-3-17(6-7-23(20,21)22)4-5-18-9-16-10-11(18)14-8-15-12(10)19/h8-9H,1,3-7H2,(H,14,15,19)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine |
J Med Chem 55: 6209-23 (2012)
Article DOI: 10.1021/jm300662d
BindingDB Entry DOI: 10.7270/Q2VX0HND |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50392251

(CHEMBL2153490)Show InChI InChI=1S/C12H17N6O4P/c13-2-1-3-17(6-7-23(20,21)22)4-5-18-9-16-10-11(18)14-8-15-12(10)19/h8-9H,1,3-7H2,(H,14,15,19)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
J Med Chem 58: 827-46 (2015)
Article DOI: 10.1021/jm501416t
BindingDB Entry DOI: 10.7270/Q2HM5B3V |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50392247
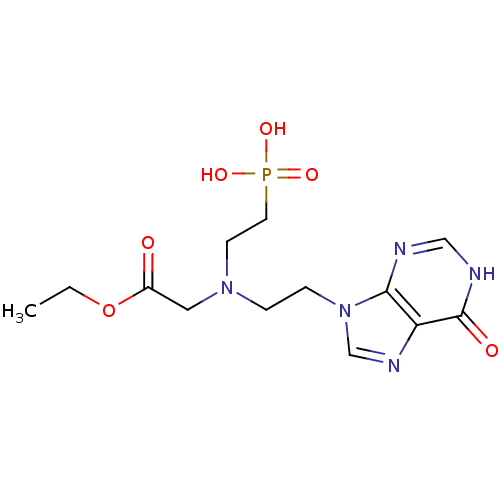
(CHEMBL2153486)Show SMILES CCOC(=O)CN(CCn1cnc2c1nc[nH]c2=O)CCP(O)(O)=O Show InChI InChI=1S/C13H20N5O6P/c1-2-24-10(19)7-17(5-6-25(21,22)23)3-4-18-9-16-11-12(18)14-8-15-13(11)20/h8-9H,2-7H2,1H3,(H,14,15,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine |
J Med Chem 55: 6209-23 (2012)
Article DOI: 10.1021/jm300662d
BindingDB Entry DOI: 10.7270/Q2VX0HND |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50392245
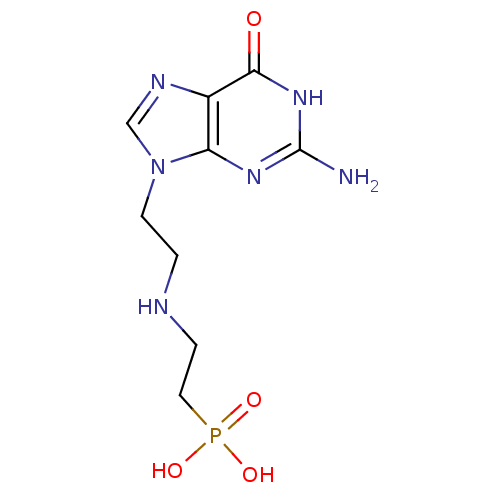
(CHEMBL2153498)Show InChI InChI=1S/C9H15N6O4P/c10-9-13-7-6(8(16)14-9)12-5-15(7)3-1-11-2-4-20(17,18)19/h5,11H,1-4H2,(H2,17,18,19)(H3,10,13,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine |
J Med Chem 55: 6209-23 (2012)
Article DOI: 10.1021/jm300662d
BindingDB Entry DOI: 10.7270/Q2VX0HND |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50392257
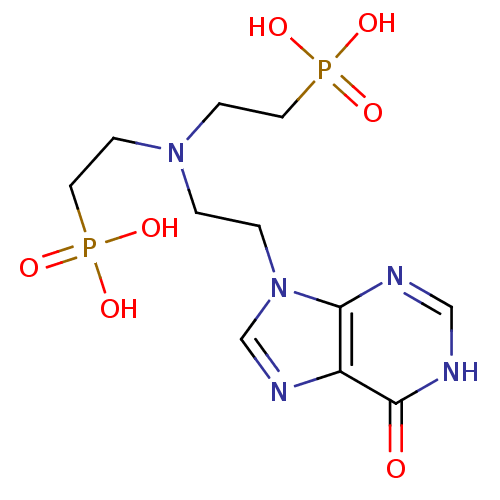
(CHEMBL2153496)Show SMILES OP(O)(=O)CCN(CCn1cnc2c1nc[nH]c2=O)CCP(O)(O)=O Show InChI InChI=1S/C11H19N5O7P2/c17-11-9-10(12-7-13-11)16(8-14-9)2-1-15(3-5-24(18,19)20)4-6-25(21,22)23/h7-8H,1-6H2,(H,12,13,17)(H2,18,19,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine |
J Med Chem 55: 6209-23 (2012)
Article DOI: 10.1021/jm300662d
BindingDB Entry DOI: 10.7270/Q2VX0HND |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50059914

(CHEMBL3394311)Show SMILES OP(O)(=O)CCCCN(CCn1cnc2c1nc[nH]c2=O)CCP(O)(O)=O Show InChI InChI=1S/C13H23N5O7P2/c19-13-11-12(14-9-15-13)18(10-16-11)5-4-17(6-8-27(23,24)25)3-1-2-7-26(20,21)22/h9-10H,1-8H2,(H,14,15,19)(H2,20,21,22)(H2,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT |
