

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50379086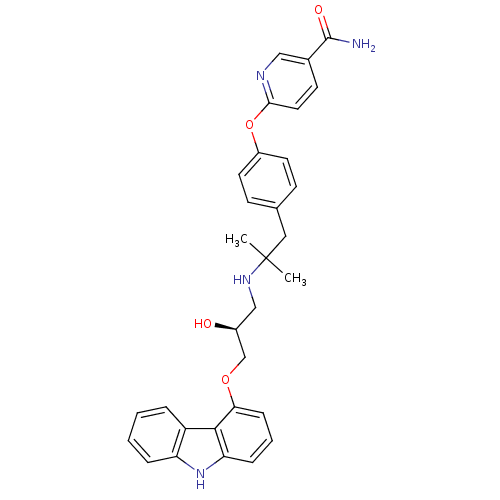 (CHEMBL2012521 | CHEMBL2012522 | LY-377604) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]Iodocyanopindolol from human adrenergic beta2 receptor expressed in insect sf9 cells by scintillation counting | ACS Med Chem Lett 2: 583-586 (2011) Article DOI: 10.1021/ml200071k BindingDB Entry DOI: 10.7270/Q20R9QDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50179190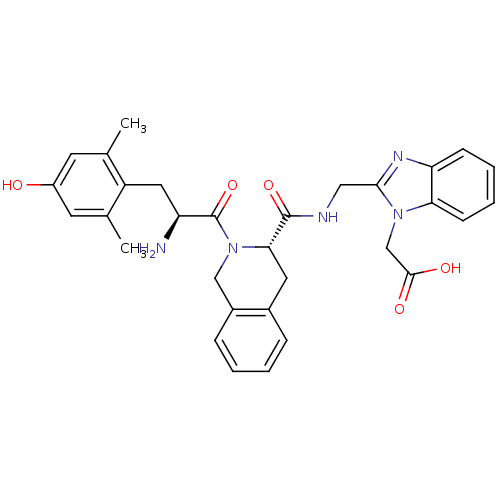 (2-(2-(((S)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari Curated by ChEMBL | Assay Description Displacement of [3H]deltorphin II from rat delta opioid receptor in brain P2 synaptosomes | J Med Chem 48: 8112-4 (2005) Article DOI: 10.1021/jm058259l BindingDB Entry DOI: 10.7270/Q24J0DQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50121649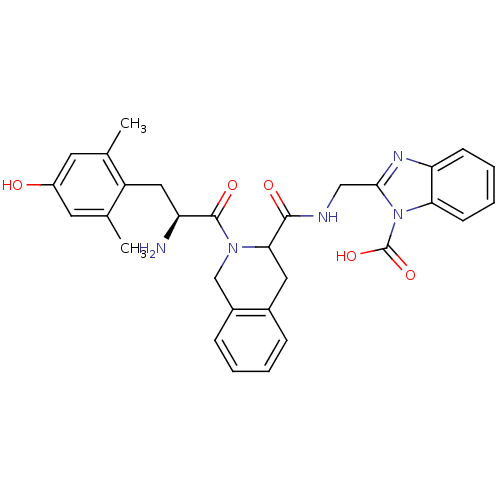 (2-[({2-[2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari Curated by ChEMBL | Assay Description Inhibition of [3H]DPDPE binding to Opioid receptor delta 1 of rat brain P2 synaptosomes | J Med Chem 45: 5556-63 (2002) BindingDB Entry DOI: 10.7270/Q2BR8SWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50194487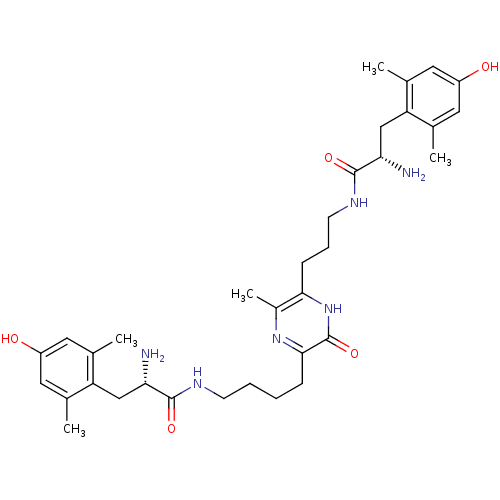 (3-[4'-(H-Dmt)-aminobutyl]-6-[3'-(H-Dmt)-aminopropy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat synaptosomes P2 fraction | Bioorg Med Chem Lett 16: 5793-6 (2006) Article DOI: 10.1016/j.bmcl.2006.08.079 BindingDB Entry DOI: 10.7270/Q26Q1WWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50379086 (CHEMBL2012521 | CHEMBL2012522 | LY-377604) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]Iodocyanopindolol from human adrenergic beta1 receptor expressed in insect sf9 cells by scintillation counting | ACS Med Chem Lett 2: 583-586 (2011) Article DOI: 10.1021/ml200071k BindingDB Entry DOI: 10.7270/Q20R9QDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor necrosis factor (Homo sapiens (Human)) | BDBM102777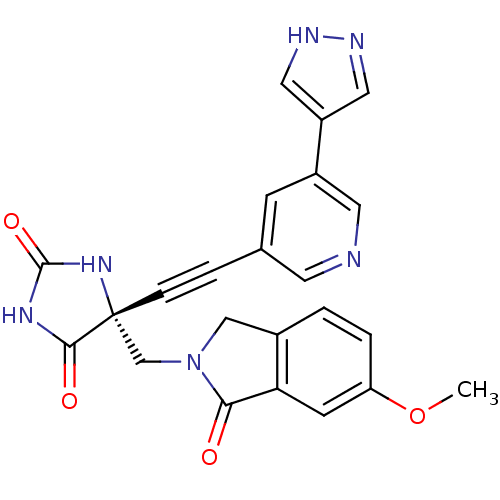 (US8541572, 2207) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0300 | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibition assay using