

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189451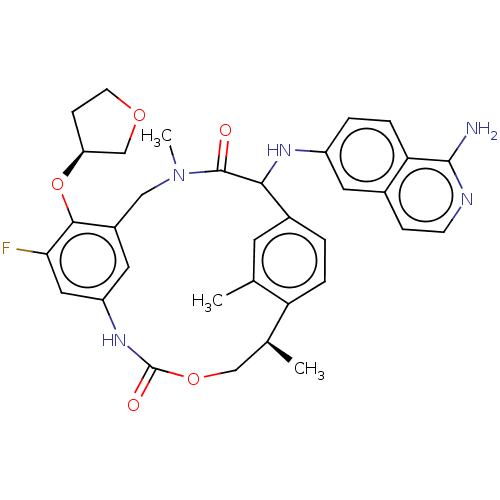 (US9174974, Example 39) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50254445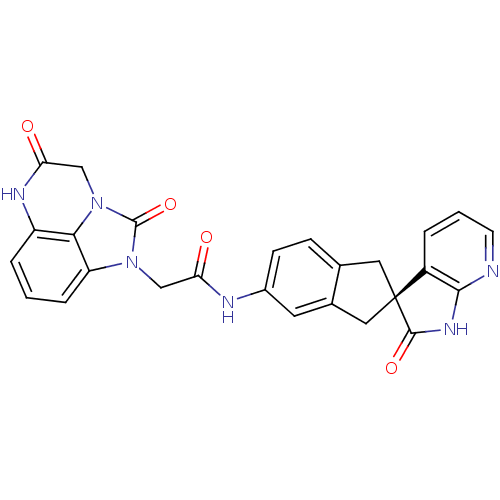 ((S)-2-(2,5-dioxo-2,4,5,6-tetrahydro-1H-imidazo[1,5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co Curated by ChEMBL | Assay Description Displacement of [125I]hCGRP from human CGRP receptor expressed in HEK293 cells coexpressing RAMP1 | Bioorg Med Chem Lett 19: 214-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.106 BindingDB Entry DOI: 10.7270/Q21C1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189454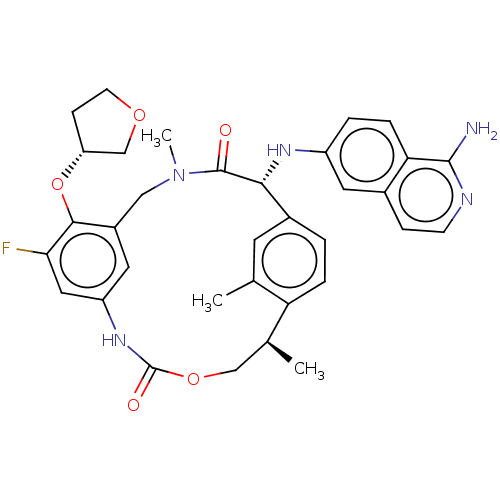 (US9174974, Example 38) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vesicular acetylcholine transporter (Homo sapiens (Human)) | BDBM50039613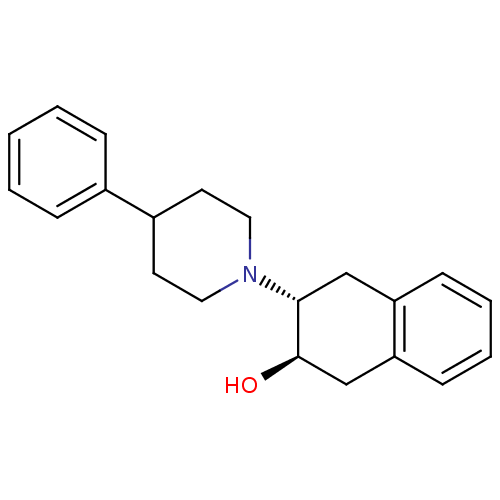 ((2R,3R)-3-(4-Phenyl-piperidin-1-yl)-1,2,3,4-tetrah...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University Curated by ChEMBL | Assay Description Binding affinity to VAChT | Bioorg Med Chem 20: 4422-9 (2012) Article DOI: 10.1016/j.bmc.2012.05.045 BindingDB Entry DOI: 10.7270/Q29K4C9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM50192770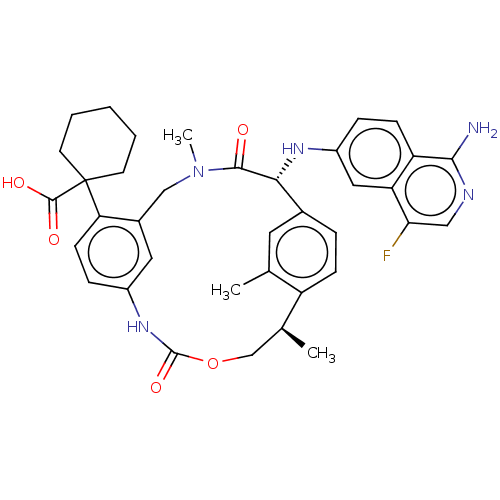 (CHEMBL3956096) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Inhibition of recombinant human TF-factor 7a using factor 10 as substrate at 37 degC | Bioorg Med Chem Lett 26: 5051-5057 (2016) Article DOI: 10.1016/j.bmcl.2016.08.088 BindingDB Entry DOI: 10.7270/Q24X59Q6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50364330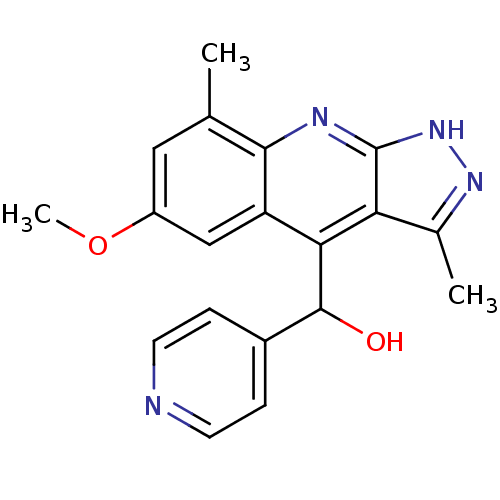 (CHEMBL1949936) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE10A assessed as hydrolysis of cAMP to AMP using [3H]cAMP substrate by scintillation proximity