J Med Chem 58: 827-46 (2015)
Article DOI: 10.1021/jm501416t
BindingDB Entry DOI: 10.7270/Q2HM5B3V |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50392256
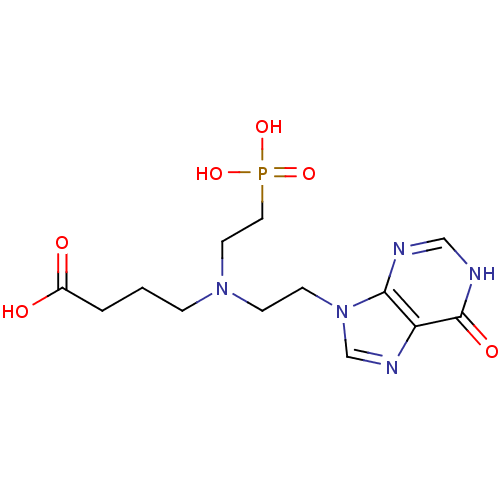
(CHEMBL2153494)Show SMILES OC(=O)CCCN(CCn1cnc2c1nc[nH]c2=O)CCP(O)(O)=O Show InChI InChI=1S/C13H20N5O6P/c19-10(20)2-1-3-17(6-7-25(22,23)24)4-5-18-9-16-11-12(18)14-8-15-13(11)21/h8-9H,1-7H2,(H,19,20)(H,14,15,21)(H2,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine |
J Med Chem 55: 6209-23 (2012)
Article DOI: 10.1021/jm300662d
BindingDB Entry DOI: 10.7270/Q2VX0HND |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
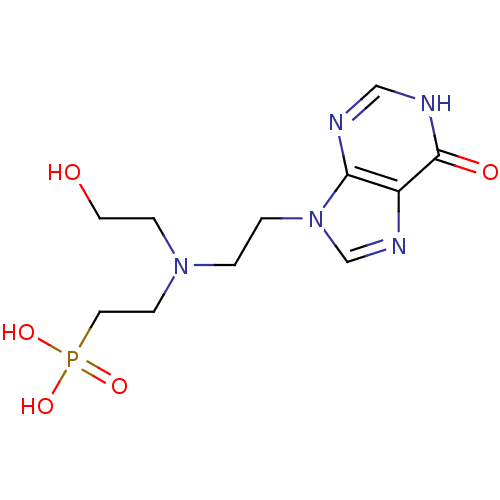
(Homo sapiens (Human)) | BDBM50392253
(CHEMBL2153495)Show InChI InChI=1S/C11H18N5O5P/c17-5-3-15(4-6-22(19,20)21)1-2-16-8-14-9-10(16)12-7-13-11(9)18/h7-8,17H,1-6H2,(H,12,13,18)(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine |
J Med Chem 55: 6209-23 (2012)
Article DOI: 10.1021/jm300662d
BindingDB Entry DOI: 10.7270/Q2VX0HND |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
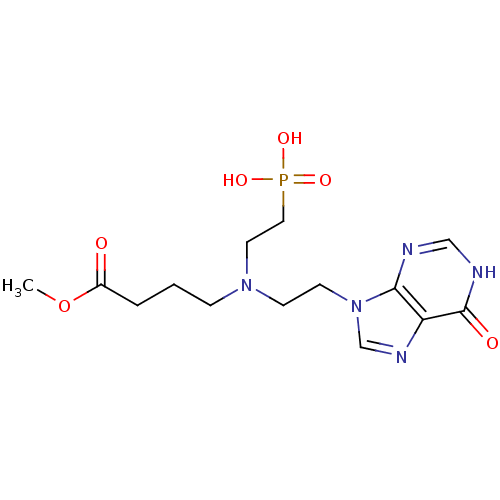
(Homo sapiens (Human)) | BDBM50392249
(CHEMBL2153488)Show SMILES COC(=O)CCCN(CCn1cnc2c1nc[nH]c2=O)CCP(O)(O)=O Show InChI InChI=1S/C14H22N5O6P/c1-25-11(20)3-2-4-18(7-8-26(22,23)24)5-6-19-10-17-12-13(19)15-9-16-14(12)21/h9-10H,2-8H2,1H3,(H,15,16,21)(H2,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine |
J Med Chem 55: 6209-23 (2012)
Article DOI: 10.1021/jm300662d
BindingDB Entry DOI: 10.7270/Q2VX0HND |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50392252
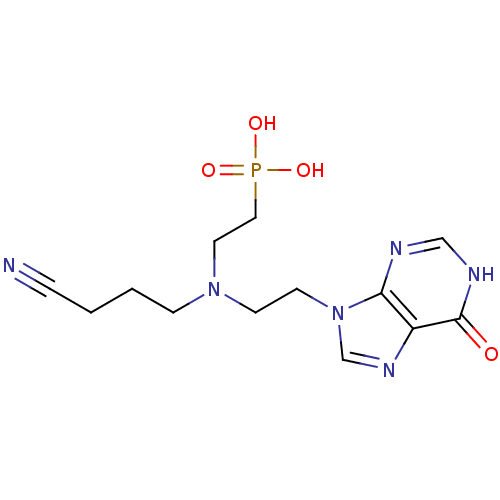
(CHEMBL2153491)Show InChI InChI=1S/C13H19N6O4P/c14-3-1-2-4-18(7-8-24(21,22)23)5-6-19-10-17-11-12(19)15-9-16-13(11)20/h9-10H,1-2,4-8H2,(H,15,16,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine |
J Med Chem 55: 6209-23 (2012)
Article DOI: 10.1021/jm300662d
BindingDB Entry DOI: 10.7270/Q2VX0HND |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50392254
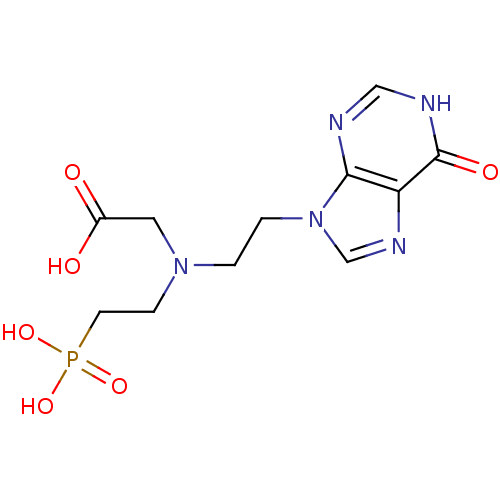
(CHEMBL2153492)Show InChI InChI=1S/C11H16N5O6P/c17-8(18)5-15(3-4-23(20,21)22)1-2-16-7-14-9-10(16)12-6-13-11(9)19/h6-7H,1-5H2,(H,17,18)(H,12,13,19)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine |
J Med Chem 55: 6209-23 (2012)
Article DOI: 10.1021/jm300662d