TNF-alpha. | US Patent US8541572 (2013) BindingDB Entry DOI: 10.7270/Q2QC024J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor necrosis factor (Homo sapiens (Human)) | BDBM102624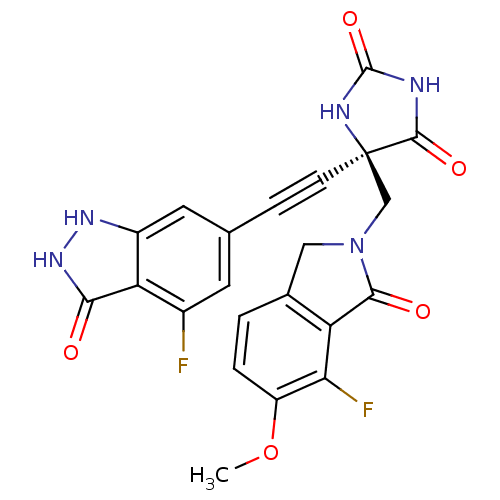 (US8541572, 303) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0300 | n/a | 383 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibition assay using TNF-alpha. | US Patent US8541572 (2013) BindingDB Entry DOI: 10.7270/Q2QC024J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50266025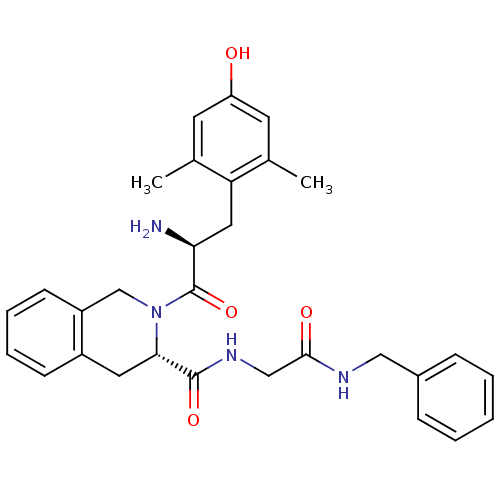 ((S)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussels Curated by ChEMBL | Assay Description Binding affinity to delta opioid receptor | J Med Chem 49: 3990-3 (2006) Article DOI: 10.1021/jm0603264 BindingDB Entry DOI: 10.7270/Q2474BPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50266025 ((S)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliary Curated by ChEMBL | Assay Description Binding affinity at Opioid receptor delta 1 using rat brain receptor (P2 synaptosome) assay | J Med Chem 45: 713-20 (2002) BindingDB Entry DOI: 10.7270/Q2639QFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50179191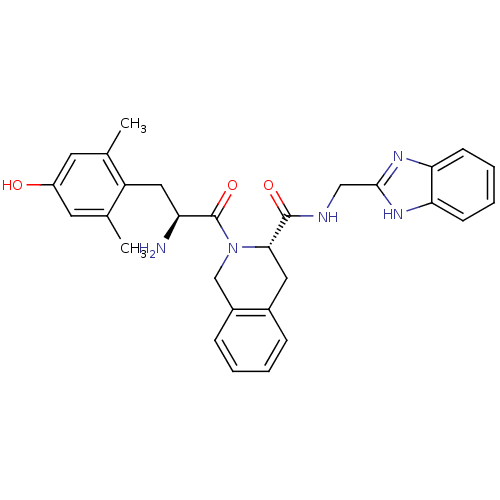 ((S)-N-((1H-benzo[d]imidazol-2-yl)methyl)-2-((S)-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliary Curated by ChEMBL | Assay Description Binding affinity at Opioid receptor delta 1 using rat brain receptor (P2 synaptosome) assay | J Med Chem 45: 713-20 (2002) BindingDB Entry DOI: 10.7270/Q2639QFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50027229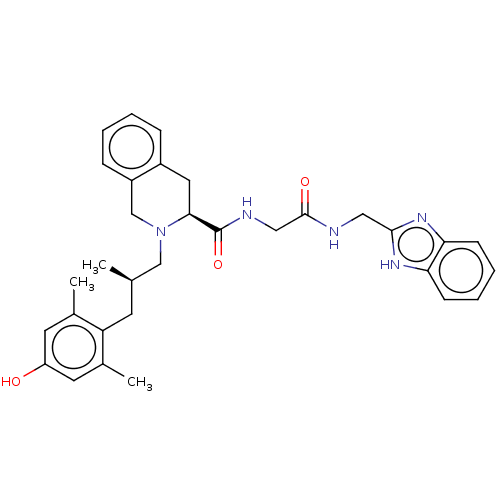 (CHEMBL2375157) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussels Curated by ChEMBL | Assay Description Binding affinity to delta opioid receptor | J Med Chem 49: 3990-3 (2006) Article DOI: 10.1021/jm0603264 BindingDB Entry DOI: 10.7270/Q2474BPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50179191 ((S)-N-((1H-benzo[d]imidazol-2-yl)methyl)-2-((S)-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari Curated by ChEMBL | Assay Description Inhibition of [3H]DPDPE binding to Opioid receptor delta 1 of rat brain P2 synaptosomes | J Med Chem 45: 5556-63 (2002) BindingDB Entry DOI: 10.7270/Q2BR8SWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50157686 (5-[({5-[(2S)-2-({2-[(2S)-2-amino-3-(4-hydroxy-2,6-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliary Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in rat brain synaptosomes P2 fraction | J Med Chem 47: 6541-6 (2004) Article DOI: 10.1021/jm040128h BindingDB Entry DOI: 10.7270/Q28W3CS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50179191 ((S)-N-((1H-benzo[d]imidazol-2-yl)methyl)-2-((S)-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari Curated by ChEMBL | Assay Description Displacement of [3H]deltorphin II from rat delta opioid receptor in brain P2 synaptosomes | J Med Chem 48: 8112-4 (2005) Article DOI: 10.1021/jm058259l BindingDB Entry DOI: 10.7270/Q24J0DQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17428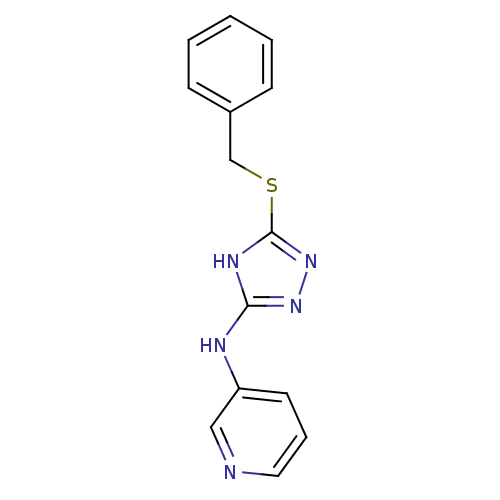 (1,2,4-Triazole Compound, 86 | N-[5-(benzylsulfanyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17355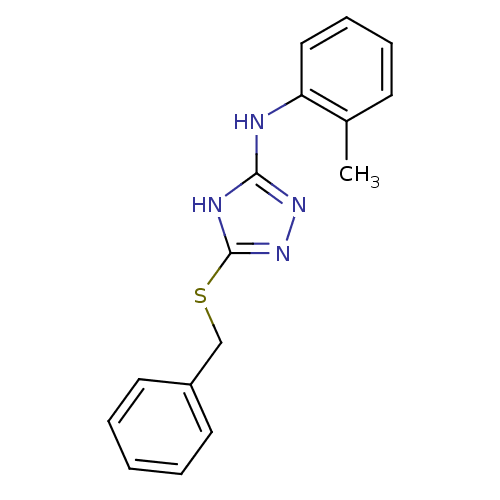 (1,2,4-Triazole Compound, 13 | 5-(benzylsulfanyl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50083160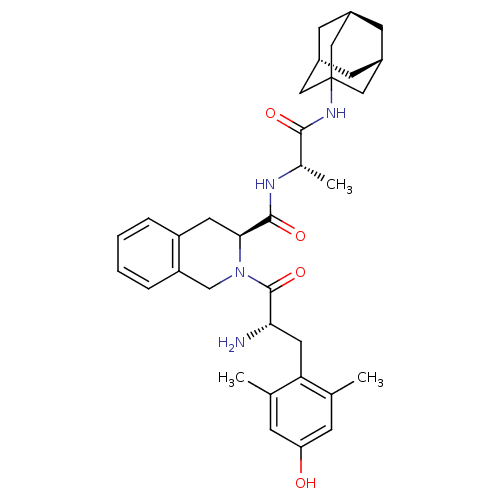 ((S)-2-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara Curated by ChEMBL | Assay Description Binding affinity at Opioid receptor delta 1 from rat brain synaptosomal preparations by H-Dmt-Tic-OH displacement. | J Med Chem 42: 5010-9 (2000) BindingDB Entry DOI: 10.7270/Q2VX0FQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50130611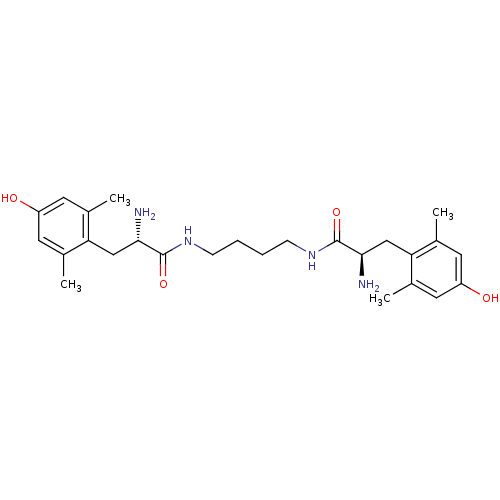 (2-Amino-N-{4-[2-amino-3-(4-hydroxy-2,6-dimethyl-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor mu 1 using [3H]-DAGO in rat brain P2 synaptosomal preparation | J Med Chem 46: 3201-9 (2003) Article DOI: 10.1021/jm020459z BindingDB Entry DOI: 10.7270/Q28C9VMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50272081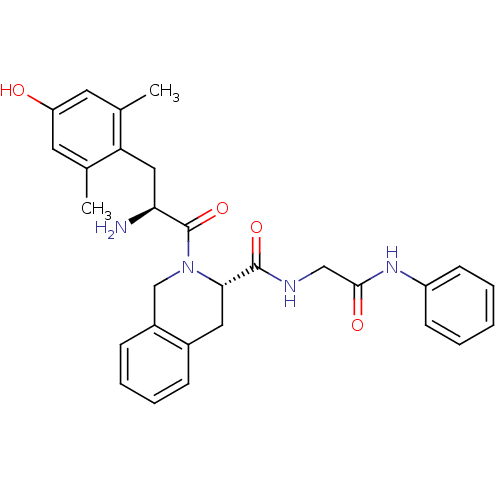 (2-[2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-propi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussels Curated by ChEMBL | Assay Description Binding affinity to delta opioid receptor | J Med Chem 49: 3990-3 (2006) Article DOI: 10.1021/jm0603264 