

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hexokinase-4 (Rattus norvegicus) | BDBM50239048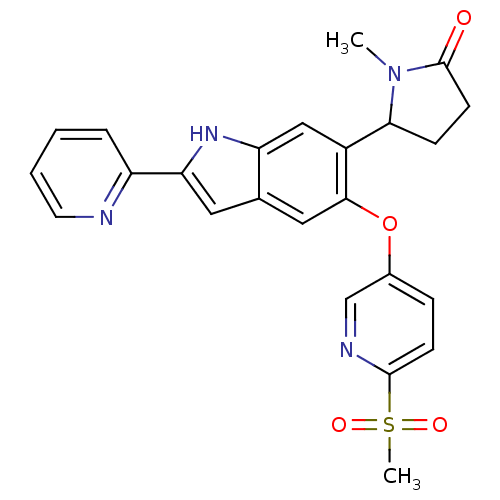 (CHEMBL4067028) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Merck& Co., Inc. Curated by ChEMBL | Assay Description Activation of glucokinase in rat INS-1 cells assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HILIC LC-M... | Bioorg Med Chem Lett 27: 2069-2073 (2017) Article DOI: 10.1016/j.bmcl.2016.10.085 BindingDB Entry DOI: 10.7270/Q2DZ0BM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50239048 (CHEMBL4067028) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Merck& Co., Inc. Curated by ChEMBL | Assay Description Activation of glucokinase in rat INS-1 cells assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HILIC LC-M... | Bioorg Med Chem Lett 27: 2069-2073 (2017) Article DOI: 10.1016/j.bmcl.2016.10.085 BindingDB Entry DOI: 10.7270/Q2DZ0BM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50239049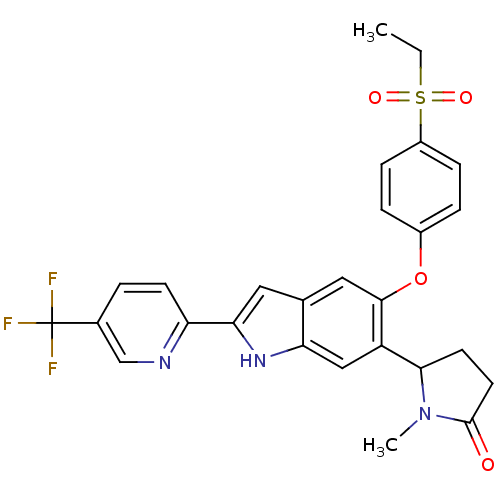 (CHEMBL4086782) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a |
Merck& Co., Inc. Curated by ChEMBL | Assay Description Activation of glucokinase in rat INS-1 cells assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HILIC LC-M... | Bioorg Med Chem Lett 27: 2069-2073 (2017) Article DOI: 10.1016/j.bmcl.2016.10.085 BindingDB Entry DOI: 10.7270/Q2DZ0BM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50239045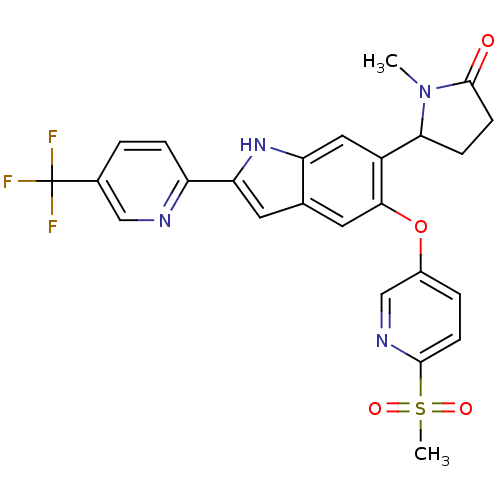 (CHEMBL4083339) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
Merck& Co., Inc. Curated by ChEMBL | Assay Description Activation of glucokinase in rat INS-1 cells assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HILIC LC-M... | Bioorg Med Chem Lett 27: 2069-2073 (2017) Article DOI: 10.1016/j.bmcl.2016.10.085 BindingDB Entry DOI: 10.7270/Q2DZ0BM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50239045 (CHEMBL4083339) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
Merck& Co., Inc. Curated by ChEMBL | Assay Description Activation of glucokinase in rat INS-1 cells assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HILIC LC-M... | Bioorg Med Chem Lett 27: 2069-2073 (2017) Article DOI: 10.1016/j.bmcl.2016.10.085 BindingDB Entry DOI: 10.7270/Q2DZ0BM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50239045 (CHEMBL4083339) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a |