BindingDB Entry DOI: 10.7270/Q2VX0HND |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50392250

(CHEMBL2153489)Show InChI InChI=1S/C11H15N6O4P/c12-1-2-16(5-6-22(19,20)21)3-4-17-8-15-9-10(17)13-7-14-11(9)18/h7-8H,2-6H2,(H,13,14,18)(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine |
J Med Chem 55: 6209-23 (2012)
Article DOI: 10.1021/jm300662d
BindingDB Entry DOI: 10.7270/Q2VX0HND |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50427807
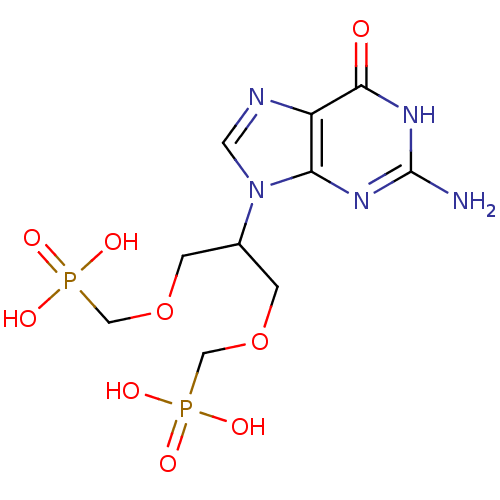
(CHEMBL2325755)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)C(COCP(O)(O)=O)COCP(O)(O)=O Show InChI InChI=1S/C10H17N5O9P2/c11-10-13-8-7(9(16)14-10)12-3-15(8)6(1-23-4-25(17,18)19)2-24-5-26(20,21)22/h3,6H,1-2,4-5H2,(H2,17,18,19)(H2,20,21,22)(H3,11,13,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal hexahistidine-tagged HGPRT |
J Med Chem 56: 2513-26 (2013)
Article DOI: 10.1021/jm301893b
BindingDB Entry DOI: 10.7270/Q2MW2JGT |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50392246
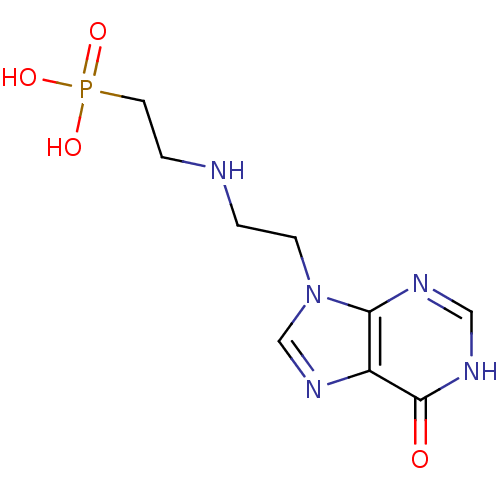
(CHEMBL2153499)Show InChI InChI=1S/C9H14N5O4P/c15-9-7-8(11-5-12-9)14(6-13-7)3-1-10-2-4-19(16,17)18/h5-6,10H,1-4H2,(H,11,12,15)(H2,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine |
J Med Chem 55: 6209-23 (2012)
Article DOI: 10.1021/jm300662d
BindingDB Entry DOI: 10.7270/Q2VX0HND |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50392248
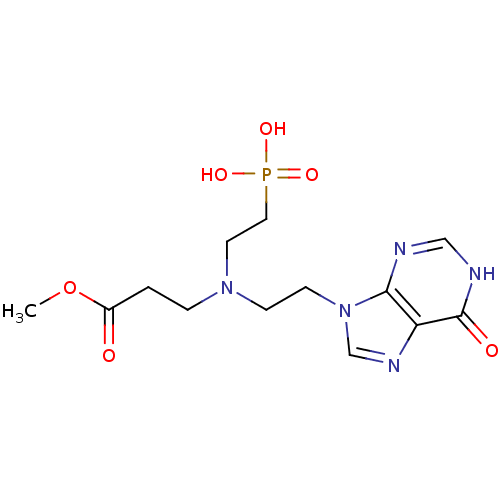
(CHEMBL2153487)Show SMILES COC(=O)CCN(CCn1cnc2c1nc[nH]c2=O)CCP(O)(O)=O Show InChI InChI=1S/C13H20N5O6P/c1-24-10(19)2-3-17(6-7-25(21,22)23)4-5-18-9-16-11-12(18)14-8-15-13(11)20/h8-9H,2-7H2,1H3,(H,14,15,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine |
J Med Chem 55: 6209-23 (2012)
Article DOI: 10.1021/jm300662d
BindingDB Entry DOI: 10.7270/Q2VX0HND |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50392255
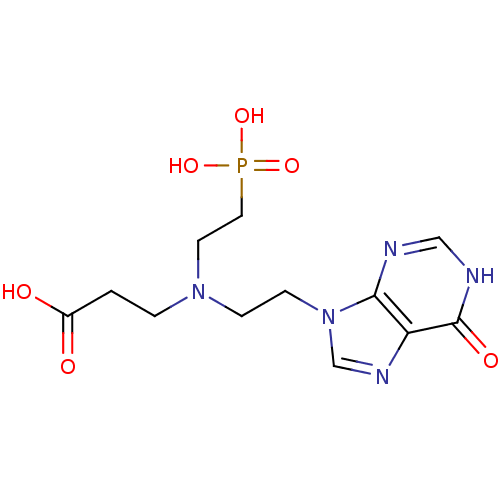
(CHEMBL2153493)Show InChI InChI=1S/C12H18N5O6P/c18-9(19)1-2-16(5-6-24(21,22)23)3-4-17-8-15-10-11(17)13-7-14-12(10)20/h7-8H,1-6H2,(H,18,19)(H,13,14,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanine |
J Med Chem 55: 6209-23 (2012)
Article DOI: 10.1021/jm300662d
BindingDB Entry DOI: 10.7270/Q2VX0HND |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 11 [51-76]/Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM449685

