

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50216168 (CHEMBL132778) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP21A2deltaH mutant expressed in Escherichia coli DH5alpha assessed as decrease in progesteron... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50358201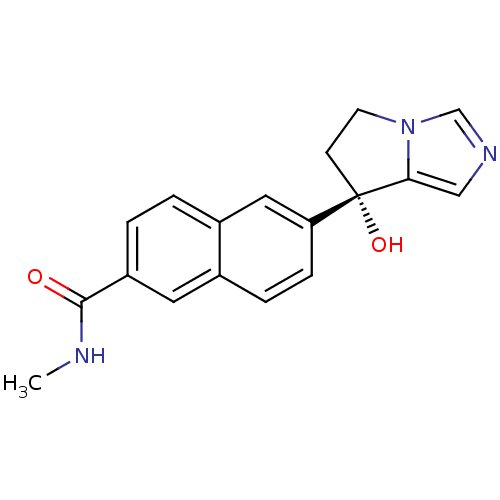 (CHEMBL1921976 | US9611270, orteronel) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50203335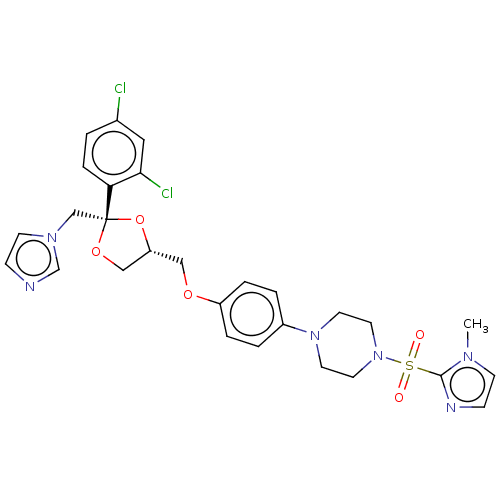 (CHEMBL3891718) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP21 (unknown origin) overexpressed in human AD293 cells assessed as reduction in 11-deoxycortisol formation preincubated ... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM314740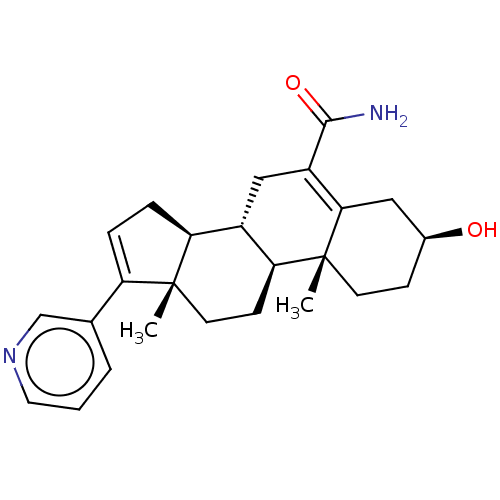 (US9611270, Example 42) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM314736 (US9611270, Example 33) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50203337 (CHEMBL3980889) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP21 (unknown origin) overexpressed in human AD293 cells assessed as reduction in 11-deoxycortisol formation preincubated ... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM314738 (US9611270, Example 38) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP21A2deltaH mutant expressed in Escherichia coli DH5alpha assessed as decrease in progesteron... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50203333 (CHEMBL3968178) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP21 (unknown origin) overexpressed in human AD293 cells assessed as reduction in 11-deoxycortisol formation preincubated ... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50203339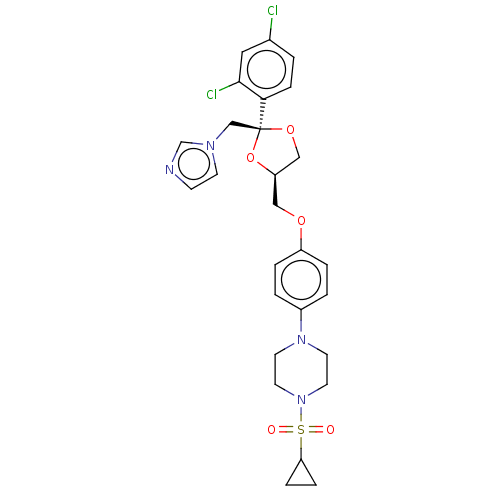 (CHEMBL3922888) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP21 (unknown origin) overexpressed in human AD293 cells assessed as reduction in 11-deoxycortisol formation preincubated ... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM25458 ((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP21A2deltaH mutant expressed in Escherichia coli DH5alpha assessed as decrease in progesteron... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50456363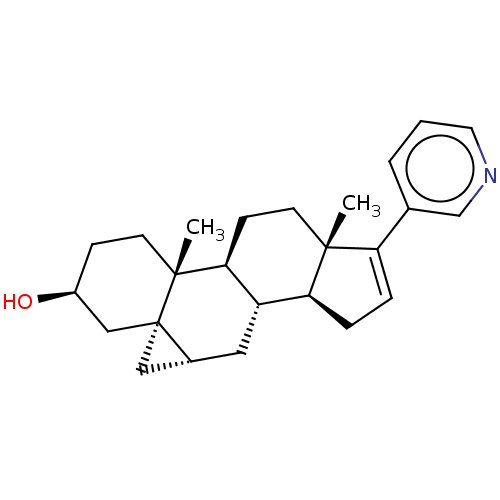 (CHEMBL4210737) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP21A2deltaH mutant expressed in Escherichia coli DH5alpha assessed as decrease in progesteron... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50203340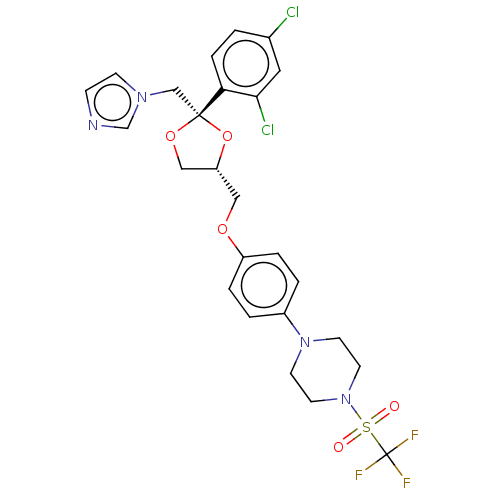 (CHEMBL3938615) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP21 (unknown origin) overexpressed in human AD293 cells assessed as reduction in 11-deoxycortisol formation preincubated ... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50203336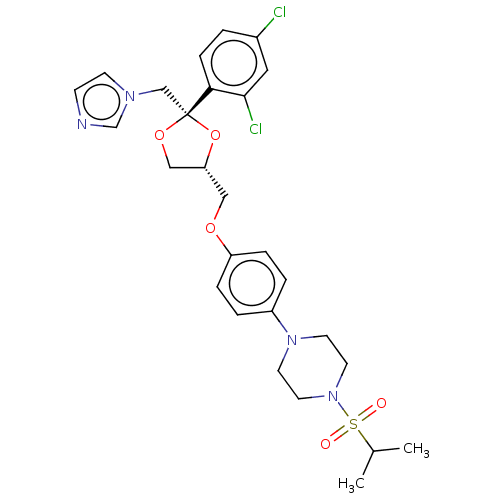 (CHEMBL3894912) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP21 (unknown origin) overexpressed in human AD293 cells assessed as reduction in 11-deoxycortisol formation preincubated ... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50435990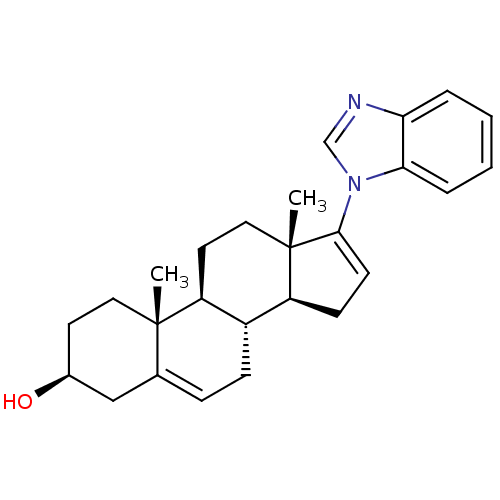 (GALETERONE | TOK-001 | US9611270, galaterone | VN/...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP21A2deltaH mutant expressed in Escherichia coli DH5alpha assessed as decrease in progesteron... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50203342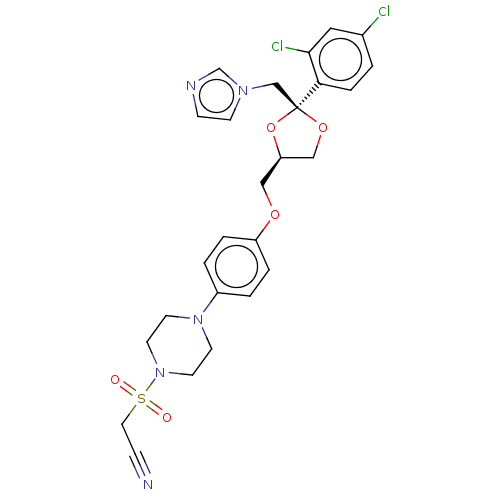 (CHEMBL3952032) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP21 (unknown origin) overexpressed in human AD293 cells assessed as reduction in 11-deoxycortisol formation preincubated ... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50203334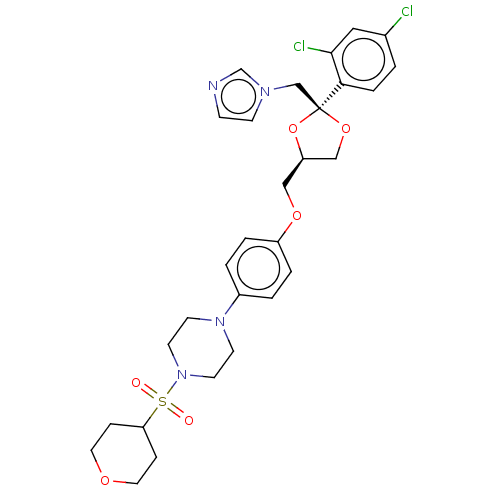 (CHEMBL3931836) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP21 (unknown origin) overexpressed in human AD293 cells assessed as reduction in 11-deoxycortisol formation preincubated ... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50203341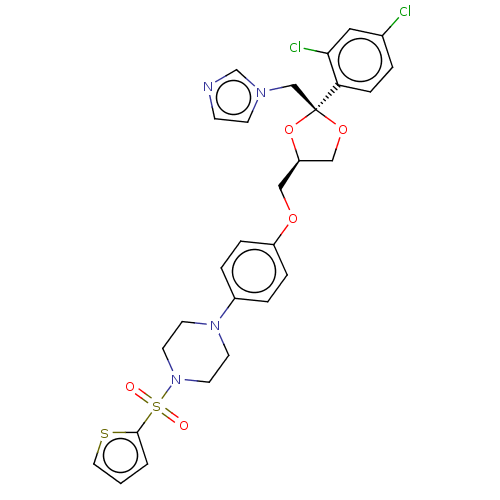 (CHEMBL3960724) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP21 (unknown origin) overexpressed in human AD293 cells assessed as reduction in 11-deoxycortisol formation preincubated ... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50203331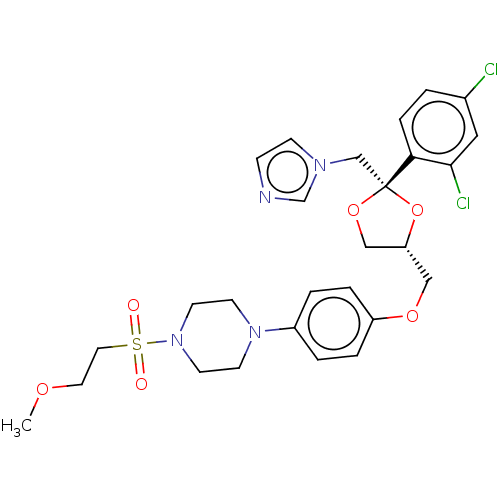 (CHEMBL3964421) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 166 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP21 (unknown origin) overexpressed in human AD293 cells assessed as reduction in 11-deoxycortisol formation preincubated ... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50456365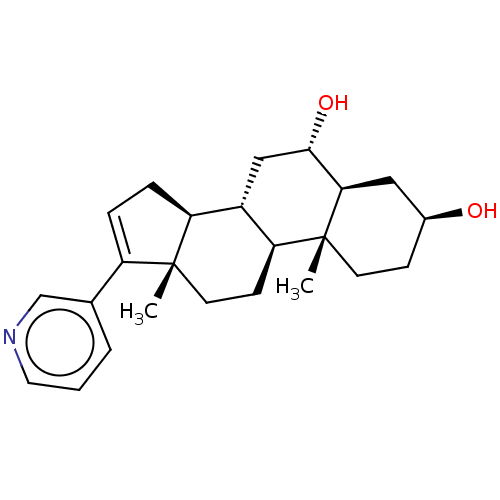 (CHEMBL4213452) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 234 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP21A2deltaH mutant expressed in Escherichia coli DH5alpha assessed as decrease in progesteron... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50435990 (GALETERONE | TOK-001 | US9611270, galaterone | VN/...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 248 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50203332 (CHEMBL3913908) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 271 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP21 (unknown origin) overexpressed in human AD293 cells assessed as reduction in 11-deoxycortisol formation preincubated ... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM25458 ((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 296 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM314732 (US9611270, Example 29) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP21A2deltaH mutant expressed in Escherichia coli DH5alpha assessed as decrease in progesteron... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM314738 (US9611270, Example 38) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 537 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM314740 (US9611270, Example 42) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 673 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP21A2deltaH mutant expressed in Escherichia coli DH5alpha assessed as decrease in progesteron... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM314739 (US9611270, Example 39 | US9611270, Example 41) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 831 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF KANSAS US Patent | Assay Description Complementary assays were utilized for the quantitative comparison of compound activity data for CYP17A1 and CYP21A2. Progesterone is a substrate for... | US Patent US9611270 (2017) BindingDB Entry DOI: 10.7270/Q2CV4KTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50156282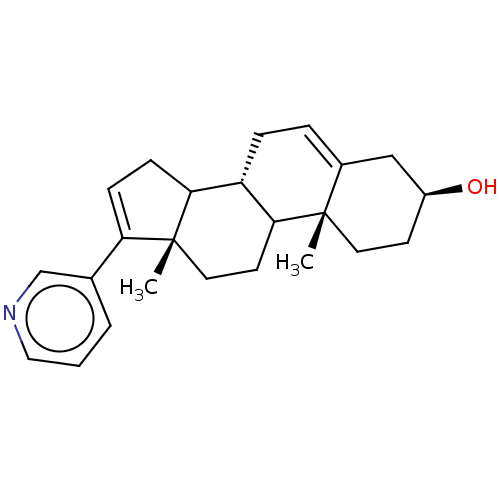 (CHEMBL3780847) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP21A2 in human H295R cells using hydroxy progesterone as substrate incubated for 120 mins by LC-MS method | ACS Med Chem Lett 7: 40-5 (2016) Article DOI: 10.1021/acsmedchemlett.5b00310 BindingDB Entry DOI: 10.7270/Q2TH8PK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50456366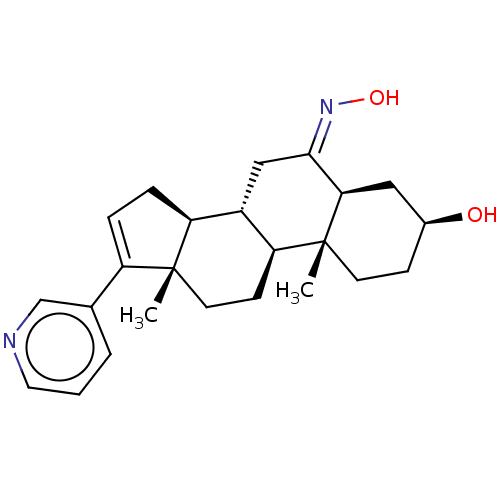 (CHEMBL4214976) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP21A2deltaH mutant expressed in Escherichia coli DH5alpha assessed as decrease in progesteron... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50203338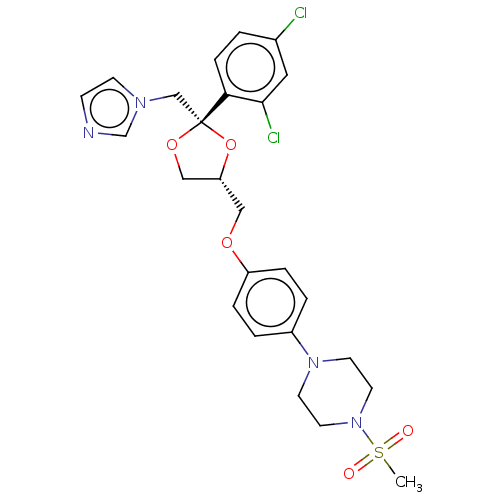 (CHEMBL3983734) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP21 (unknown origin) overexpressed in human AD293 cells assessed as reduction in 11-deoxycortisol formation preincubated ... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM314733 (US9611270, Example 30) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged recombinant human CYP21A2deltaH mutant expressed in Escherichia coli DH5alpha assessed as decrease in progesteron... | J Med Chem 61: 4946-4960 (2018) Article DOI: 10.1021/acs.jmedchem.8b00419 BindingDB Entry DOI: 10.7270/Q2D2217B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM31768 (CHEMBL295698 | Ketoconazole | Nizoral | Panfungol) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of recombinant CYP21 (unknown origin) overexpressed in human AD293 cells assessed as reduction in 