Merck& Co., Inc. Curated by ChEMBL | Assay Description Activation of glucokinase in primary rat hepatocytes assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HI... | Bioorg Med Chem Lett 27: 2069-2073 (2017) Article DOI: 10.1016/j.bmcl.2016.10.085 BindingDB Entry DOI: 10.7270/Q2DZ0BM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50239045 (CHEMBL4083339) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a |
Merck& Co., Inc. Curated by ChEMBL | Assay Description Activation of glucokinase in primary rat hepatocytes assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HI... | Bioorg Med Chem Lett 27: 2069-2073 (2017) Article DOI: 10.1016/j.bmcl.2016.10.085 BindingDB Entry DOI: 10.7270/Q2DZ0BM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50239046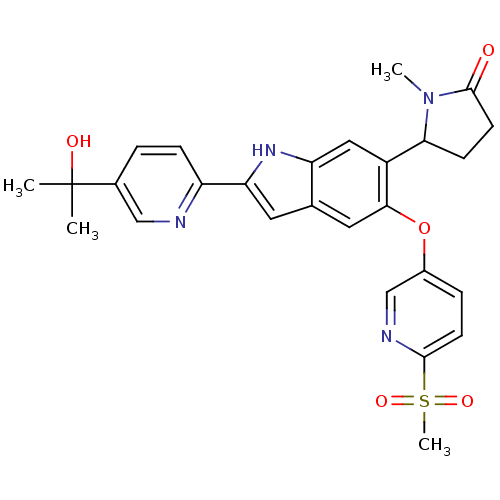 (CHEMBL4068048) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a |
Merck& Co., Inc. Curated by ChEMBL | Assay Description Activation of glucokinase in rat INS-1 cells assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HILIC LC-M... | Bioorg Med Chem Lett 27: 2069-2073 (2017) Article DOI: 10.1016/j.bmcl.2016.10.085 BindingDB Entry DOI: 10.7270/Q2DZ0BM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50239046 (CHEMBL4068048) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a |
Merck& Co., Inc. Curated by ChEMBL | Assay Description Activation of glucokinase in rat INS-1 cells assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HILIC LC-M... | Bioorg Med Chem Lett 27: 2069-2073 (2017) Article DOI: 10.1016/j.bmcl.2016.10.085 BindingDB Entry DOI: 10.7270/Q2DZ0BM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50239046 (CHEMBL4068048) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a |
Merck& Co., Inc. Curated by ChEMBL | Assay Description Activation of glucokinase in primary rat hepatocytes assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HI... | Bioorg Med Chem Lett 27: 2069-2073 (2017) Article DOI: 10.1016/j.bmcl.2016.10.085 BindingDB Entry DOI: 10.7270/Q2DZ0BM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50239046 (CHEMBL4068048) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a |