(US10703733, Example 298 | US11643400, Example 298)Show SMILES CO[C@H]1CC[C@H](O)[C@H](C)C[S@@](=O)(NC(=O)c2cn(C)nc2OC)=NC(=O)c2ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C[C@@H]4CC[C@@H]14)c3c2 |r| Show InChI InChI=1S/C38H48ClN5O7S/c1-23-20-52(48,42-36(47)29-19-43(2)40-37(29)50-4)41-35(46)25-8-13-34-31(17-25)44(18-26-7-10-28(26)33(49-3)14-12-32(23)45)21-38(22-51-34)15-5-6-24-16-27(39)9-11-30(24)38/h8-9,11,13,16-17,19,23,26,28,32-33,45H,5-7,10,12,14-15,18,20-22H2,1-4H3,(H,41,42,46,47,48)/t23-,26+,28-,32+,33+,38+,52+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
US Patent
| Assay Description
The AlphaLISA assay was performed in a 384-well Proxiplate in a total volume of 40 μL. The reaction mixture contained 0.0625 nM 6×His-Mcl-1 (171... |
US Patent US10703733 (2020)
BindingDB Entry DOI: 10.7270/Q2XK8JK4 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 11 [51-76]/Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM449685

(US10703733, Example 298 | US11643400, Example 298)Show SMILES CO[C@H]1CC[C@H](O)[C@H](C)C[S@@](=O)(NC(=O)c2cn(C)nc2OC)=NC(=O)c2ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C[C@@H]4CC[C@@H]14)c3c2 |r| Show InChI InChI=1S/C38H48ClN5O7S/c1-23-20-52(48,42-36(47)29-19-43(2)40-37(29)50-4)41-35(46)25-8-13-34-31(17-25)44(18-26-7-10-28(26)33(49-3)14-12-32(23)45)21-38(22-51-34)15-5-6-24-16-27(39)9-11-30(24)38/h8-9,11,13,16-17,19,23,26,28,32-33,45H,5-7,10,12,14-15,18,20-22H2,1-4H3,(H,41,42,46,47,48)/t23-,26+,28-,32+,33+,38+,52+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24B357N |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 11 [51-76]/Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM602144
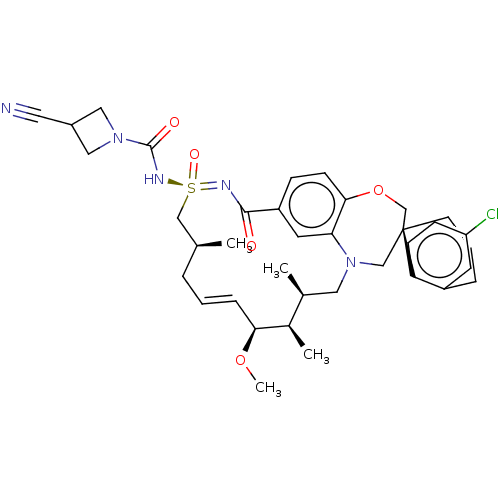
(US11643400, Example 233)Show SMILES CO[C@H]1\C=C\C[C@H](C)C[S@@](=O)(NC(=O)N2CC(C2)C#N)=NC(=O)c2ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C[C@H](C)[C@H]1C)c3c2 |r,t:3| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24B357N |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM449620
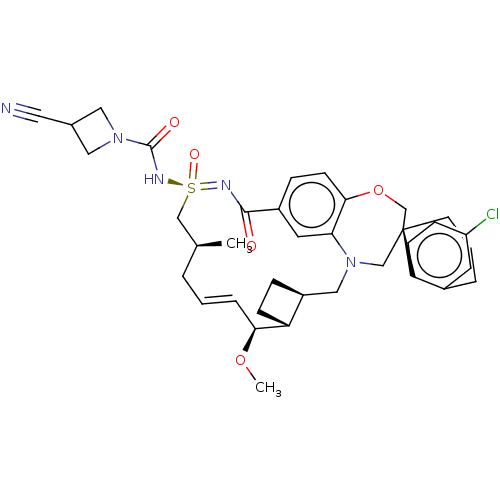