BindingDB Entry DOI: 10.7270/Q2474BPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50272081 (2-[2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-propi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliary Curated by ChEMBL | Assay Description Binding affinity at Opioid receptor delta 1 using rat brain receptor (P2 synaptosome) assay | J Med Chem 45: 713-20 (2002) BindingDB Entry DOI: 10.7270/Q2639QFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50221787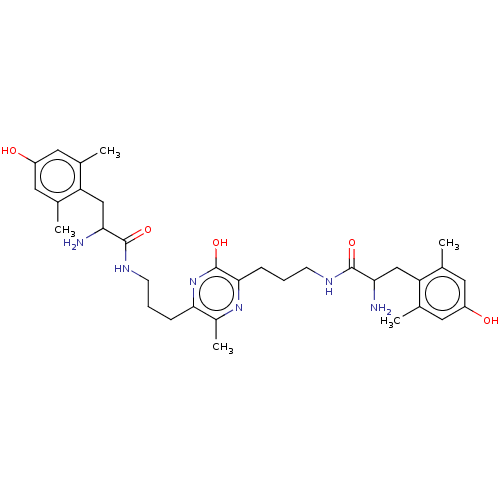 (CHEMBL3216418) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Environmental Health Sciences Curated by ChEMBL | Assay Description Compound was tested for Opioid receptor mu 1 agonism in isolated tissues from guinea pig ileum | J Med Chem 47: 2599-610 (2004) Article DOI: 10.1021/jm0304616 BindingDB Entry DOI: 10.7270/Q2RB755V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50121648 (2-({2-[2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari Curated by ChEMBL | Assay Description Inhibition of [3H]DPDPE binding to Opioid receptor delta 1 of rat brain P2 synaptosomes | J Med Chem 45: 5556-63 (2002) BindingDB Entry DOI: 10.7270/Q2BR8SWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50212613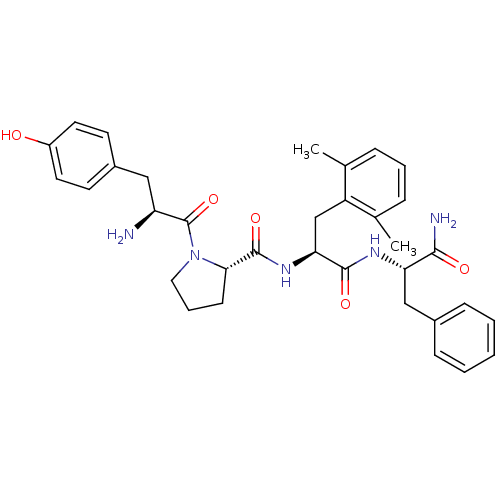 (CHEMBL266122 | Tyr-Pro-Dmp-Phe-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain P2 synaptosome membrane | J Med Chem 50: 2753-66 (2007) Article DOI: 10.1021/jm061238m BindingDB Entry DOI: 10.7270/Q25B0264 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50049378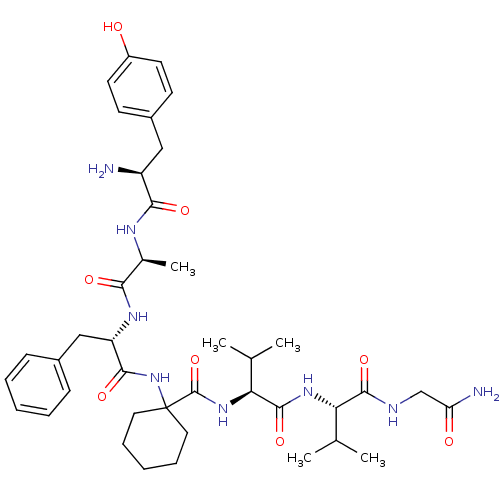 (1-((S)-2-{(S)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara Curated by ChEMBL | Assay Description Displacement of [3H]-DPDPE (0.63 nM) from Opioid receptor delta 1 | J Med Chem 39: 773-80 (1996) Article DOI: 10.1021/jm950490j BindingDB Entry DOI: 10.7270/Q2QV3KKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50121645 (2-[(2S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari Curated by ChEMBL | Assay Description Inhibition of [3H]DPDPE binding to Opioid receptor delta 1 of rat brain P2 synaptosomes | J Med Chem 45: 5556-63 (2002) BindingDB Entry DOI: 