Merck& Co., Inc. Curated by ChEMBL | Assay Description Activation of glucokinase in primary rat hepatocytes assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HI... | Bioorg Med Chem Lett 27: 2069-2073 (2017) Article DOI: 10.1016/j.bmcl.2016.10.085 BindingDB Entry DOI: 10.7270/Q2DZ0BM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50239047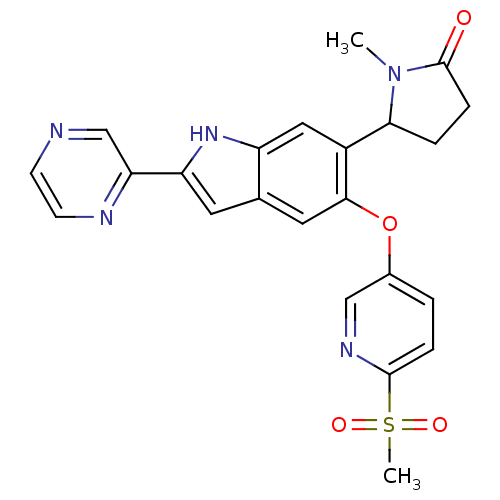 (CHEMBL4078915) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a |
Merck& Co., Inc. Curated by ChEMBL | Assay Description Activation of glucokinase in rat INS-1 cells assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HILIC LC-M... | Bioorg Med Chem Lett 27: 2069-2073 (2017) Article DOI: 10.1016/j.bmcl.2016.10.085 BindingDB Entry DOI: 10.7270/Q2DZ0BM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50239047 (CHEMBL4078915) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a |
Merck& Co., Inc. Curated by ChEMBL | Assay Description Activation of glucokinase in rat INS-1 cells assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HILIC LC-M... | Bioorg Med Chem Lett 27: 2069-2073 (2017) Article DOI: 10.1016/j.bmcl.2016.10.085 BindingDB Entry DOI: 10.7270/Q2DZ0BM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50239048 (CHEMBL4067028) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a |
Merck& Co., Inc. Curated by ChEMBL | Assay Description Activation of glucokinase in primary rat hepatocytes assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HI... | Bioorg Med Chem Lett 27: 2069-2073 (2017) Article DOI: 10.1016/j.bmcl.2016.10.085 BindingDB Entry DOI: 10.7270/Q2DZ0BM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50239048 (CHEMBL4067028) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a |
Merck& Co., Inc. Curated by ChEMBL | Assay Description Activation of glucokinase in primary rat hepatocytes assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HI... | Bioorg Med Chem Lett 27: 2069-2073 (2017) Article DOI: 10.1016/j.bmcl.2016.10.085 BindingDB Entry DOI: 10.7270/Q2DZ0BM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50239044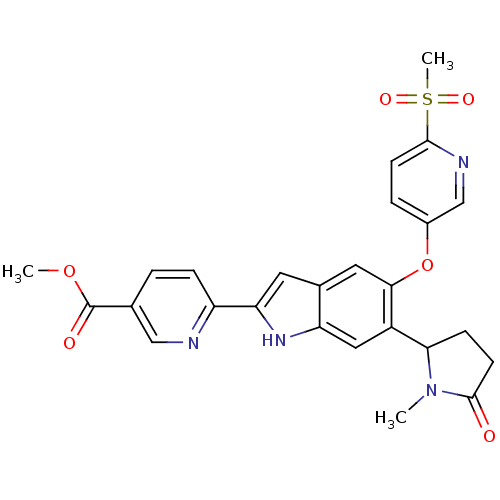 (CHEMBL4104680) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a |