(US10703733, Example 233 | US10988451, Example 233)Show SMILES CO[C@H]1\C=C\C[C@H](C)C[S@@](=O)(NC(=O)N2CC(C2)C#N)=NC(=O)c2ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C[C@@H]4CC[C@@H]14)c3c2 |r,t:3| Show InChI InChI=1S/C37H44ClN5O5S/c1-24-5-3-7-33(47-2)30-11-8-28(30)20-43-22-37(14-4-6-26-15-29(38)10-12-31(26)37)23-48-34-13-9-27(16-32(34)43)35(44)40-49(46,21-24)41-36(45)42-18-25(17-39)19-42/h3,7,9-10,12-13,15-16,24-25,28,30,33H,4-6,8,11,14,18-23H2,1-2H3,(H,40,41,44,45,46)/b7-3+/t24-,28-,30+,33-,37-,49-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
US Patent
| Assay Description
The AlphaLISA assay was performed in a 384-well Proxiplate in a total volume of 40 μL. The reaction mixture contained 0.0625 nM 6× His-Mcl-1 (17... |
US Patent US10988451 (2021)
BindingDB Entry DOI: 10.7270/Q2M048KN |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 11 [51-76]/Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM449620

(US10703733, Example 233 | US10988451, Example 233)Show SMILES CO[C@H]1\C=C\C[C@H](C)C[S@@](=O)(NC(=O)N2CC(C2)C#N)=NC(=O)c2ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C[C@@H]4CC[C@@H]14)c3c2 |r,t:3| Show InChI InChI=1S/C37H44ClN5O5S/c1-24-5-3-7-33(47-2)30-11-8-28(30)20-43-22-37(14-4-6-26-15-29(38)10-12-31(26)37)23-48-34-13-9-27(16-32(34)43)35(44)40-49(46,21-24)41-36(45)42-18-25(17-39)19-42/h3,7,9-10,12-13,15-16,24-25,28,30,33H,4-6,8,11,14,18-23H2,1-2H3,(H,40,41,44,45,46)/b7-3+/t24-,28-,30+,33-,37-,49-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
US Patent
| Assay Description
The AlphaLISA assay was performed in a 384-well Proxiplate in a total volume of 40 μL. The reaction mixture contained 0.0625 nM 6×His-Mcl-1 (171... |
US Patent US10703733 (2020)
BindingDB Entry DOI: 10.7270/Q2XK8JK4 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM449686
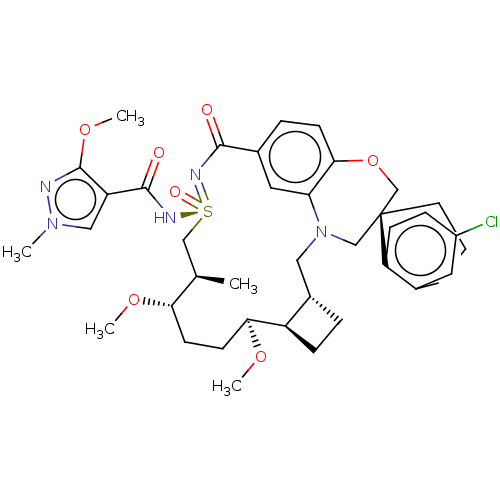
(US10703733, Example 299 | US10988451, Example 298 ...)Show SMILES CO[C@H]1CC[C@H](OC)[C@H](C)C[S@@](=O)(NC(=O)c2cn(C)nc2OC)=NC(=O)c2ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C[C@@H]4CC[C@@H]14)c3c2 |r| Show InChI InChI=1S/C39H50ClN5O7S/c1-24-21-53(48,43-37(47)30-20-44(2)41-38(30)51-5)42-36(46)26-9-13-35-32(18-26)45(19-27-8-11-29(27)34(50-4)15-14-33(24)49-3)22-39(23-52-35)16-6-7-25-17-28(40)10-12-31(25)39/h9-10,12-13,17-18,20,24,27,29,33-34H,6-8,11,14-16,19,21-23H2,1-5H3,(H,42,43,46,47,48)/t24-,27+,29-,33+,34+,39+,53+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