11-deoxycortisol formation preincubated ... | Bioorg Med Chem Lett 26: 5825-5829 (2016) Article DOI: 10.1016/j.bmcl.2016.10.016 BindingDB Entry DOI: 10.7270/Q2X63PW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50004442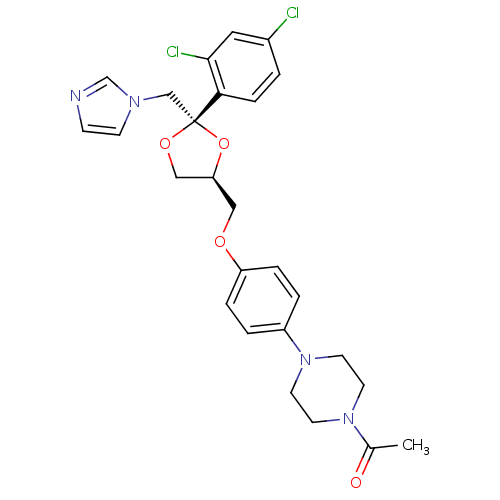 ((2S,4S)-ketoconazole | 1-acetyl-4-(4-{[(2S,4S)-2-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 4.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Inhibition of Progesterone 21-hydroxylase cytochrome P450 21 | J Med Chem 35: 2818-25 (1992) BindingDB Entry DOI: 10.7270/Q24Q7SZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50004441 ((2R,4R)-ketoconazole | 1-acetyl-4-(4-{[(2R,4R)-2-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Inhibition of Progesterone 21-hydroxylase cytochrome P450 21 | J Med Chem 35: 2818-25 (1992) BindingDB Entry DOI: 10.7270/Q24Q7SZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50391846 (CHEMBL2147041 | US9133160, 31) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP21A2 in human H295R cells using hydroxy progesterone as substrate incubated for 120 mins by LC-MS method | ACS Med Chem Lett 7: 40-5 (2016) Article DOI: 10.1021/acsmedchemlett.5b00310 BindingDB Entry DOI: 10.7270/Q2TH8PK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM31768 (CHEMBL295698 | Ketoconazole | Nizoral | Panfungol) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 9.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Inhibition of Progesterone 21-hydroxylase cytochrome P450 21 | J Med Chem 35: 2818-25 (1992) BindingDB Entry DOI: 10.7270/Q24Q7SZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50249048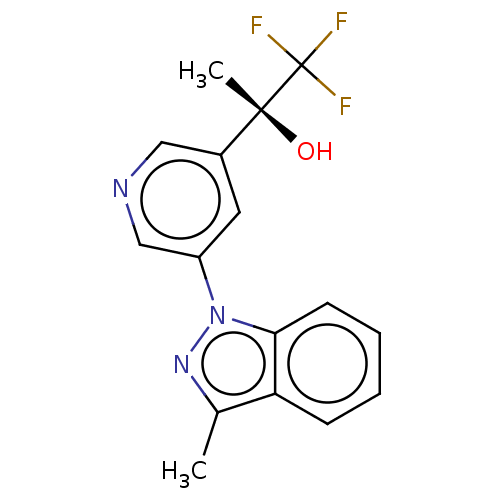 (CHEMBL4080256) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human CYP21 expressed in African green monkey COS7 cells using 17-hydroxypregnenolone as substrate preincubated for 1 hr followed by su... | Bioorg Med Chem Lett 27: 2384-2388 (2017) Article DOI: 10.1016/j.bmcl.2017.04.021 BindingDB Entry DOI: 10.7270/Q2MC92F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50249046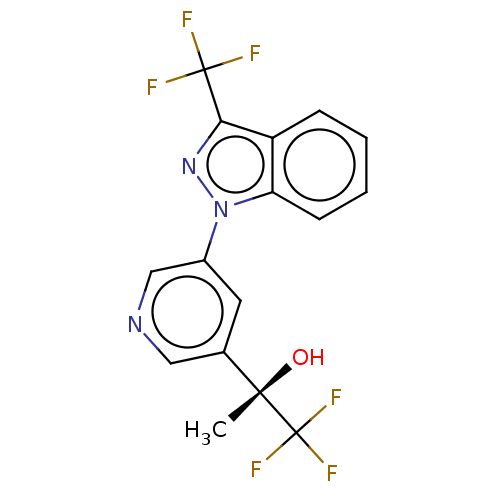 (CHEMBL4071689) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human CYP21 expressed in African green monkey COS7 cells using 17-hydroxypregnenolone as substrate preincubated for 1 hr followed by su... | Bioorg Med Chem Lett 27: 2384-2388 (2017) Article DOI: 10.1016/j.bmcl.2017.04.021 BindingDB Entry DOI: 10.7270/Q2MC92F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50249044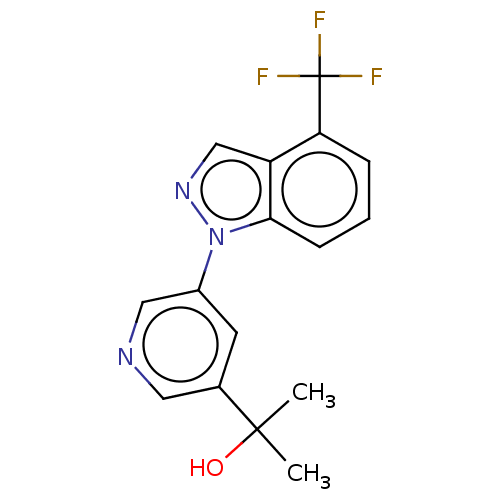 (CHEMBL4067000) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human CYP21 expressed in African green monkey COS7 cells using 17-hydroxypregnenolone as substrate preincubated for 1 hr followed by su... | Bioorg Med Chem Lett 27: 2384-2388 (2017) Article DOI: 10.1016/j.bmcl.2017.04.021 BindingDB Entry DOI: 10.7270/Q2MC92F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50092153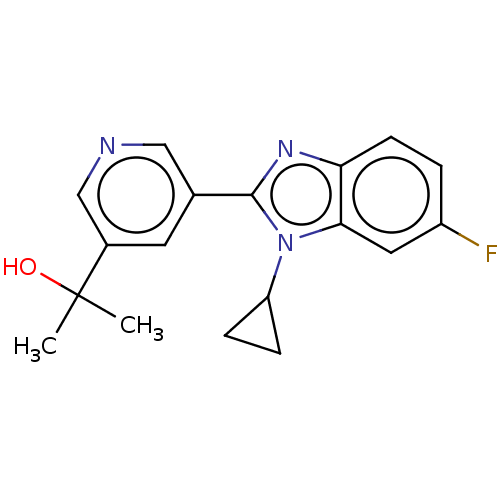 (CHEMBL3582478) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human CYP21 expressed in COS7 cells incubated for 1 hr before 17-hydroxypregnenolone substrate addition by HTRF-based assay | ACS Med Chem Lett 6: 573-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00054 BindingDB Entry DOI: 10.7270/Q2K64KT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50092137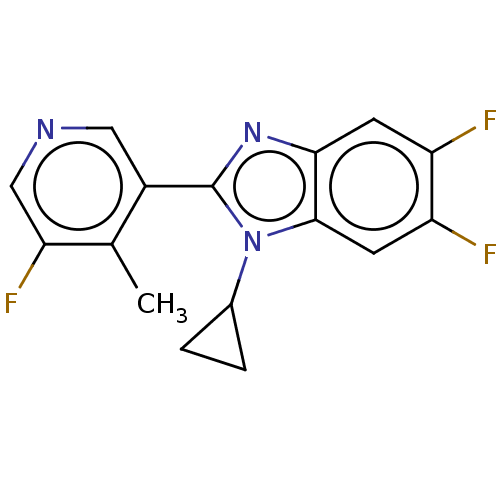 (CHEMBL3582481) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human CYP21 expressed in COS7 cells incubated for 1 hr before 17-hydroxypregnenolone substrate addition by HTRF-based assay | ACS Med Chem Lett 6: 573-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00054 BindingDB Entry DOI: 10.7270/Q2K64KT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50206961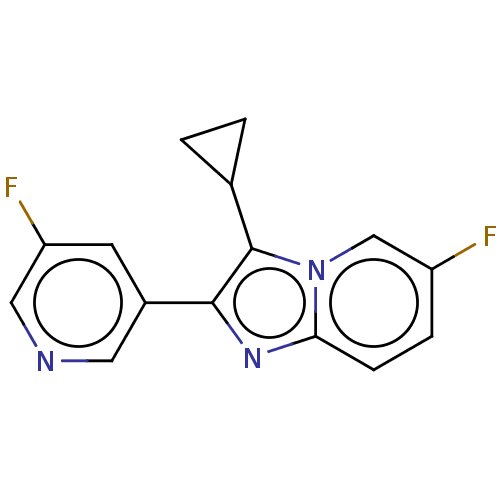 (CHEMBL3893614 | US9518055, Example 31) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck USA Curated by ChEMBL | Assay Description Inhibition of human CYP21 expressed in African green monkey COS7 cells using 17-hydroxypregnenolone as substrate preincubated for 1 hr followed by su... | Bioorg