

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50350311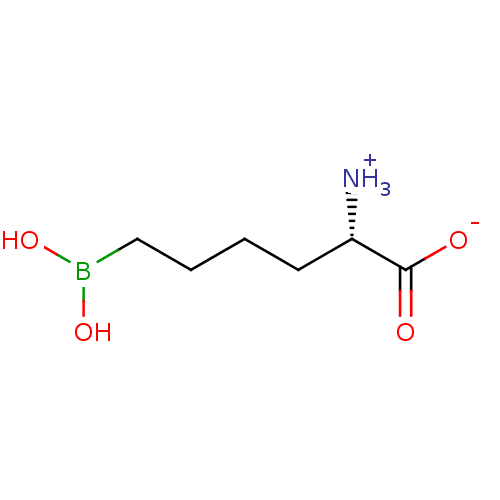 (CHEMBL1812661) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University Curated by ChEMBL | Assay Description Binding affinity to human arginase 2 | J Med Chem 54: 5432-43 (2011) Article DOI: 10.1021/jm200443b BindingDB Entry DOI: 10.7270/Q2TH8N21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50350311 (CHEMBL1812661) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Arg2 at pH 7.5 | Citation and Details Article DOI: 10.1016/j.bmc.2020.115658 BindingDB Entry DOI: 10.7270/Q2WS8XZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50561046 (CHEMBL4790798) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Arg2 using L-arginine as substrate after 60 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.bmc.2020.115658 BindingDB Entry DOI: 10.7270/Q2WS8XZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50561046 (CHEMBL4790798) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Arg1 using L-arginine as substrate after 60 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.bmc.2020.115658 BindingDB Entry DOI: 10.7270/Q2WS8XZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50561045 (CHEMBL4758805) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Arg2 using L-arginine as substrate after 60 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.bmc.2020.115658 BindingDB Entry DOI: 10.7270/Q2WS8XZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50561045 (CHEMBL4758805) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Arg1 using L-arginine as substrate after 60 mins by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.bmc.2020.115658 BindingDB Entry DOI: 10.7270/Q2WS8XZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50561034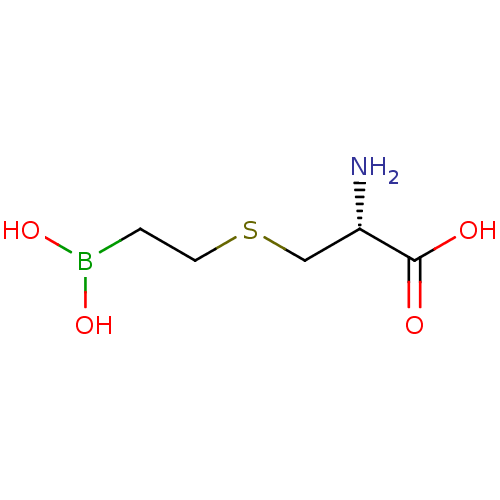 (CHEMBL4244287) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Arg2 at pH 7.5 | Citation and Details Article DOI: 10.1016/j.bmc.2020.115658 BindingDB Entry DOI: 