Merck& Co., Inc. Curated by ChEMBL | Assay Description Activation of glucokinase in rat INS-1 cells assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HILIC LC-M... | Bioorg Med Chem Lett 27: 2069-2073 (2017) Article DOI: 10.1016/j.bmcl.2016.10.085 BindingDB Entry DOI: 10.7270/Q2DZ0BM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50239040 (CHEMBL4086771) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Merck& Co., Inc. Curated by ChEMBL | Assay Description Activation of glucokinase in primary rat hepatocytes assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HI... | Bioorg Med Chem Lett 27: 2069-2073 (2017) Article DOI: 10.1016/j.bmcl.2016.10.085 BindingDB Entry DOI: 10.7270/Q2DZ0BM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50239040 (CHEMBL4086771) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Merck& Co., Inc. Curated by ChEMBL | Assay Description Activation of glucokinase in primary rat hepatocytes assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HI... | Bioorg Med Chem Lett 27: 2069-2073 (2017) Article DOI: 10.1016/j.bmcl.2016.10.085 BindingDB Entry DOI: 10.7270/Q2DZ0BM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50239044 (CHEMBL4104680) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Merck& Co., Inc. Curated by ChEMBL | Assay Description Activation of glucokinase in primary rat hepatocytes assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HI... | Bioorg Med Chem Lett 27: 2069-2073 (2017) Article DOI: 10.1016/j.bmcl.2016.10.085 BindingDB Entry DOI: 10.7270/Q2DZ0BM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50239040 (CHEMBL4086771) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Merck& Co., Inc. Curated by ChEMBL | Assay Description Activation of glucokinase in rat INS-1 cells assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HILIC LC-M... | Bioorg Med Chem Lett 27: 2069-2073 (2017) Article DOI: 10.1016/j.bmcl.2016.10.085 BindingDB Entry DOI: 10.7270/Q2DZ0BM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50239040 (CHEMBL4086771) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Merck& Co., Inc. Curated by ChEMBL | Assay Description Activation of glucokinase in rat INS-1 cells assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HILIC LC-M... | Bioorg Med Chem Lett 27: 2069-2073 (2017) Article DOI: 10.1016/j.bmcl.2016.10.085 BindingDB Entry DOI: 10.7270/Q2DZ0BM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50239043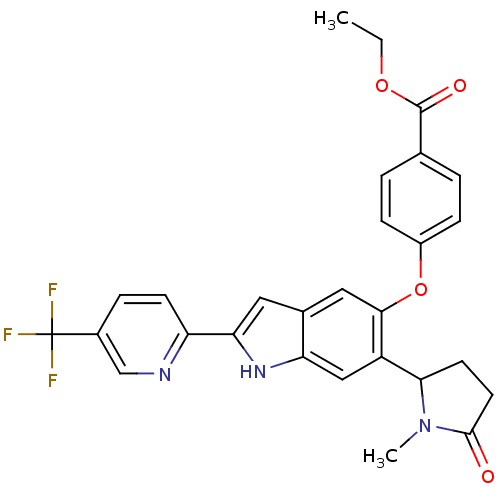 (CHEMBL4067037) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