10.7270/Q2BR8SWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17388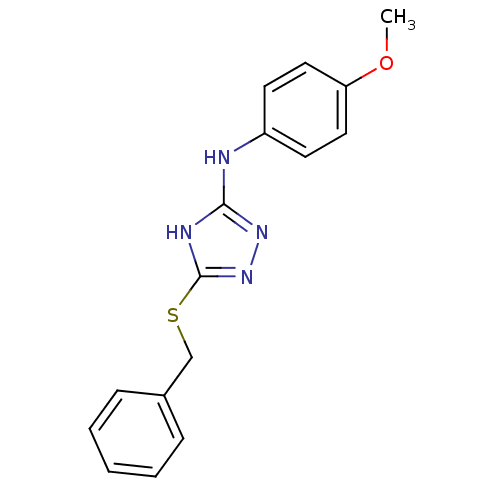 (1,2,4-Triazole Compound, 46 | 5-(benzylsulfanyl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50130617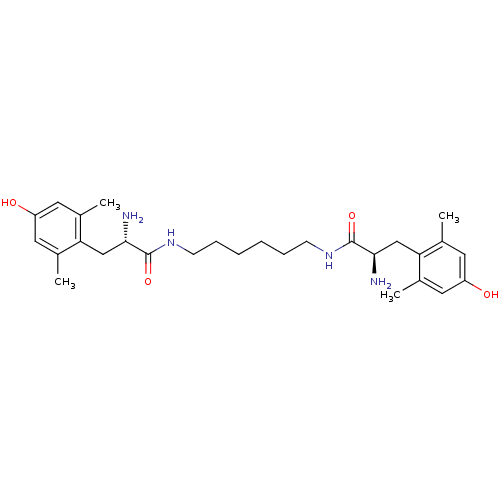 (2-Amino-N-{6-[2-amino-3-(4-hydroxy-2,6-dimethyl-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor mu 1 using [3H]-DAGO in rat brain P2 synaptosomal preparation | J Med Chem 46: 3201-9 (2003) Article DOI: 10.1021/jm020459z BindingDB Entry DOI: 10.7270/Q28C9VMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50194488 (6-[4'-(H-Dmt)-aminobutyl]-3-[3'-(H-Dmt)-aminopropy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat synaptosomes P2 fraction | Bioorg Med Chem Lett 16: 5793-6 (2006) Article DOI: 10.1016/j.bmcl.2006.08.079 BindingDB Entry DOI: 10.7270/Q26Q1WWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor necrosis factor (Homo sapiens (Human)) | BDBM102623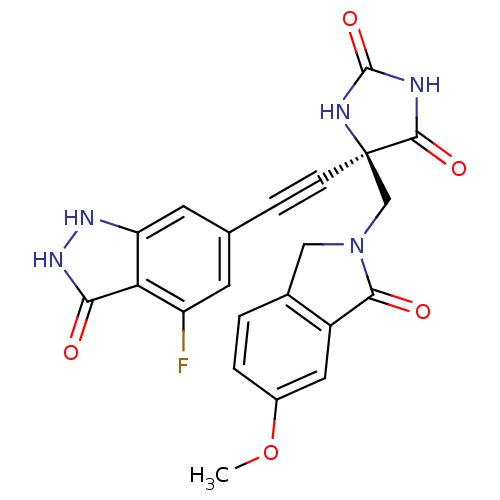 (US8541572, 302) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0521 | n/a | 723 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibition assay using TNF-alpha. | US Patent US8541572 (2013) BindingDB Entry DOI: 10.7270/Q2QC024J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor necrosis factor (Homo sapiens (Human)) | BDBM102666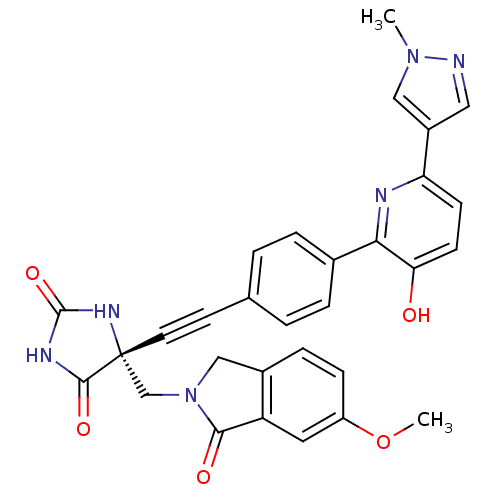 (US8541572, 973) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0524 | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibition assay using TNF-alpha. | US Patent US8541572 (2013) BindingDB Entry DOI: 10.7270/Q2QC024J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50199865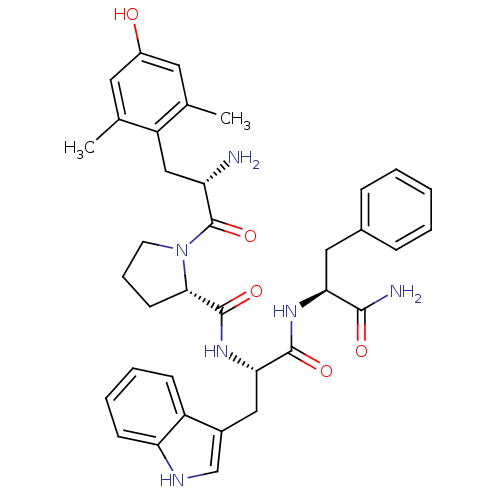 ((S)-N-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain P2 synaptosome | Bioorg Med Chem 15: 1237-51 (2007) Article DOI: 10.1016/j.bmc.2006.11.019 BindingDB Entry DOI: 10.7270/Q2348K1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50049376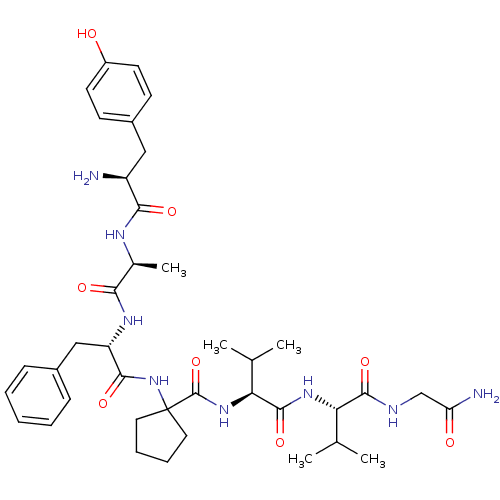 (1-((S)-2-{(S)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara Curated by ChEMBL | Assay Description Displacement of [3H]-DPDPE (0.63 nM) from Opioid receptor delta 1 | J Med Chem 39: 773-80 (1996) Article DOI: 10.1021/jm950490j BindingDB