US Patent
| Assay Description
The AlphaLISA assay was performed in a 384-well Proxiplate in a total volume of 40 μL. The reaction mixture contained 0.0625 nM 6× His-Mcl-1 (17... |
US Patent US10988451 (2021)
BindingDB Entry DOI: 10.7270/Q2M048KN |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM449659

(US10703733, Example 272 | US10988451, Example 272 ...)Show SMILES CO[C@H]1\C=C\C[C@H](C)C[S@@](=O)(NC(=O)N2CC3(C2)CN(C)C(=O)O3)=NC(=O)c2ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C[C@@H]4CC[C@@H]14)c3c2 |r,t:3| Show InChI InChI=1S/C39H48ClN5O7S/c1-25-6-4-8-33(50-3)30-12-9-28(30)18-44-20-38(15-5-7-26-16-29(40)11-13-31(26)38)24-51-34-14-10-27(17-32(34)44)35(46)41-53(49,19-25)42-36(47)45-22-39(23-45)21-43(2)37(48)52-39/h4,8,10-11,13-14,16-17,25,28,30,33H,5-7,9,12,15,18-24H2,1-3H3,(H,41,42,46,47,49)/b8-4+/t25-,28-,30+,33-,38-,53-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
US Patent
| Assay Description
The AlphaLISA assay was performed in a 384-well Proxiplate in a total volume of 40 μL. The reaction mixture contained 0.0625 nM 6× His-Mcl-1 (17... |
US Patent US10988451 (2021)
BindingDB Entry DOI: 10.7270/Q2M048KN |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 11 [51-76]/Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM449659

(US10703733, Example 272 | US10988451, Example 272 ...)Show SMILES CO[C@H]1\C=C\C[C@H](C)C[S@@](=O)(NC(=O)N2CC3(C2)CN(C)C(=O)O3)=NC(=O)c2ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C[C@@H]4CC[C@@H]14)c3c2 |r,t:3| Show InChI InChI=1S/C39H48ClN5O7S/c1-25-6-4-8-33(50-3)30-12-9-28(30)18-44-20-38(15-5-7-26-16-29(40)11-13-31(26)38)24-51-34-14-10-27(17-32(34)44)35(46)41-53(49,19-25)42-36(47)45-22-39(23-45)21-43(2)37(48)52-39/h4,8,10-11,13-14,16-17,25,28,30,33H,5-7,9,12,15,18-24H2,1-3H3,(H,41,42,46,47,49)/b8-4+/t25-,28-,30+,33-,38-,53-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
US Patent
| Assay Description
The AlphaLISA assay was performed in a 384-well Proxiplate in a total volume of 40 μL. The reaction mixture contained 0.0625 nM 6×His-Mcl-1 (171... |
US Patent US10703733 (2020)
BindingDB Entry DOI: 10.7270/Q2XK8JK4 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 11 [51-76]/Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM449659