10.7270/Q2WS8XZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50008099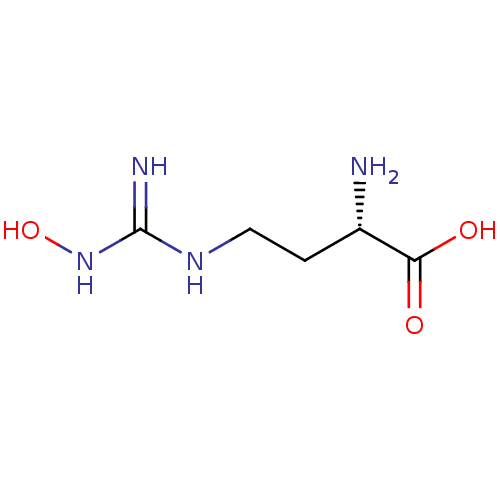 (CHEMBL1234777) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Arg2 at pH 7.5 | Citation and Details Article DOI: 10.1016/j.bmc.2020.115658 BindingDB Entry DOI: 10.7270/Q2WS8XZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50008099 (CHEMBL1234777) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of arginase 2 (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2017.12.044 BindingDB Entry DOI: 10.7270/Q2736THR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50294581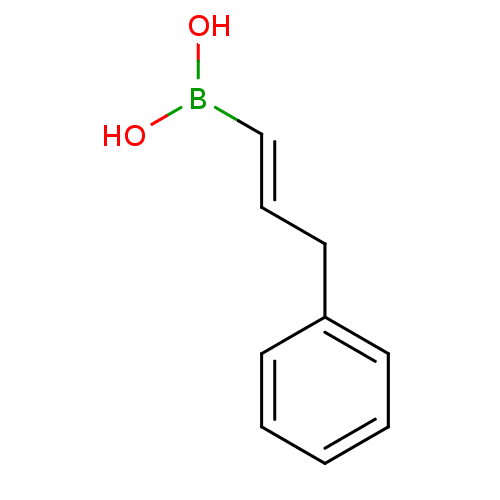 (3-phenylprop-1-enylboronic acid | CHEMBL539140) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM23417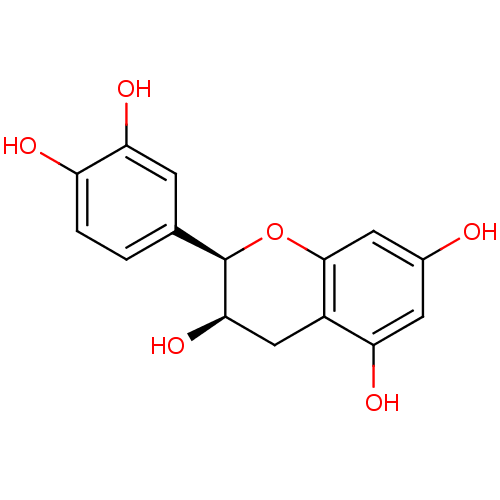 (α-CA inhibitor, 4 | (-)-Epicatechin | (2R,3R)...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Noncompetitive inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substr... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50561034 (CHEMBL4244287) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM23416 (α-CA inhibitor, 3 | (+)-Catechin | (2R,3S)-2-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Noncompetitive inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substr... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50241867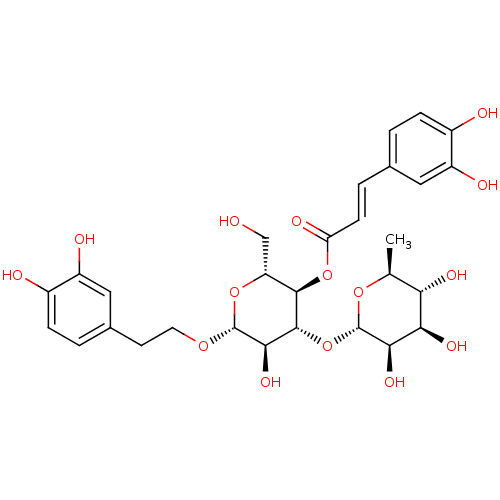 (((2R,3R,4R,5R,6R)-6-(3,4-dihydroxyphenethoxy)-5-hy...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Competitive inhibition of Leishmania amazonensis arginase assessed as inhibition constant for enzyme-inhibitor complex using L-arginine as substrate ... | J Nat Prod 79: 1459-63 (2016) Article DOI: 10.1021/acs.jnatprod.5b00875 BindingDB Entry DOI: 10.7270/Q2G73J78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50446567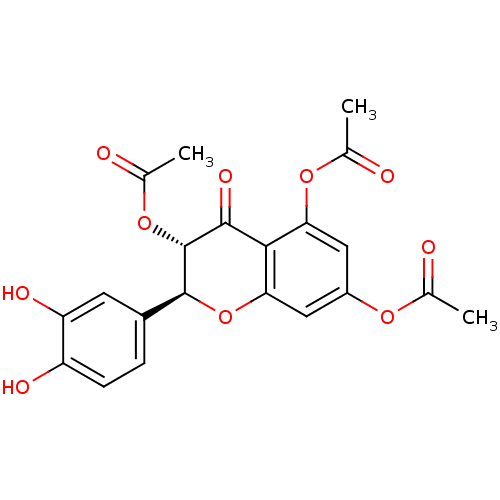 (CHEMBL3109443) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Noncompetitive inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substr... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM15236 (3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Noncompetitive inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substr... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50521944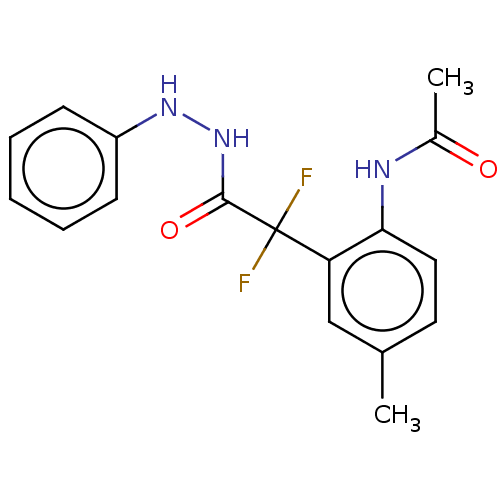 (CHEMBL4204512) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro Curated by ChEMBL | Assay Description Competitive inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli assessed as reduction in urea production by measu... | Bioorg Med Chem 27: 3853-3859 (2019) Article DOI: 10.1016/j.bmc.2019.07.022 BindingDB Entry DOI: 10.7270/Q27D2ZJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50230418 (CHEMBL260629 | N(gamma)-hydroxy-L-arginine | N-OME...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Arg2 at pH 7.5 | Citation and Details Article DOI: 10.1016/j.bmc.2020.115658 BindingDB Entry DOI: 10.7270/Q2WS8XZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Schistosoma mansoni (flatworms)) | BDBM130378 (2(S)-amino-6-boronohexanoic acid (ABH)) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
University of Pennsylvania | Assay Description Briefly, 0.5-50 mM L-arginine (pH 8.5) was added to a solution of 50 mM 4-(2-hydroxyethyl)piperazine-1-propanesulfonic acid (EPPS) (pH 8.5) and 100 &... | Biochemistry 53: 4671-4684 (2014) Article DOI: 10.1021/bi5004519 BindingDB Entry DOI: 10.7270/Q2RR1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Schistosoma mansoni (flatworms)) | BDBM130379 (N-hydroxy-L-arginine (NOHA)) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
University of Pennsylvania | Assay Description Briefly, 0.5-50 mM L-arginine (pH 8.5) was added to a solution of 50 mM 4-(2-hydroxyethyl)piperazine-1-propanesulfonic acid (EPPS) (pH 8.5) and 100 &... | Biochemistry 53: 4671-4684 (2014) Article DOI: 10.1021/bi5004519 BindingDB Entry DOI: 10.7270/Q2RR1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50446568 (CHEMBL3109439) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Noncompetitive inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substr... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50316603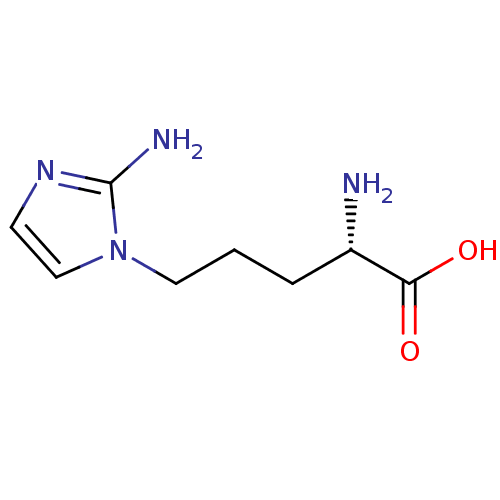 (2-(S)-amino-5-(2-aminoimidazol-1-yl)pentanoic acid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant full length arginase 1 expressed in Escherichia coli BL21(DE3) by fixed point assay | J Med Chem 53: 4266-76 (2010) Article DOI: 10.1021/jm100306a BindingDB Entry DOI: 10.7270/Q2SQ90JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50521942 (CHEMBL4216180) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro Curated by ChEMBL | Assay Description Competitive inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli assessed as reduction in urea production by measu... | Bioorg Med Chem 27: 3853-3859 (2019) Article DOI: 10.1016/j.bmc.2019.07.022 BindingDB Entry DOI: 10.7270/Q27D2ZJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM84978 (Quercitrin | cid_5280459) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Noncompetitive inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substr... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Plasmodium falciparum) | BDBM50350311 (CHEMBL1812661) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum arginase using L-arginine as substrate by colorimetric assay | J Med Chem 54: 5432-43 (2011) Article DOI: 10.1021/jm200443b BindingDB Entry DOI: 10.7270/Q2TH8N21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50510706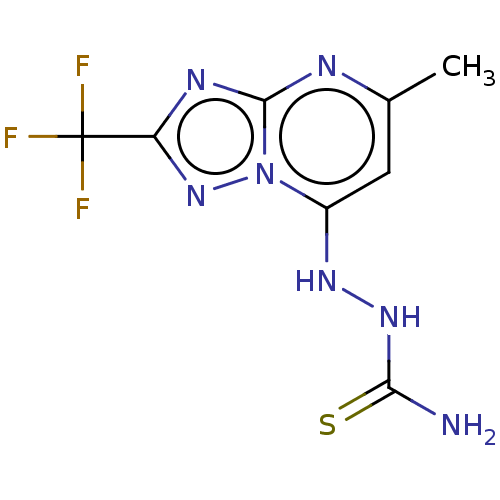 (CHEMBL4515068) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em Farmacos Curated by ChEMBL | Assay Description Non-competitive inhibition of Leishmania amazonensis arginase using L-arginine as substrate incubated for 15 mins | Bioorg Med Chem 27: 3061-3069 (2019) Article DOI: 10.1016/j.bmc.2019.05.026 BindingDB Entry DOI: 10.7270/Q2SX6HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50375814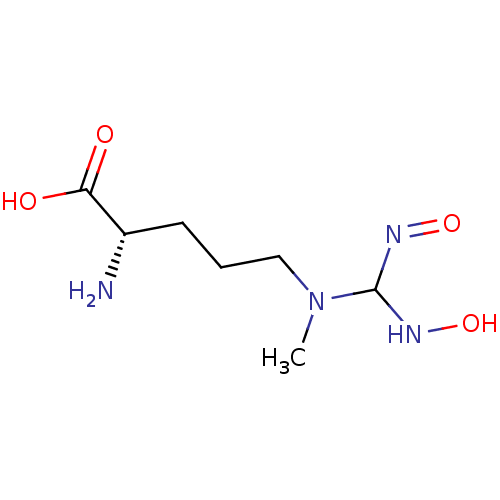 (CHEMBL260628) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Christian-Albrechts-University of Kiel Curated by ChEMBL | Assay