Entry DOI: 10.7270/Q2QV3KKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50049377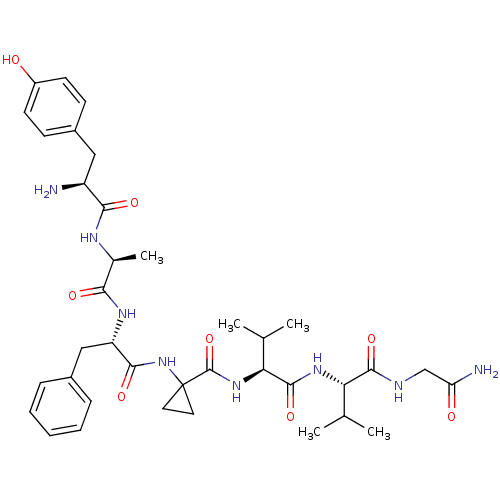 (1-((S)-2-{(S)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara Curated by ChEMBL | Assay Description Displacement of [3H]-DPDPE (0.63 nM) from Opioid receptor delta 1 | J Med Chem 39: 773-80 (1996) Article DOI: 10.1021/jm950490j BindingDB Entry DOI: 10.7270/Q2QV3KKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor necrosis factor (Homo sapiens (Human)) | BDBM102663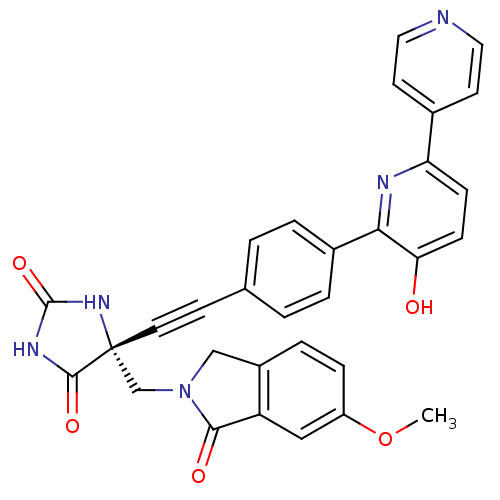 (US8541572, 970) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0556 | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibition assay using TNF-alpha. | US Patent US8541572 (2013) BindingDB Entry DOI: 10.7270/Q2QC024J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50083154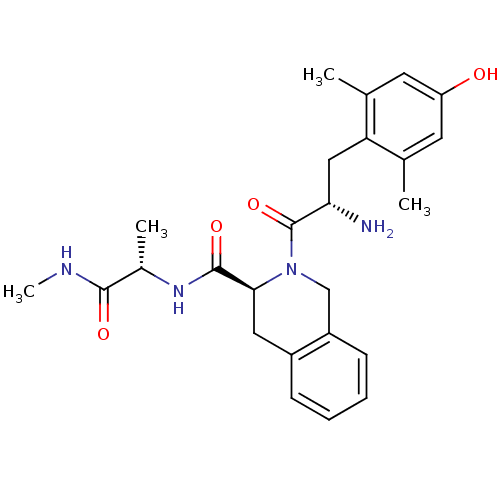 ((S)-2-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara Curated by ChEMBL | Assay Description Binding affinity at Opioid receptor delta 1 from rat brain synaptosomal preparations by [3H]N,N-(Me)2-Dmt-Tic-OH displacement. | J Med Chem 42: 5010-9 (2000) BindingDB Entry DOI: 10.7270/Q2VX0FQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor necrosis factor (Homo sapiens (Human)) | BDBM102802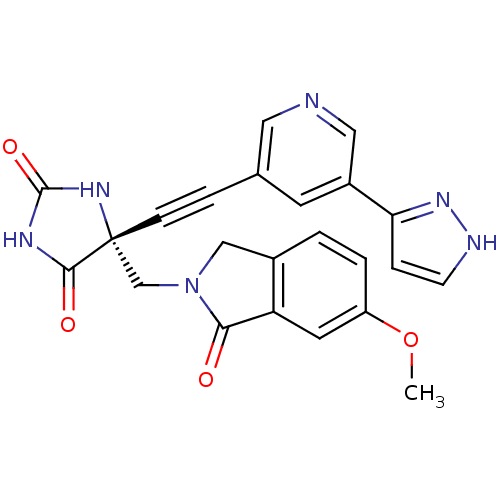 (US8541572, 2234) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0600 | n/a | 678 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibition assay using TNF-alpha. | US Patent US8541572 (2013) BindingDB Entry DOI: 10.7270/Q2QC024J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50157684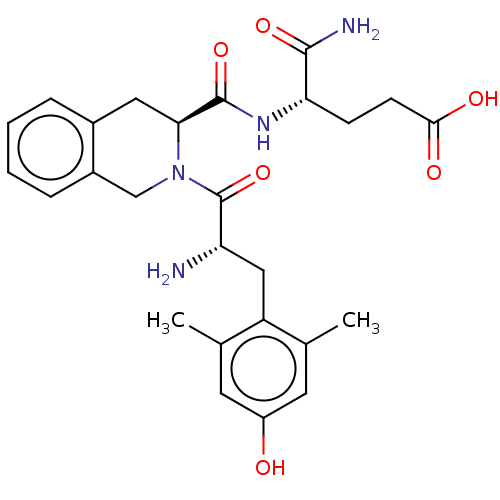 (CHEMBL2369958 | H-Dmt-Tic-Glu-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliary Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain synaptosomes P2 fraction | J Med Chem 47: 6541-6 (2004) Article DOI: 10.1021/jm040128h BindingDB Entry DOI: 10.7270/Q28W3CS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50332270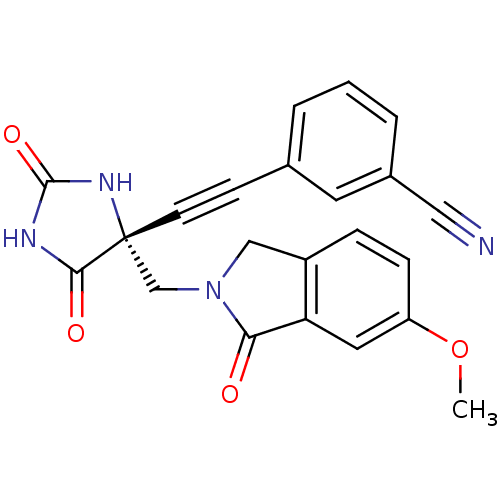 ((R)-3-((4-((6-methoxy-1-oxoisoindolin-2-yl)methyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of TACE | Bioorg Med Chem Lett 20: 7283-7 (2010) Article DOI: 10.1016/j.bmcl.2010.10.081 BindingDB Entry DOI: 10.7270/Q2Z89CP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50178759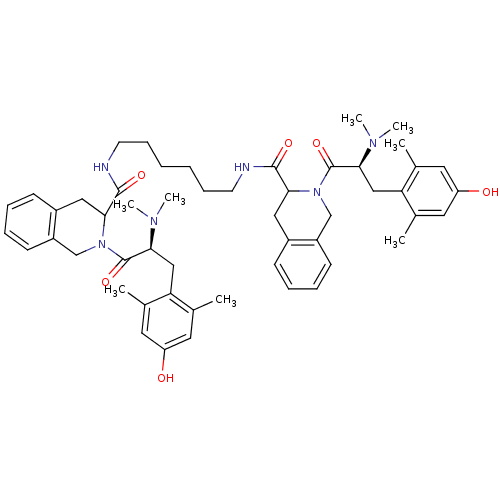 (1,6-bis-(N,N-dimethyl-Dmt-Tic-NH)hexane | CHEMBL37...