Merck& Co., Inc. Curated by ChEMBL | Assay Description Activation of glucokinase in rat INS-1 cells assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HILIC LC-M... | Bioorg Med Chem Lett 27: 2069-2073 (2017) Article DOI: 10.1016/j.bmcl.2016.10.085 BindingDB Entry DOI: 10.7270/Q2DZ0BM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50239047 (CHEMBL4078915) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
Merck& Co., Inc. Curated by ChEMBL | Assay Description Activation of glucokinase in primary rat hepatocytes assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HI... | Bioorg Med Chem Lett 27: 2069-2073 (2017) Article DOI: 10.1016/j.bmcl.2016.10.085 BindingDB Entry DOI: 10.7270/Q2DZ0BM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50239047 (CHEMBL4078915) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
Merck& Co., Inc. Curated by ChEMBL | Assay Description Activation of glucokinase in primary rat hepatocytes assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HI... | Bioorg Med Chem Lett 27: 2069-2073 (2017) Article DOI: 10.1016/j.bmcl.2016.10.085 BindingDB Entry DOI: 10.7270/Q2DZ0BM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50321004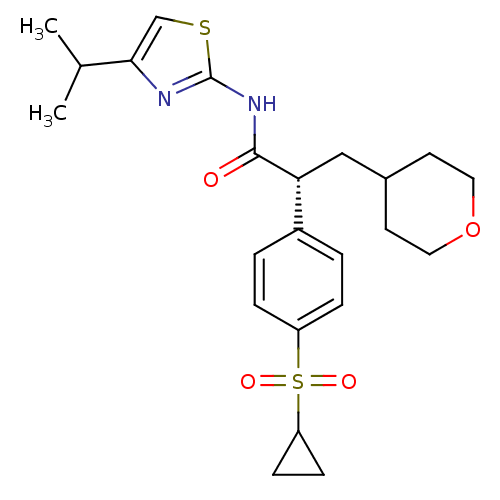 ((R)-2-(4-(Cyclopropylsulfonyl)phenyl)-N-(4-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Induction of glucokinase-mediated 2-deoxy-D-[3H]glucose uptake in Sprague-Dawley rat hepatocytes after 4 hrs by liquid scintillation countingl | Bioorg Med Chem 18: 3875-84 (2010) Article DOI: 10.1016/j.bmc.2010.04.038 BindingDB Entry DOI: 10.7270/Q2H995CS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50394682 (CHEMBL2165619) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 153 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Activation of glucokinase in rat INS-1 cells assessed as stimulation of glucose-induced insulin secretion | J Med Chem 55: 1318-33 (2012) Article DOI: 10.1021/jm2014887 BindingDB Entry DOI: 10.7270/Q2NG4RRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50239049 (CHEMBL4086782) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 174 | n/a | n/a | n/a | n/a |