Med Chem Lett 27: 143-146 (2017) Article DOI: 10.1016/j.bmcl.2016.12.003 BindingDB Entry DOI: 10.7270/Q20K2BK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50206959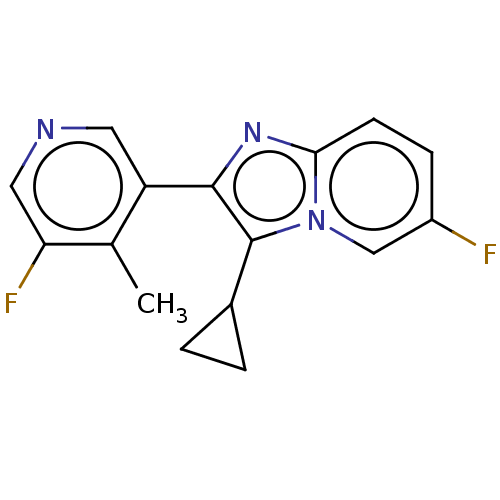 (CHEMBL3924257) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck USA Curated by ChEMBL | Assay Description Inhibition of human CYP21 expressed in African green monkey COS7 cells using 17-hydroxypregnenolone as substrate preincubated for 1 hr followed by su... | Bioorg Med Chem Lett 27: 143-146 (2017) Article DOI: 10.1016/j.bmcl.2016.12.003 BindingDB Entry DOI: 10.7270/Q20K2BK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50206955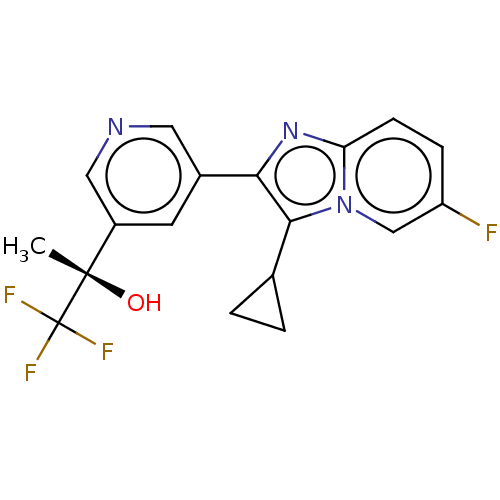 (CHEMBL3923127) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck USA Curated by ChEMBL | Assay Description Inhibition of human CYP21 expressed in African green monkey COS7 cells using 17-hydroxypregnenolone as substrate preincubated for 1 hr followed by su... | Bioorg Med Chem Lett 27: 143-146 (2017) Article DOI: 10.1016/j.bmcl.2016.12.003 BindingDB Entry DOI: 10.7270/Q20K2BK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50249045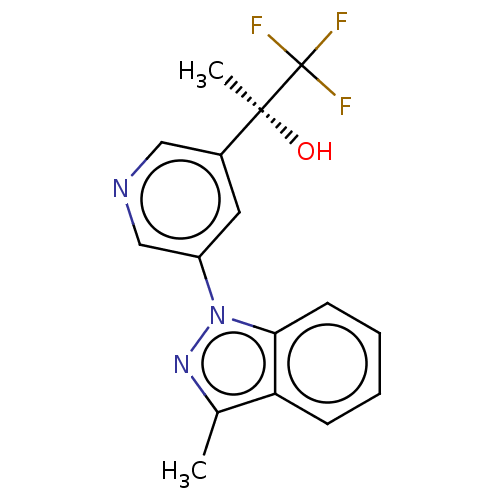 (CHEMBL4098297) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human CYP21 expressed in African green monkey COS7 cells using 17-hydroxypregnenolone as substrate preincubated for 1 hr followed by su... | Bioorg Med Chem Lett 27: 2384-2388 (2017) Article DOI: 10.1016/j.bmcl.2017.04.021 BindingDB Entry DOI: 10.7270/Q2MC92F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50249047 (CHEMBL4080420) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human CYP21 expressed in African green monkey COS7 cells using 17-hydroxypregnenolone as substrate preincubated for 1 hr followed by su... | Bioorg Med Chem Lett 27: 2384-2388 (2017) Article DOI: 10.1016/j.bmcl.2017.04.021 BindingDB Entry DOI: 10.7270/Q2MC92F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50092142 (CHEMBL3582477) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human CYP21 