(US10703733, Example 272 | US10988451, Example 272 ...)Show SMILES CO[C@H]1\C=C\C[C@H](C)C[S@@](=O)(NC(=O)N2CC3(C2)CN(C)C(=O)O3)=NC(=O)c2ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C[C@@H]4CC[C@@H]14)c3c2 |r,t:3| Show InChI InChI=1S/C39H48ClN5O7S/c1-25-6-4-8-33(50-3)30-12-9-28(30)18-44-20-38(15-5-7-26-16-29(40)11-13-31(26)38)24-51-34-14-10-27(17-32(34)44)35(46)41-53(49,19-25)42-36(47)45-22-39(23-45)21-43(2)37(48)52-39/h4,8,10-11,13-14,16-17,25,28,30,33H,5-7,9,12,15,18-24H2,1-3H3,(H,41,42,46,47,49)/b8-4+/t25-,28-,30+,33-,38-,53-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24B357N |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 11 [51-76]/Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM602151
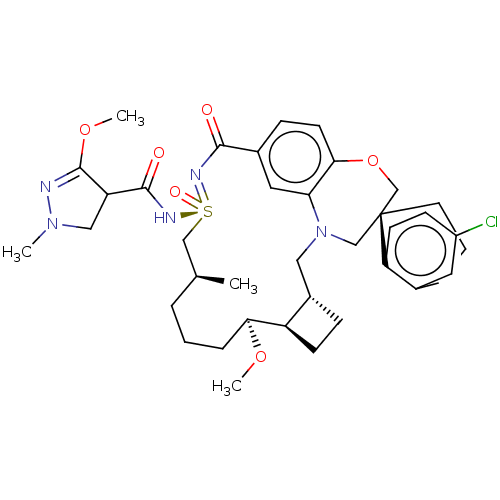
(US11643400, Example 240)Show SMILES CO[C@H]1CCC[C@H](C)C[S@@](=O)(NC(=O)C2CN(C)N=C2OC)=NC(=O)c2ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C[C@@H]4CC[C@@H]14)c3c2 |r,c:18| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24B357N |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM449627

(US10703733, Example 240 | US10988451, Example 240)Show SMILES CO[C@H]1CCC[C@H](C)C[S@@](=O)(NC(=O)c2cn(C)nc2OC)=NC(=O)c2ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C[C@@H]4CC[C@@H]14)c3c2 |r| Show InChI InChI=1S/C38H48ClN5O6S/c1-24-7-5-9-33(48-3)29-13-10-27(29)19-44-22-38(16-6-8-25-17-28(39)12-14-31(25)38)23-50-34-15-11-26(18-32(34)44)35(45)41-51(47,21-24)42-36(46)30-20-43(2)40-37(30)49-4/h11-12,14-15,17-18,20,24,27,29,33H,5-10,13,16,19,21-23H2,1-4H3,(H,41,42,45,46,47)/t24-,27-,29+,33-,38-,51-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
US Patent
| Assay Description
The AlphaLISA assay was performed in a 384-well Proxiplate in a total volume of 40 μL. The reaction mixture contained 0.0625 nM 6× His-Mcl-1 (17... |
US Patent US10988451 (2021)
BindingDB Entry DOI: 10.7270/Q2M048KN |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 11 [51-76]/Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM449627