assay | Bioorg Med Chem Lett 22: 1335-9 (2012) Article DOI: 10.1016/j.bmcl.2011.12.080 BindingDB Entry DOI: 10.7270/Q2RF5VGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50607169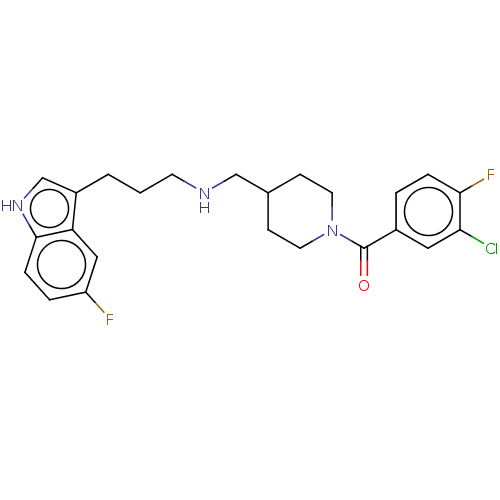 (CHEMBL5220371) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.129006 BindingDB Entry DOI: 10.7270/Q2ZC8701 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50364327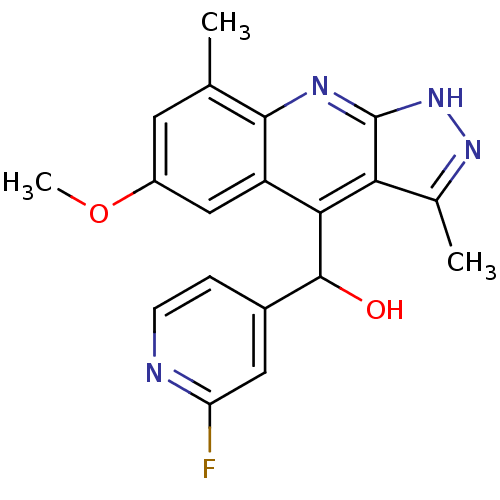 (CHEMBL1949939) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE10A assessed as hydrolysis of cAMP to AMP using [3H]cAMP substrate by scintillation proximity assay | Bioorg Med Chem Lett 22: 1335-9 (2012) Article DOI: 10.1016/j.bmcl.2011.12.080 BindingDB Entry DOI: 10.7270/Q2RF5VGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50600748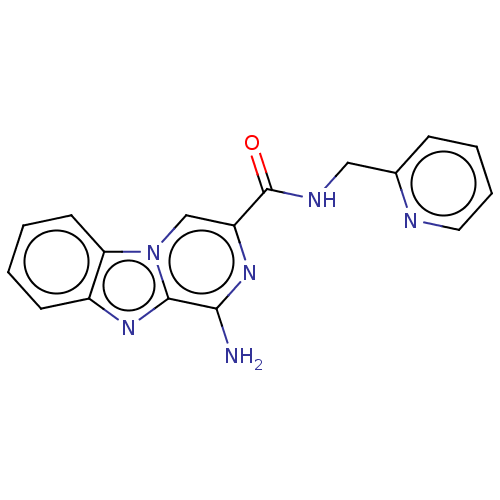 (CHEMBL5176737) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00101 BindingDB Entry DOI: 10.7270/Q2417231 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Rattus norvegicus) | BDBM50165931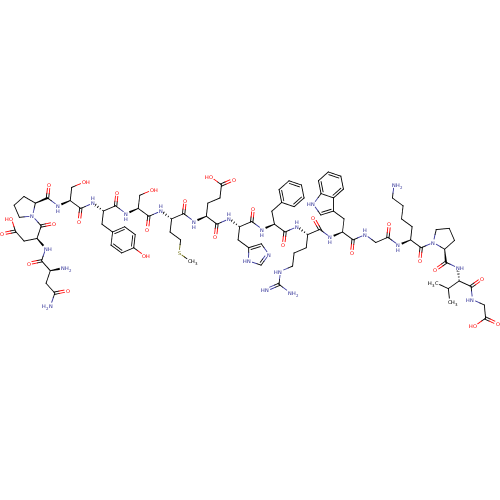 (CHEMBL415165 | NDP-SYSMEHFRWGKPVG) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for rat melanocortin-4 receptor | J Med Chem 48: 3095-8 (2005) Article DOI: 10.1021/jm0501432 BindingDB Entry DOI: 10.7270/Q2251HQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040861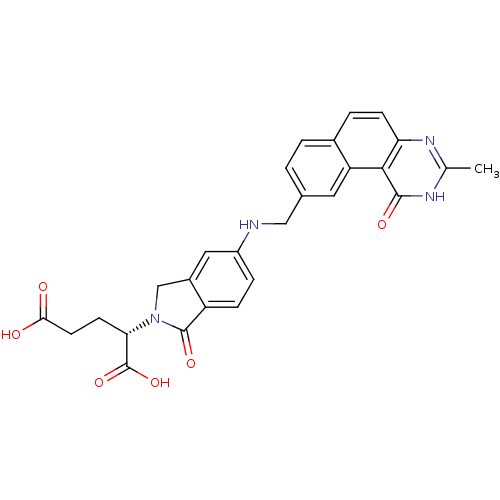 ((S)-2-(5-(((1,2-DIHYDRO-3-METHYL-1-OXOBENZO(F)QUIN...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of TS by spectrophotometry | Bioorg Med Chem 19: 3585-94 (2011) Article DOI: 10.1016/j.bmc.2011.03.067 BindingDB Entry DOI: 10.7270/Q22V2GGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50165935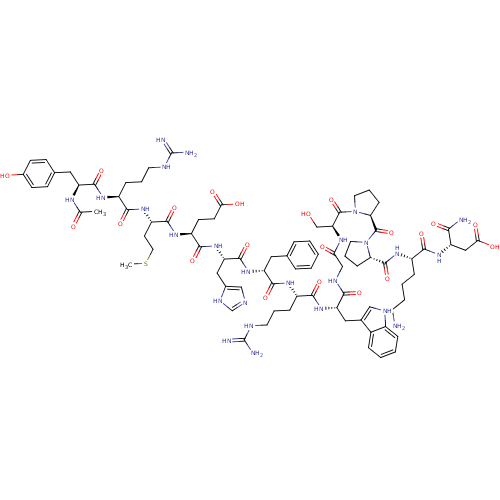 (Ac-YRMEHdFRWGSPPKD-NH2 | CHEMBL414718) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-1 