Description Inhibition of bovine liver arginase | Bioorg Med Chem 16: 2305-12 (2008) Article DOI: 10.1016/j.bmc.2007.11.066 BindingDB Entry DOI: 10.7270/Q2KW5GXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50521943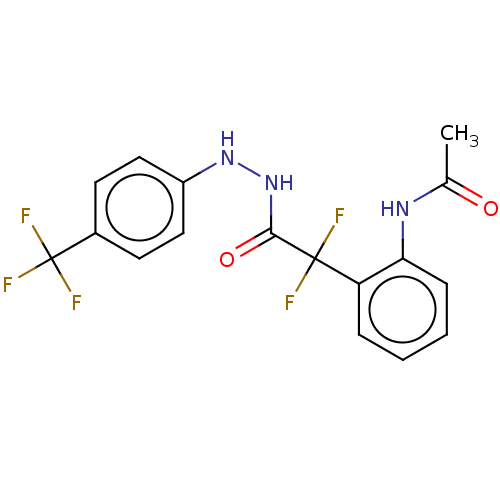 (CHEMBL4574177) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro Curated by ChEMBL | Assay Description Competitive inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli assessed as reduction in urea production by measu... | Bioorg Med Chem 27: 3853-3859 (2019) Article DOI: 10.1016/j.bmc.2019.07.022 BindingDB Entry DOI: 10.7270/Q27D2ZJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50230418 (CHEMBL260629 | N(gamma)-hydroxy-L-arginine | N-OME...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Christian-Albrechts-University of Kiel Curated by ChEMBL | Assay Description Inhibition of bovine liver arginase | Bioorg Med Chem 16: 2305-12 (2008) Article DOI: 10.1016/j.bmc.2007.11.066 BindingDB Entry DOI: 10.7270/Q2KW5GXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50521947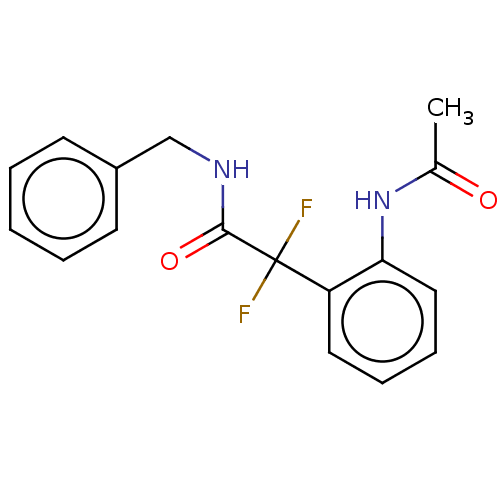 (CHEMBL4530523) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro Curated by ChEMBL | Assay Description Competitive inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli assessed as reduction in urea production by measu... | Bioorg Med Chem 27: 3853-3859 (2019) Article DOI: 10.1016/j.bmc.2019.07.022 BindingDB Entry DOI: 10.7270/Q27D2ZJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Rattus norvegicus) | BDBM50354832 (CHEMBL1834160) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50553165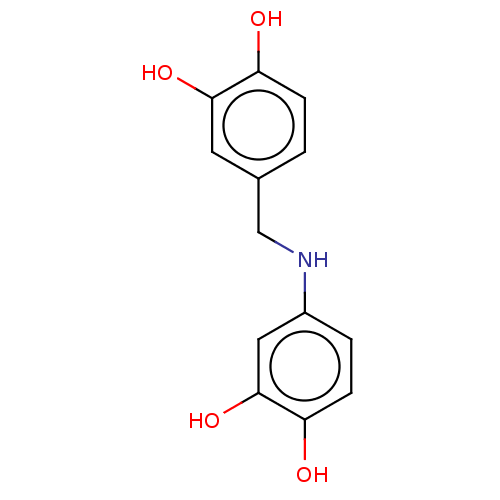 (CHEMBL4754674) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed type inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50553165 (CHEMBL4754674) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed type inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by Dixon plot analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50553165 (CHEMBL4754674) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed type inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by Cornish-Bowden plot analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50608139 (CHEMBL5287570) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50045936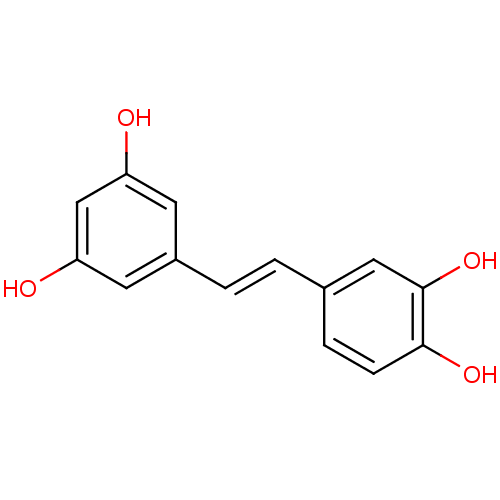 ((E)-4-(3,5-dihydroxystyryl)benzene-1,2-diol | (E)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed type inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by Cornish-Bowden plot analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50045936 ((E)-4-(3,5-dihydroxystyryl)benzene-1,2-diol | (E)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed type inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Bos taurus) | BDBM50045936 ((E)-4-(3,5-dihydroxystyryl)benzene-1,2-diol | (E)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed type inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by Dixon plot analysis | Citation and Details Article DOI: 10.1039/d0md00011f BindingDB Entry DOI: 10.7270/Q261140V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Plasmodium falciparum) | BDBM50350309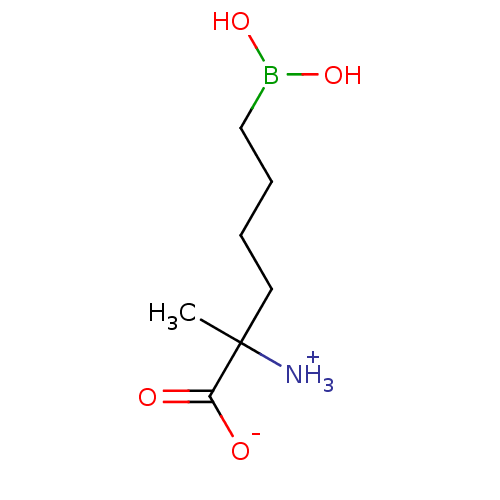 (CHEMBL1812662) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum arginase using L-arginine as substrate by colorimetric assay | J Med Chem 54: 5432-43 (2011) Article DOI: 10.1021/jm200443b BindingDB Entry DOI: 10.7270/Q2TH8N21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50462601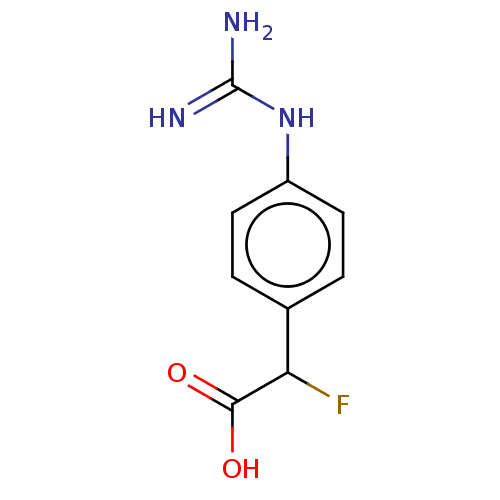 (CHEMBL4250607) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.75E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University Curated by ChEMBL | Assay Description Irreversible inhibition of human arginase 1 using thioarginine as substrate measured up to 360 mins by UV micro plate method | Bioorg Med Chem 26: 3939-3946 (2018) Article DOI: 10.1016/j.bmc.2018.06.015 BindingDB Entry DOI: 10.7270/Q2TX3J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50316607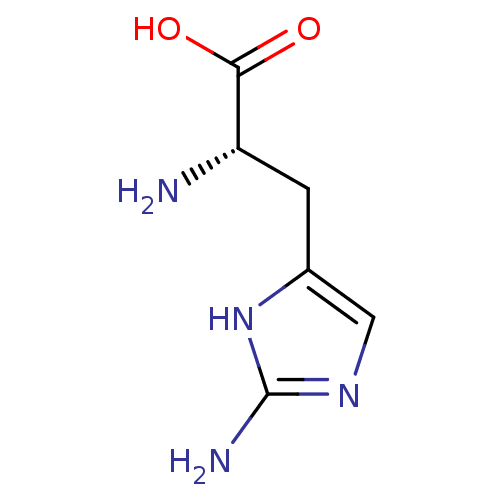 (2-amino-L-histidine | CHEMBL1099167 | L-2-aminohis...