Merck& Co., Inc. Curated by ChEMBL | Assay Description Inhibition of cGMP PDE II enzyme | Bioorg Med Chem Lett 27: 2069-2073 (2017) Article DOI: 10.1016/j.bmcl.2016.10.085 BindingDB Entry DOI: 10.7270/Q2DZ0BM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50394681 (CHEMBL2165620) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 190 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Induction of glucokinase translocation from nucleus to cytoplasm in cryopreserved rat hepatocytes after 1 hr by Hoechst staining-based fluorescence m... | J Med Chem 55: 1318-33 (2012) Article DOI: 10.1021/jm2014887 BindingDB Entry DOI: 10.7270/Q2NG4RRT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50239043 (CHEMBL4067037) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 232 | n/a | n/a | n/a | n/a |
Merck& Co., Inc. Curated by ChEMBL | Assay Description Activation of glucokinase in primary rat hepatocytes assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HI... | Bioorg Med Chem Lett 27: 2069-2073 (2017) Article DOI: 10.1016/j.bmcl.2016.10.085 BindingDB Entry DOI: 10.7270/Q2DZ0BM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161691 (CHEMBL180172 | N-{3-[3-(5-Chloro-thiazol-2-yl)-1-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50401849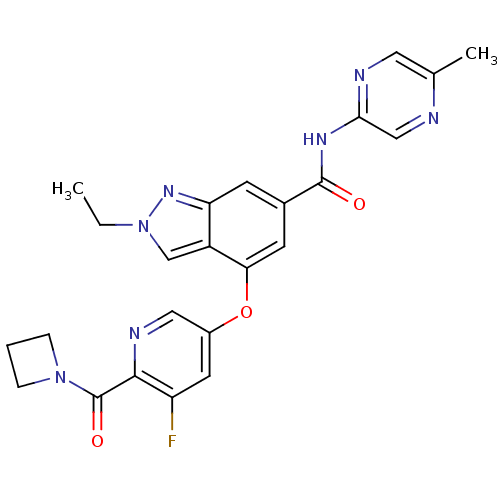 (CHEMBL2204663) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 350 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Activation of recombinant rat glucokinase assessed measuring rate of glucose 6-phosphate formation using G6PDH/NADP coupling | Bioorg Med Chem Lett 22: 7100-5 (2012) Article DOI: 10.1016/j.bmcl.2012.09.082 BindingDB Entry DOI: 10.7270/Q2GM88H7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161692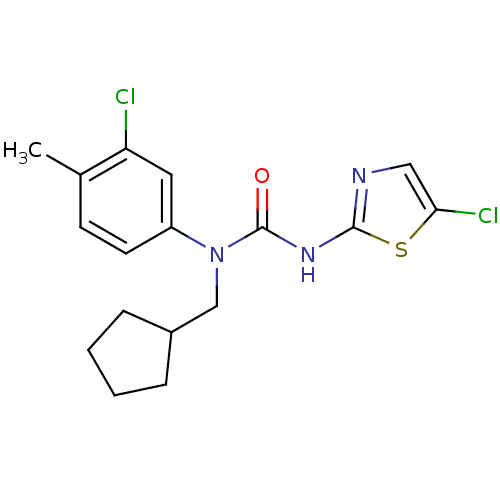 (1-(3-Chloro-4-methyl-phenyl)-3-(5-chloro-thiazol-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161695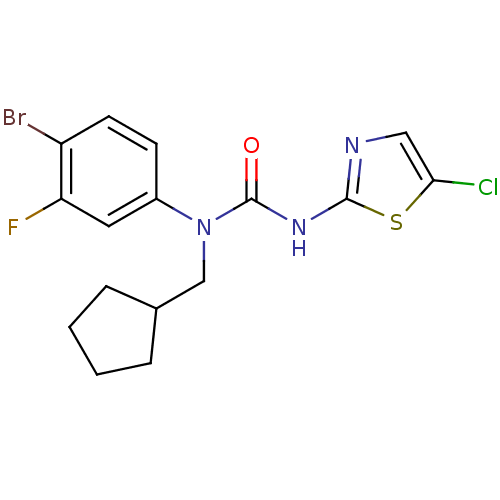 (1-(4-Bromo-3-fluoro-phenyl)-3-(5-chloro-thiazol-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 700 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50239042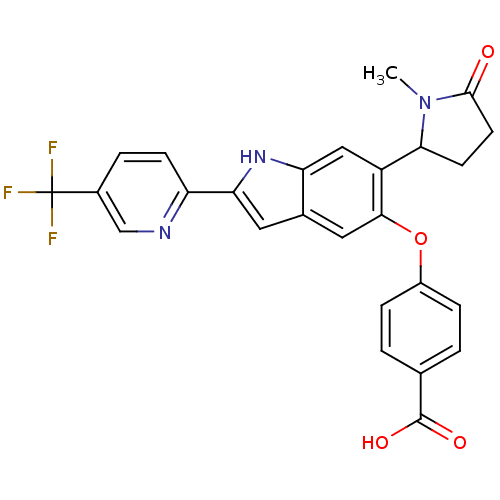 (CHEMBL4094511) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 728 | n/a | n/a | n/a | n/a |