receptor expressed in HEK293 cells | J Med Chem 48: 3095-8 (2005) Article DOI: 10.1021/jm0501432 BindingDB Entry DOI: 10.7270/Q2251HQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50607170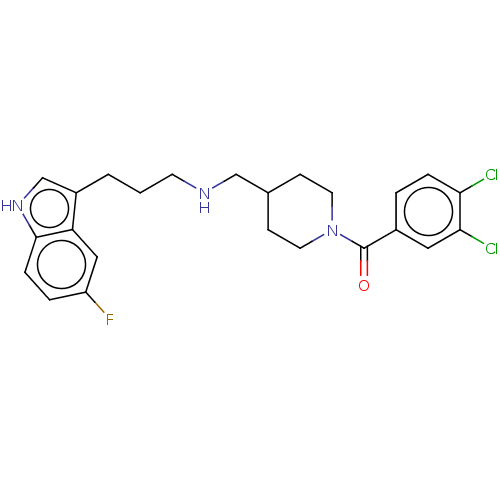 (CHEMBL5220872) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.129006 BindingDB Entry DOI: 10.7270/Q2ZC8701 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50165931 (CHEMBL415165 | NDP-SYSMEHFRWGKPVG) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-1 receptor expressed in HEK293 cells | J Med Chem 48: 3095-8 (2005) Article DOI: 10.1021/jm0501432 BindingDB Entry DOI: 10.7270/Q2251HQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM197114 (US9216182, 1.60) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ... | US Patent US9216182 (2015) BindingDB Entry DOI: 10.7270/Q2125RGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50165935 (Ac-YRMEHdFRWGSPPKD-NH2 | CHEMBL414718) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-3 receptor expressed in HEK293 cells | J Med Chem 48: 3095-8 (2005) Article DOI: 10.1021/jm0501432 BindingDB Entry DOI: 10.7270/Q2251HQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189450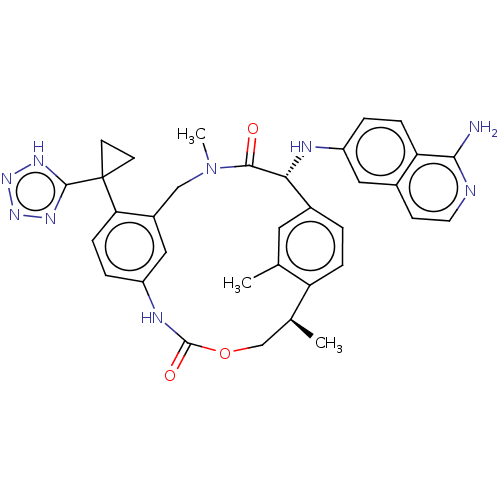 (US9174974, Example 36) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189447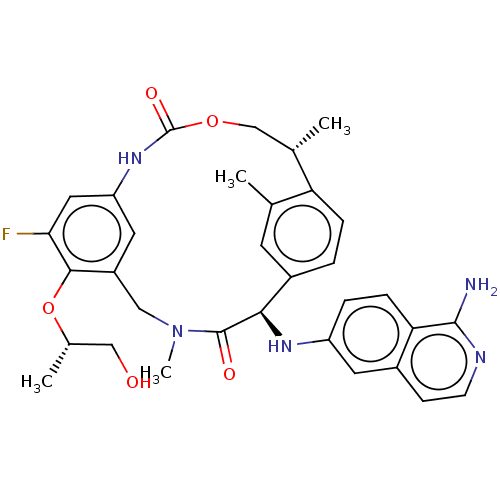 (US9174974, Example 33) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189448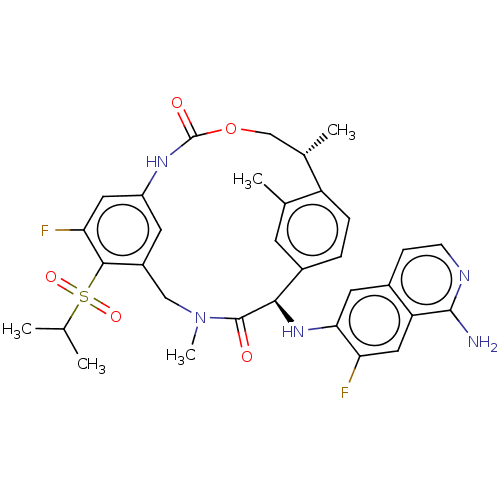 (US9174974, Example 34) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM50192768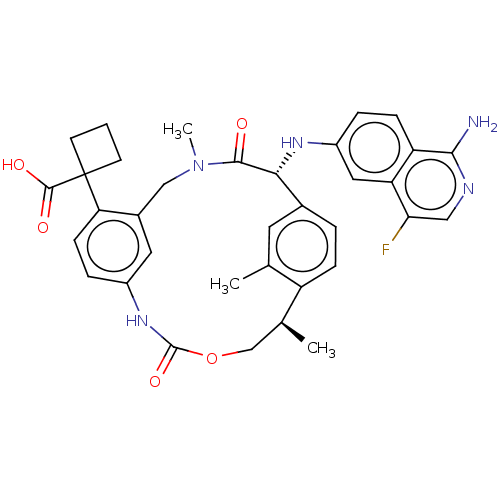 (CHEMBL3900166) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Inhibition of recombinant human TF-factor 7a using factor 10 as substrate at 37 degC | Bioorg Med Chem Lett 26: 5051-5057 (2016) Article DOI: 10.1016/j.bmcl.2016.08.088 BindingDB Entry DOI: 10.7270/Q24X59Q6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189453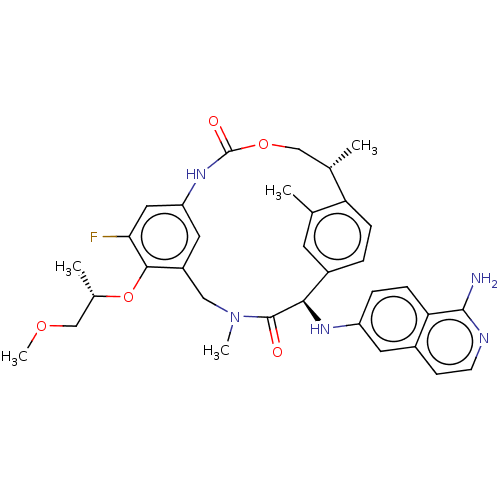 (US9174974, Example 37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50165929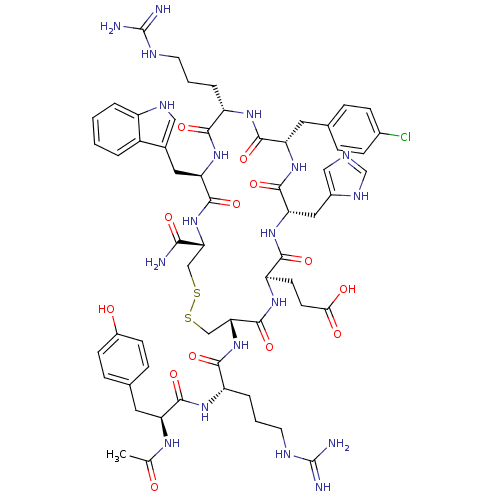 (Ac-YR[CEH(pCl-dF)RWC]-NH2 | CHEMBL415661) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells | J Med Chem 48: 3095-8 (2005) Article DOI: 10.1021/jm0501432 BindingDB Entry DOI: 10.7270/Q2251HQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189449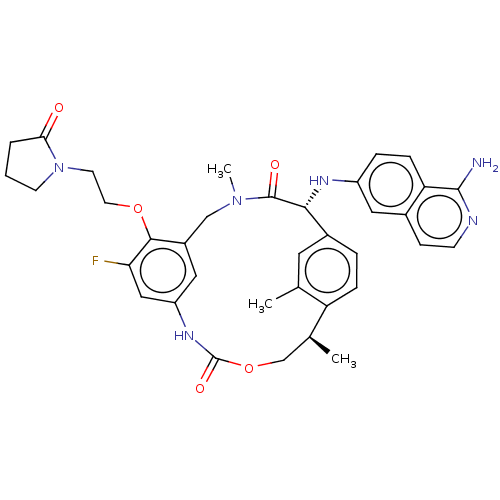 (US9174974, Example 35) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189446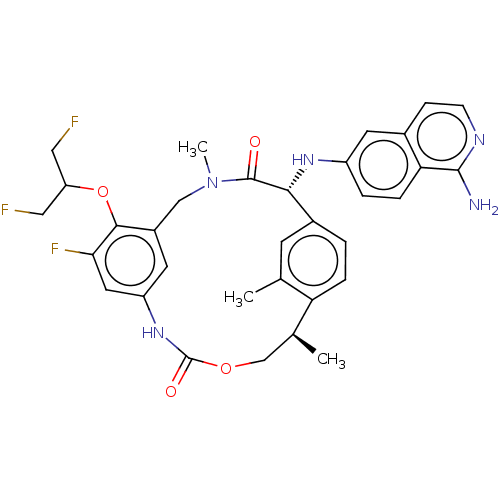 (US9174974, Example 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM123054 (US8742134, (1)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.160 | -55.3 | 0.230 | n/a | n/a | n/a | n/a | n/a | 22 |
Theron Pharmaceuticals, Inc. US Patent | Assay Description Radioligand binding studies were carried out with M3 receptor cell homogenates as described (Peralta et al., The EMBO Journal 6, 3923-3929, (1987)). ... | US Patent US8742134 (2014) BindingDB Entry DOI: 10.7270/Q20000SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM189445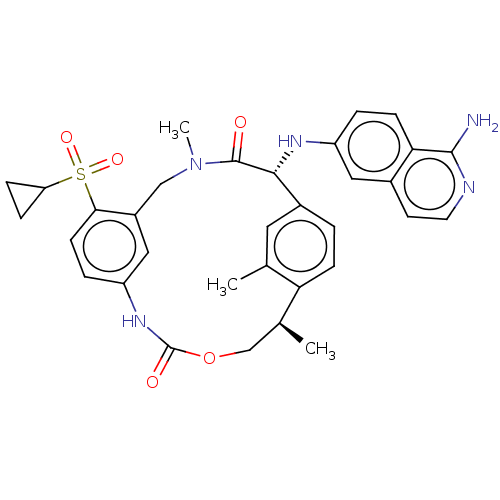 (US9174974, Example 31) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Inhibition of human TF-factor 7a (366 to 11 residues) using factor 10 as substrate after 60 mins | Bioorg Med Chem Lett 26: 5051-5057 (2016) Article DOI: 10.1016/j.bmcl.2016.08.088 BindingDB Entry DOI: 10.7270/Q24X59Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM189445 (US9174974, Example 31) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of full-length human TF/recombinant human factor 7a assessed as decrease in conversion of factor 10 to factor 10a by measuring S2765 hydro... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189443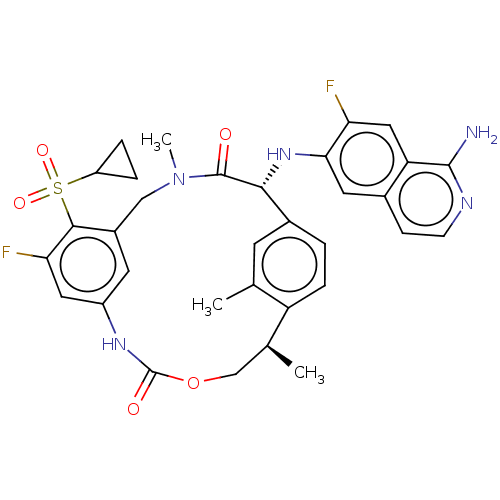 (US9174974, Example 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50165931 (CHEMBL415165 | NDP-SYSMEHFRWGKPVG) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-5 receptor expressed in HEK293 cells | J Med Chem 48: 3095-8 (2005) Article DOI: 10.1021/jm0501432 BindingDB Entry DOI: 10.7270/Q2251HQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189445 (US9174974, Example 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189444 (US9174974, Example 30) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM123055 (US8742134, (2) | US8742134, (7)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.180 | -55.1 | 0.25 | n/a | n/a | n/a | n/a | n/a | 22 |
Theron Pharmaceuticals, Inc. US Patent | Assay Description Radioligand binding studies were carried out with M3 receptor cell homogenates as described (Peralta et al., The EMBO Journal 6, 3923-3929, (1987)). ... | US Patent US8742134 (2014) BindingDB Entry DOI: 10.7270/Q20000SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM123056 (US8742134, (3) | US8742134, (8)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.180 | -55.1 | 0.25 | n/a | n/a | n/a | n/a | n/a | 22 |
Theron Pharmaceuticals, Inc. US Patent | Assay Description Radioligand binding studies were carried out with M3 receptor cell homogenates as described (Peralta et al., The EMBO Journal 6, 3923-3929, (1987)). ... | US Patent US8742134 (2014) BindingDB Entry DOI: 10.7270/Q20000SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001023 ((2R,6R,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu using [3H]-DAMGO as radioligand. | J Med Chem 35: 2812-8 (1992) BindingDB Entry DOI: 10.7270/Q29W0G3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM26236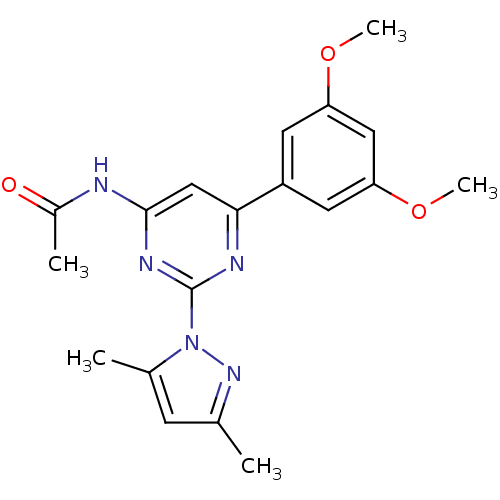 (CHEMBL487569 | N-[6-(3,5-dimethoxyphenyl)-2-(3,5-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | -54.