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant full length arginase 1 expressed in Escherichia coli BL21(DE3) by fixed point assay | J Med Chem 53: 4266-76 (2010) Article DOI: 10.1021/jm100306a BindingDB Entry DOI: 10.7270/Q2SQ90JK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50462601 (CHEMBL4250607) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.22E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University Curated by ChEMBL | Assay Description Irreversible inhibition of human arginase 2 using thioarginine as substrate measured up to 360 mins by UV micro plate method | Bioorg Med Chem 26: 3939-3946 (2018) Article DOI: 10.1016/j.bmc.2018.06.015 BindingDB Entry DOI: 10.7270/Q2TX3J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50462600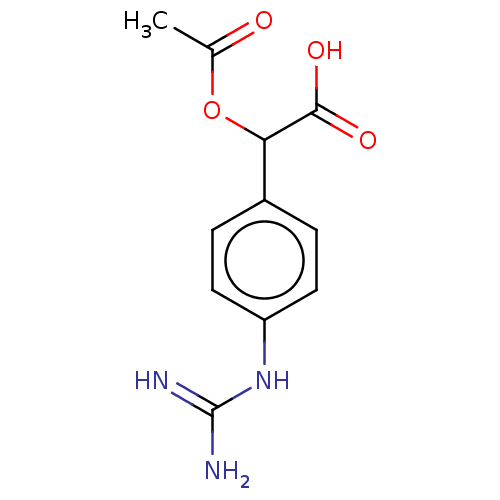 (CHEMBL4238387) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.82E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University Curated by ChEMBL | Assay Description Irreversible inhibition of human arginase 1 using thioarginine as substrate measured up to 360 mins by UV micro plate method | Bioorg Med Chem 26: 3939-3946 (2018) Article DOI: 10.1016/j.bmc.2018.06.015 BindingDB Entry DOI: 10.7270/Q2TX3J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50462600 (CHEMBL4238387) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.37E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University Curated by ChEMBL | Assay Description Irreversible inhibition of human arginase 2 using thioarginine as substrate measured up to 360 mins by UV micro plate method | Bioorg Med Chem 26: 3939-3946 (2018) Article DOI: 10.1016/j.bmc.2018.06.015 BindingDB Entry DOI: 10.7270/Q2TX3J1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50316604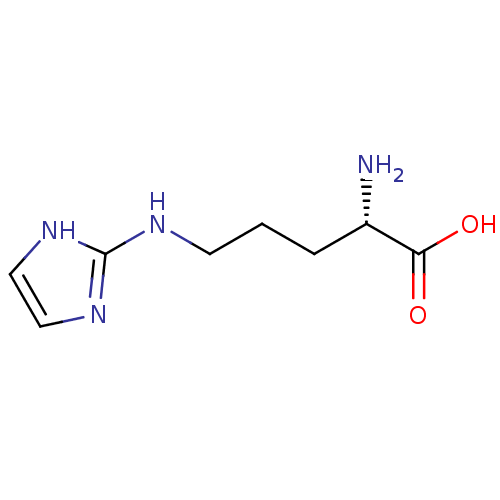 ((S)-2-amino-5-(imidazol-2-ylamino)pentanoic acid |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant full length arginase 1 expressed in Escherichia coli BL21(DE3) by fixed point assay | J Med Chem 