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University Curated by ChEMBL | Assay Description Displacement of [3H]deltorphin-II from delta opioid receptor in rat brain synaptosomal membranes | J Med Chem 48: 8035-44 (2005) Article DOI: 10.1021/jm050377l BindingDB Entry DOI: 10.7270/Q2C53KD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50157684 (CHEMBL2369958 | H-Dmt-Tic-Glu-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliary Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in rat brain synaptosomes P2 fraction | J Med Chem 47: 6541-6 (2004) Article DOI: 10.1021/jm040128h BindingDB Entry DOI: 10.7270/Q28W3CS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor necrosis factor (Homo sapiens (Human)) | BDBM102680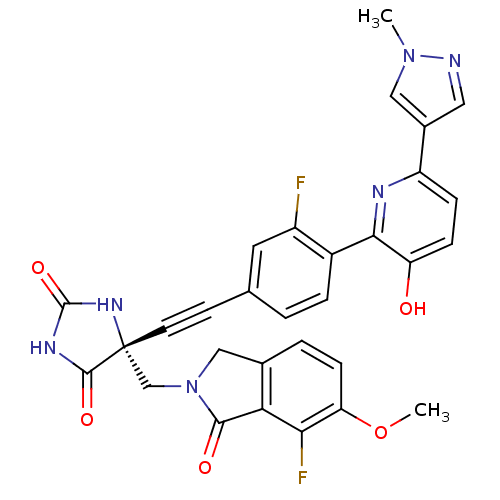 (US8541572, 987) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0627 | n/a | 75.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibition assay using TNF-alpha. | US Patent US8541572 (2013) BindingDB Entry DOI: 10.7270/Q2QC024J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor necrosis factor (Homo sapiens (Human)) | BDBM102845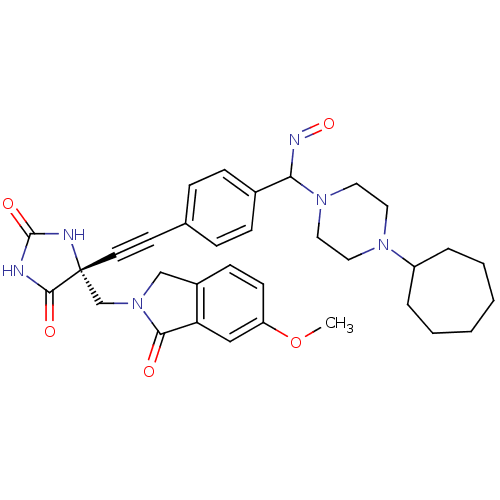 (US8541572, 927) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0630 | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibition assay using TNF-alpha. | US Patent US8541572 (2013) BindingDB Entry DOI: 10.7270/Q2QC024J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50159681 ((S)-1-[2-Amino-3-(2-ethyl-4-hydroxy-6-methyl-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO binding to mu-Opioid receptor | J Med Chem 48: 586-92 (2005) Article DOI: 10.1021/jm049384k BindingDB Entry DOI: 10.7270/Q26T0M40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50121645 (2-[(2S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari Curated by ChEMBL | Assay Description Inhibition of [3H]DPDPE binding to Opioid receptor delta 1 of rat brain P2 synaptosomes | J Med Chem 45: 5556-63 (2002) BindingDB Entry DOI: 10.7270/Q2BR8SWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor necrosis factor (Homo sapiens (Human)) | BDBM102664 (US8541572, 971) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0651 | n/a | 80.