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Neurocrine Bioscience | Assay Description The membranes prepared from HEK cells transfected with adenosine receptors were used in binding assays. Nonspecific binding was determined in the pre... | J Med Chem 51: 7099-7110 (2008) Article DOI: 10.1021/jm800851u BindingDB Entry DOI: 10.7270/Q20R9MQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM197113 (US9216182, 1.59) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ... | US Patent US9216182 (2015) BindingDB Entry DOI: 10.7270/Q2125RGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM197116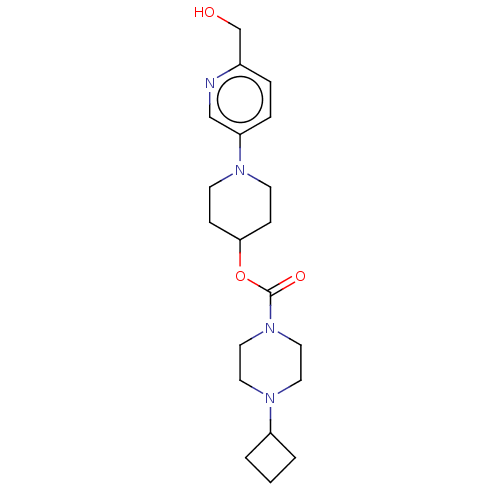 (US9216182, 1.62) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ... | US Patent US9216182 (2015) BindingDB Entry DOI: 10.7270/Q2125RGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM197117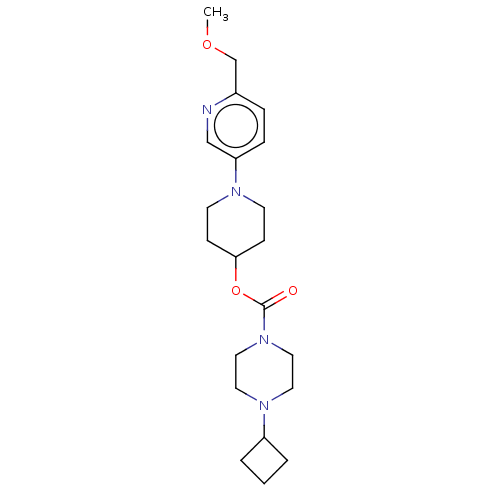 (US9216182, 1.63) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The potency of compounds of the invention as H3 receptor antagonists can be assessed by measuring the blockade of (R)-alpha-methylhistamine-mediated ... | US Patent US9216182 (2015) BindingDB Entry DOI: 10.7270/Q2125RGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189452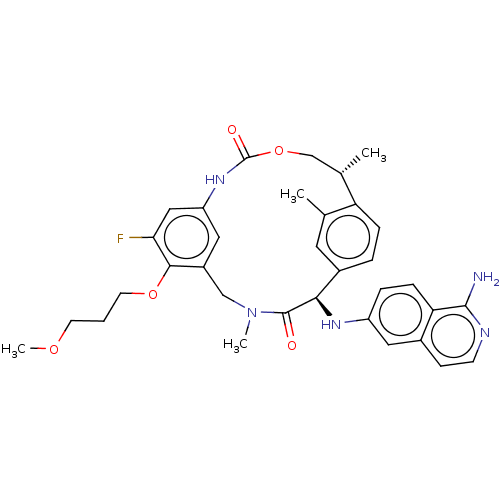 (US9174974, Example 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50364339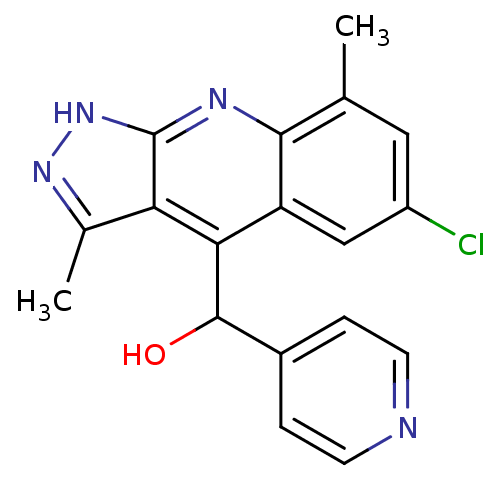 (CHEMBL1950085) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE10A assessed as hydrolysis of cAMP to AMP using [3H]cAMP substrate by scintillation proximity assay | Bioorg Med Chem Lett 22: 1335-9 (2012) Article DOI: 10.1016/j.bmcl.2011.12.080 BindingDB Entry DOI: 10.7270/Q2RF5VGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50237067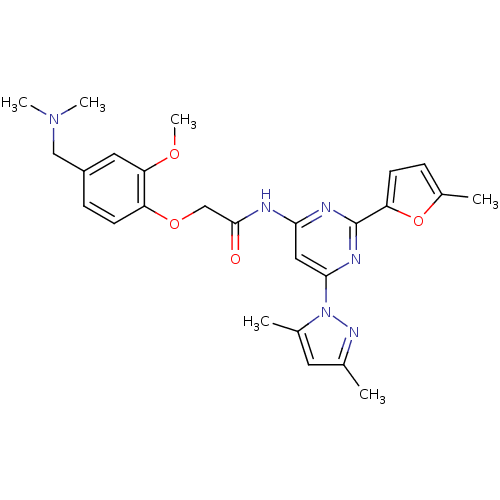 (CHEMBL429125 | N-(6-(3,5-dimethyl-1H-pyrazol-1-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 1778-83 (2008) Article DOI: 10.1016/j.bmcl.2008.02.032 BindingDB Entry DOI: 10.7270/Q2VD6Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50529312 (CHEMBL4444213) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.129006 BindingDB Entry DOI: 10.7270/Q2ZC8701 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50372562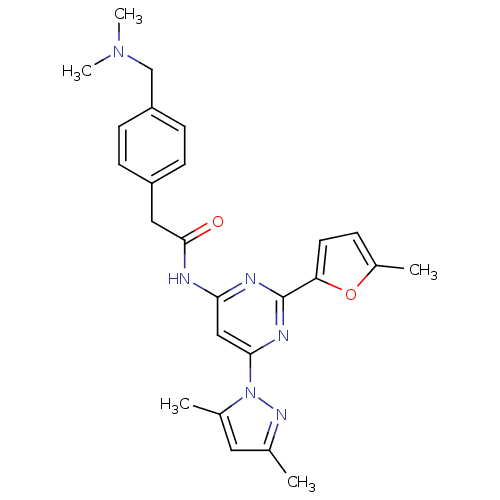 (CHEMBL255739) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 1269-73 (2008) Article DOI: 10.1016/j.bmcl.2008.01.036 BindingDB Entry DOI: 10.7270/Q2K35VGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001019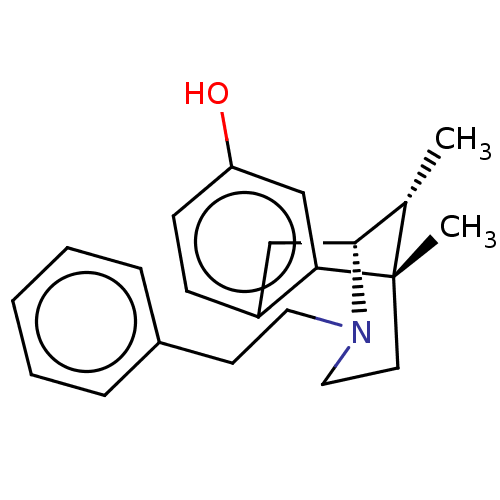 (6,11-Dimethyl-3-phenethyl-1,2,3,4,5,6-hexahydro-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu using [3H]-DAMGO as radioligand. | J Med Chem 35: 2812-8 (1992) BindingDB Entry DOI: 10.7270/Q29W0G3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM26236 (CHEMBL487569 | N-[6-(3,5-dimethoxyphenyl)-2-(3,5-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 5402-5 (2008) Article DOI: 10.1016/j.bmcl.2008.09.048 BindingDB Entry DOI: 10.7270/Q28K78ZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50255069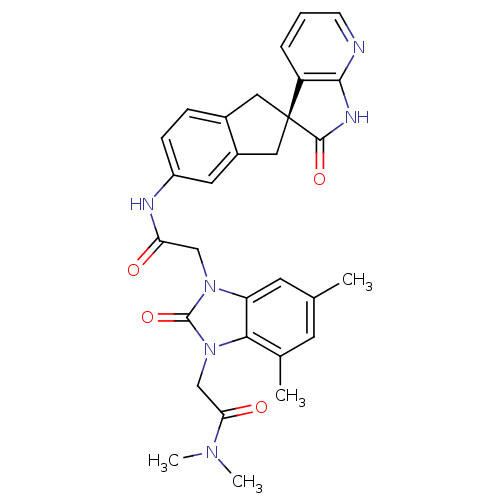 ((S)-2-(5,7-dimethyl-2-oxo-3-(2-oxo-2-(2'-oxo-1,1',...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co Curated by ChEMBL | Assay Description Displacement of [125I]hCGRP from human CGRP receptor expressed in HEK293 cells coexpressing RAMP1 | Bioorg Med Chem Lett 19: 214-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.106 