(US10703733, Example 240 | US10988451, Example 240)Show SMILES CO[C@H]1CCC[C@H](C)C[S@@](=O)(NC(=O)c2cn(C)nc2OC)=NC(=O)c2ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C[C@@H]4CC[C@@H]14)c3c2 |r| Show InChI InChI=1S/C38H48ClN5O6S/c1-24-7-5-9-33(48-3)29-13-10-27(29)19-44-22-38(16-6-8-25-17-28(39)12-14-31(25)38)23-50-34-15-11-26(18-32(34)44)35(45)41-51(47,21-24)42-36(46)30-20-43(2)40-37(30)49-4/h11-12,14-15,17-18,20,24,27,29,33H,5-10,13,16,19,21-23H2,1-4H3,(H,41,42,45,46,47)/t24-,27-,29+,33-,38-,51-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
US Patent
| Assay Description
The AlphaLISA assay was performed in a 384-well Proxiplate in a total volume of 40 μL. The reaction mixture contained 0.0625 nM 6×His-Mcl-1 (171... |
US Patent US10703733 (2020)
BindingDB Entry DOI: 10.7270/Q2XK8JK4 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 11 [51-76]/Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM449730
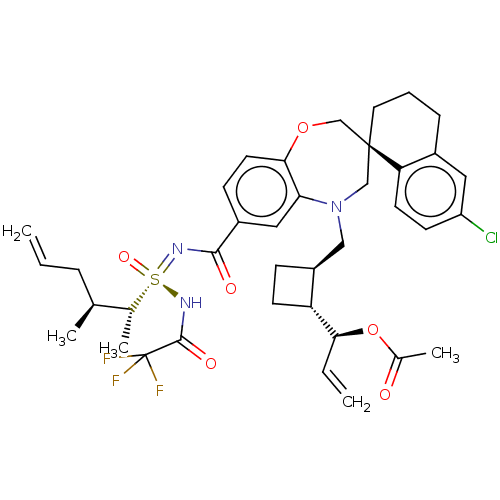
(US10703733, Example 343 | US10988451, Example 343 ...)Show SMILES C[C@@H](CC=C)[C@@H](C)[S@@](=O)(NC(=O)C(F)(F)F)=NC(=O)c1ccc2OC[C@]3(CCCc4cc(Cl)ccc34)CN(C[C@@H]3CC[C@H]3[C@@H](OC(C)=O)C=C)c2c1 |r| Show InChI InChI=1S/C38H45ClF3N3O6S/c1-6-9-23(3)24(4)52(49,44-36(48)38(40,41)42)43-35(47)27-12-16-34-32(19-27)45(20-28-11-14-30(28)33(7-2)51-25(5)46)21-37(22-50-34)17-8-10-26-18-29(39)13-15-31(26)37/h6-7,12-13,15-16,18-19,23-24,28,30,33H,1-2,8-11,14,17,20-22H2,3-5H3,(H,43,44,47,48,49)/t23-,24+,28-,30+,33-,37-,52-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24B357N |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 11 [51-76]/Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM449730

(US10703733, Example 343 | US10988451, Example 343 ...)Show SMILES C[C@@H](CC=C)[C@@H](C)[S@@](=O)(NC(=O)C(F)(F)F)=NC(=O)c1ccc2OC[C@]3(CCCc4cc(Cl)ccc34)CN(C[C@@H]3CC[C@H]3[C@@H](OC(C)=O)C=C)c2c1 |r| Show InChI InChI=1S/C38H45ClF3N3O6S/c1-6-9-23(3)24(4)52(49,44-36(48)38(40,41)42)43-35(47)27-12-16-34-32(19-27)45(20-28-11-14-30(28)33(7-2)51-25(5)46)21-37(22-50-34)17-8-10-26-18-29(39)13-15-31(26)37/h6-7,12-13,15-16,18-19,23-24,28,30,33H,1-2,8-11,14,17,20-22H2,3-5H3,(H,43,44,47,48,49)/t23-,24+,28-,30+,33-,37-,52-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
US Patent
| Assay Description
The AlphaLISA assay was performed in a 384-well Proxiplate in a total volume of 40 μL. The reaction mixture contained 0.0625 nM 6×His-Mcl-1 (171... |
US Patent US10703733 (2020)
BindingDB Entry DOI: 10.7270/Q2XK8JK4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data