expressed in COS7 cells incubated for 1 hr before 17-hydroxypregnenolone substrate addition by HTRF-based assay | ACS Med Chem Lett 6: 573-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00054 BindingDB Entry DOI: 10.7270/Q2K64KT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50092139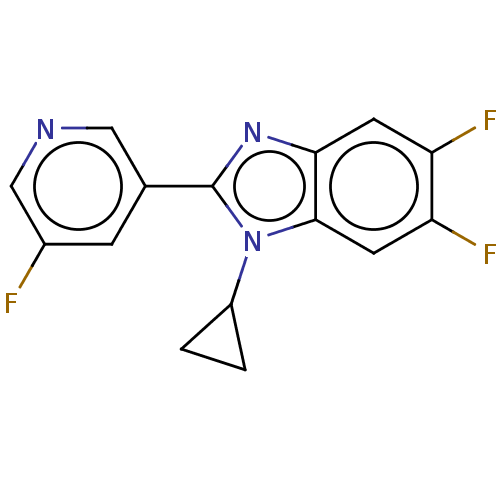 (CHEMBL3582472) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human CYP21 expressed in COS7 cells incubated for 1 hr before 17-hydroxypregnenolone substrate addition by HTRF-based assay | ACS Med Chem Lett 6: 573-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00054 BindingDB Entry DOI: 10.7270/Q2K64KT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50206963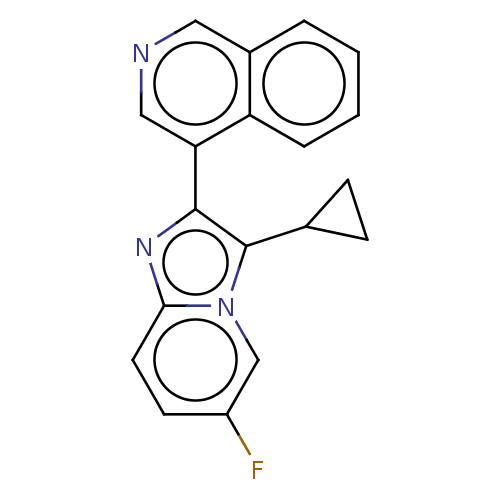 (CHEMBL3948337 | US9518055, Example 41) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck USA Curated by ChEMBL | Assay Description Inhibition of human CYP21 expressed in African green monkey COS7 cells using 17-hydroxypregnenolone as substrate preincubated for 1 hr followed by su... | Bioorg Med Chem Lett 27: 143-146 (2017) Article DOI: 10.1016/j.bmcl.2016.12.003 BindingDB Entry DOI: 10.7270/Q20K2BK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM50206975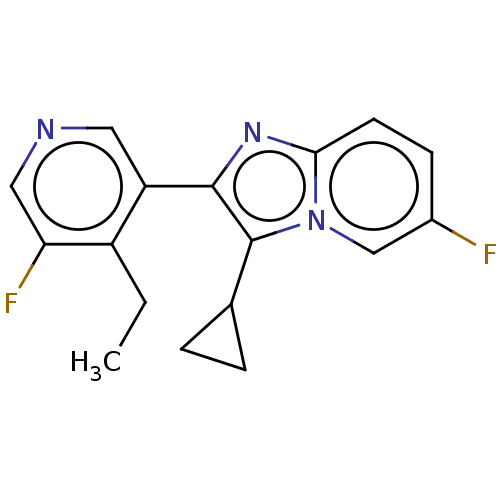 (CHEMBL3919759) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck USA Curated by ChEMBL | Assay Description Inhibition of human CYP21 expressed in African green monkey COS7 cells using 17-hydroxypregnenolone as substrate preincubated for 1 hr followed by su... | Bioorg Med Chem Lett 27: 143-146 (2017) Article DOI: 10.1016/j.bmcl.2016.12.003 BindingDB Entry DOI: 10.7270/Q20K2BK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 21-hydroxylase (Homo sapiens (Human)) | BDBM31768 (CHEMBL295698 | Ketoconazole | Nizoral | Panfungol) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Inhibition of cytochrome P450 progesterone 2-alpha-hydroxylase | J Med Chem 35: 2818-25 (1992) BindingDB Entry DOI: 10.7270/Q24Q7SZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||