BindingDB Entry DOI: 10.7270/Q21C1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50165932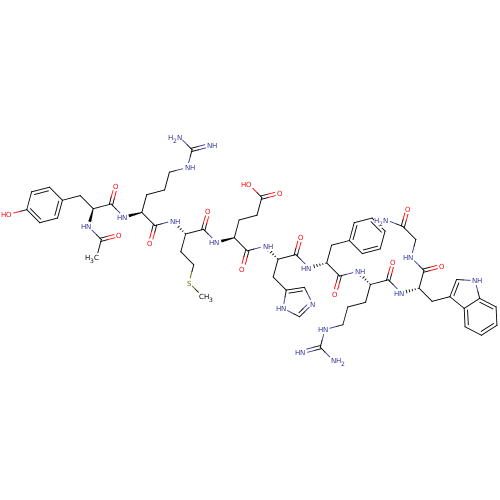 (Ac-YRMEHdFRWG-NH2 | CHEMBL266879) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-1 receptor expressed in HEK293 cells | J Med Chem 48: 3095-8 (2005) Article DOI: 10.1021/jm0501432 BindingDB Entry DOI: 10.7270/Q2251HQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM123057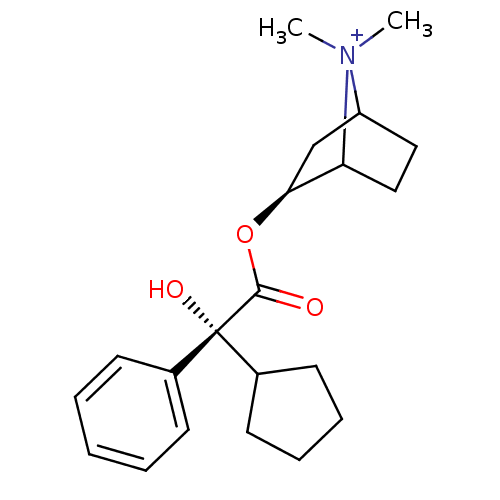 (US8742134, (4) | US8742134, (5) | US8742134, (6)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.220 | -54.6 | 0.300 | n/a | n/a | n/a | n/a | n/a | 22 |
Theron Pharmaceuticals, Inc. US Patent | Assay Description Radioligand binding studies were carried out with M3 receptor cell homogenates as described (Peralta et al., The EMBO Journal 6, 3923-3929, (1987)). ... | US Patent US8742134 (2014) BindingDB Entry DOI: 10.7270/Q20000SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vesicular acetylcholine transporter (Homo sapiens (Human)) | BDBM50046952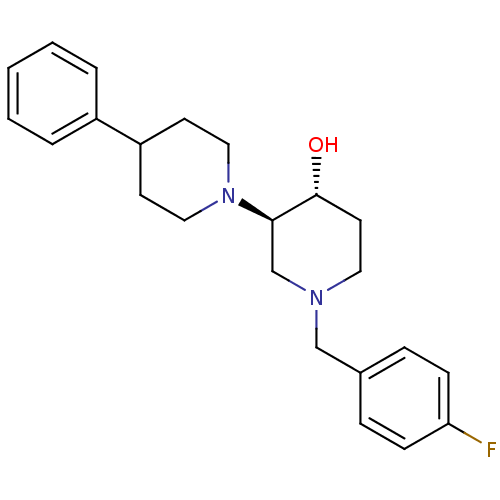 ((3'R,4'R)-1'-(4-Fluoro-benzyl)-4-phenyl-[1,3']bipi...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University Curated by ChEMBL | Assay Description Binding affinity to VAChT | Bioorg Med Chem 20: 4422-9 (2012) Article DOI: 10.1016/j.bmc.2012.05.045 BindingDB Entry DOI: 10.7270/Q29K4C9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM50192767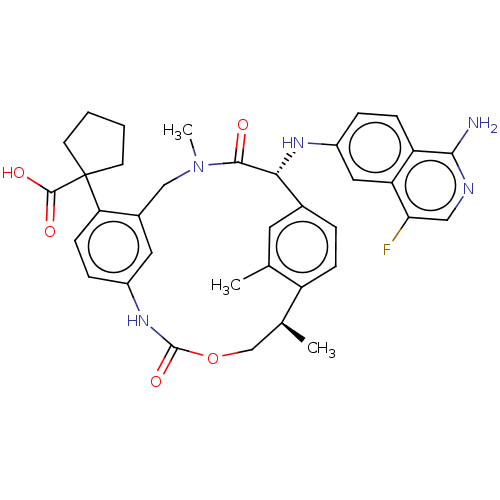 (CHEMBL3984725) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Inhibition of recombinant human TF-factor 7a using factor 10 as substrate at 37 degC | Bioorg Med Chem Lett 26: 5051-5057 (2016) Article DOI: 10.1016/j.bmcl.2016.08.088 BindingDB Entry DOI: 10.7270/Q24X59Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 26888 total ) | Next | Last >> |