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Inhibition assay using TNF-alpha. | US Patent US8541572 (2013) BindingDB Entry DOI: 10.7270/Q2QC024J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50083169 ((S)-2-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara Curated by ChEMBL | Assay Description Binding affinity at Opioid receptor delta 1 from rat brain synaptosomal preparations by [3H]N,N-(Me)2-Dmt-Tic-OH displacement. | J Med Chem 42: 5010-9 (2000) BindingDB Entry DOI: 10.7270/Q2VX0FQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50108919 (2-[2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-propi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari Curated by ChEMBL | Assay Description Inhibition of [3H]DPDPE binding to Opioid receptor delta 1 of rat brain P2 synaptosomes | J Med Chem 45: 5556-63 (2002) BindingDB Entry DOI: 10.7270/Q2BR8SWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50108919 (2-[2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-propi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliary Curated by ChEMBL | Assay Description Binding affinity at Opioid receptor delta 1 using rat brain receptor (P2 synaptosome) assay | J Med Chem 45: 713-20 (2002) BindingDB Entry DOI: 10.7270/Q2639QFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50212609 (CHEMBL228409 | Dmt-Pro-Dmp-Phe-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain P2 synaptosome membrane | J Med Chem 50: 2753-66 (2007) Article DOI: 10.1021/jm061238m BindingDB Entry DOI: 10.7270/Q25B0264 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001459 (3-[(2-{2-[2-Amino-3-(4-hydroxy-phenyl)-propionylam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara Curated by ChEMBL | Assay Description Tested for inhibition of amplification of electrically induced twitch in guinea pig ileum (GPI) | J Med Chem 36: 3748-56 (1994) BindingDB Entry DOI: 10.7270/Q2D799HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 15513 total ) | Next | Last >> |