53: 4266-76 (2010) Article DOI: 10.1021/jm100306a BindingDB Entry DOI: 10.7270/Q2SQ90JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Plasmodium falciparum) | BDBM50350310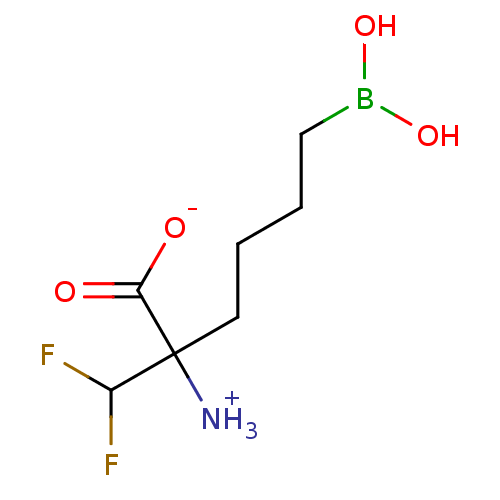 (CHEMBL1812663) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum arginase using L-arginine as substrate by colorimetric assay | J Med Chem 54: 5432-43 (2011) Article DOI: 10.1021/jm200443b BindingDB Entry DOI: 10.7270/Q2TH8N21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50316606 ((2S)-2-amino-4-(2-amino-1H-imidazol-5-yl)butanoic ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 3.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant full length arginase 1 expressed in Escherichia coli BL21(DE3) by fixed point assay | J Med Chem 53: 4266-76 (2010) Article DOI: 10.1021/jm100306a BindingDB Entry DOI: 10.7270/Q2SQ90JK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50316608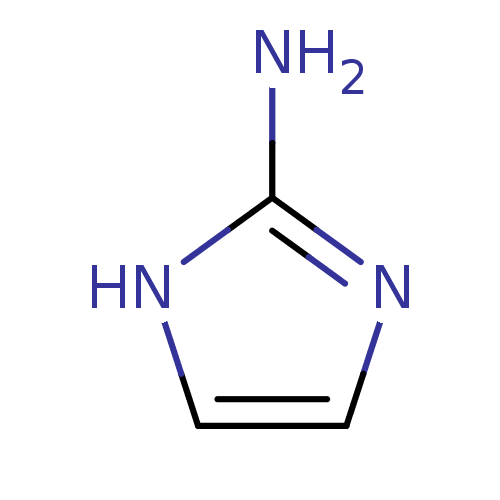 (1H-Imidazol-2-yl-ammonium | 1H-Imidazol-2-ylamine ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | MMDB Article PubMed | 3.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant full length arginase 1 expressed in Escherichia coli BL21(DE3) by fixed point assay | J Med Chem 53: 4266-76 (2010) Article DOI: 10.1021/jm100306a BindingDB Entry DOI: 10.7270/Q2SQ90JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50316603 (2-(S)-amino-5-(2-aminoimidazol-1-yl)pentanoic acid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | 3.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50316605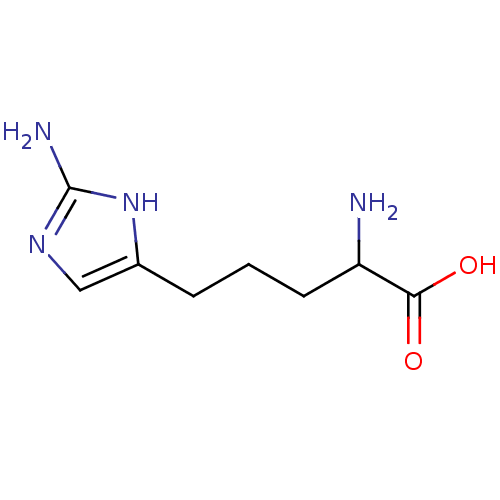 (2-amino-5-(2-aminoimidazol-4-yl)pentanoic acid | C...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >8.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant full length arginase 1 expressed in Escherichia coli BL21(DE3) by fixed point assay | J Med Chem 53: 4266-76 (2010) Article DOI: 10.1021/jm100306a BindingDB Entry DOI: 10.7270/Q2SQ90JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1114 total ) | Next | Last >> |