Merck& Co., Inc. Curated by ChEMBL | Assay Description Activation of glucokinase in primary rat hepatocytes assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HI... | Bioorg Med Chem Lett 27: 2069-2073 (2017) Article DOI: 10.1016/j.bmcl.2016.10.085 BindingDB Entry DOI: 10.7270/Q2DZ0BM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50394684 (CHEMBL2165615) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Induction of glucokinase translocation from nucleus to cytoplasm in cryopreserved rat hepatocytes after 1 hr by Hoechst staining-based fluorescence m... | J Med Chem 55: 1318-33 (2012) Article DOI: 10.1021/jm2014887 BindingDB Entry DOI: 10.7270/Q2NG4RRT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161674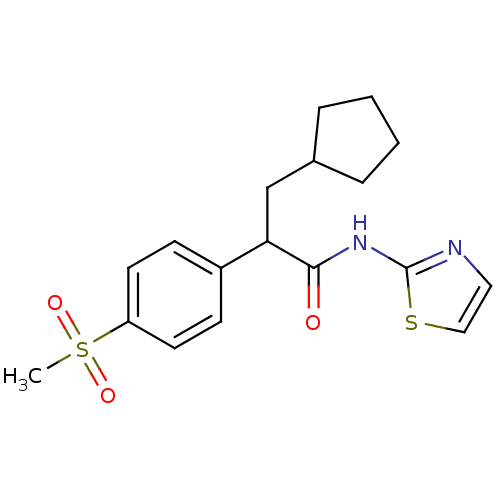 (3-Cyclopentyl-2-(4-methanesulfonyl-phenyl)-N-thiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 15 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161670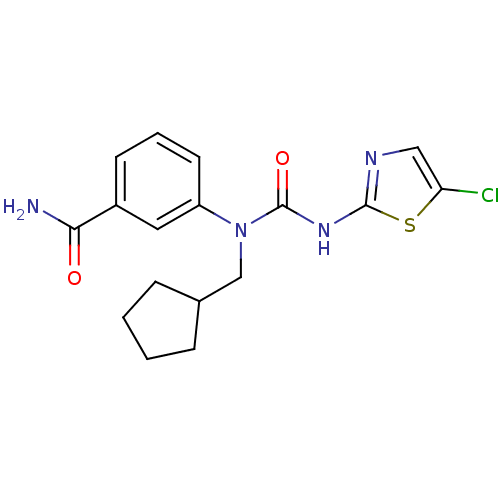 (3-[3-(5-Chloro-thiazol-2-yl)-1-cyclopentylmethyl-u...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161686 (3-(5-Chloro-thiazol-2-yl)-1-cyclopentylmethyl-1-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161682 (3-(5-Chloro-thiazol-2-yl)-1-cyclopentylmethyl-1-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161687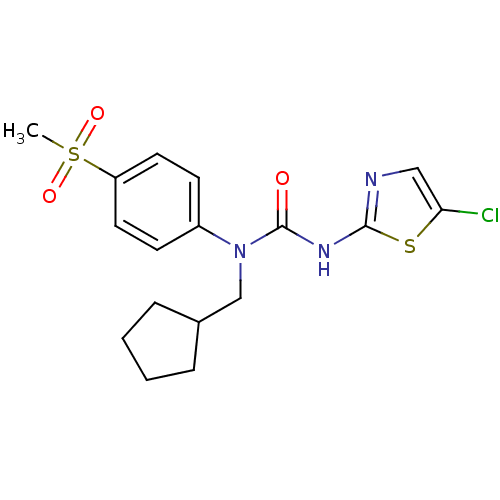 (3-(5-Chloro-thiazol-2-yl)-1-cyclopentylmethyl-1-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161677 (4-[3-(5-Chloro-thiazol-2-yl)-1-cyclopentylmethyl-u...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161672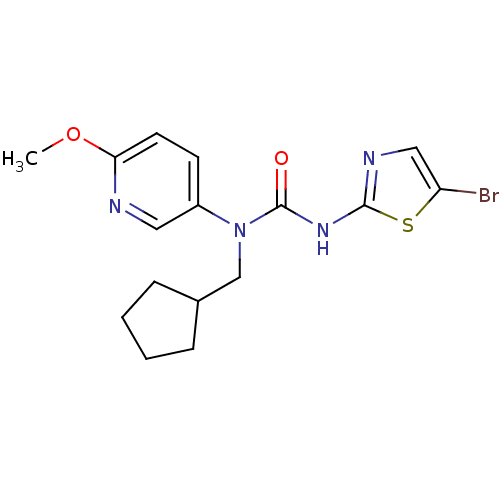 (3-(5-Bromo-thiazol-2-yl)-1-cyclopentylmethyl-1-(6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50239042 (CHEMBL4094511) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.11E+3 | n/a | n/a | n/a | n/a |
Merck& Co., Inc. Curated by ChEMBL | Assay Description Activation of glucokinase in rat INS-1 cells assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HILIC LC-M... | Bioorg Med Chem Lett 27: 2069-2073 (2017) Article DOI: 10.1016/j.bmcl.2016.10.085 BindingDB Entry DOI: 10.7270/Q2DZ0BM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161674 (3-Cyclopentyl-2-(4-methanesulfonyl-phenyl)-N-thiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161679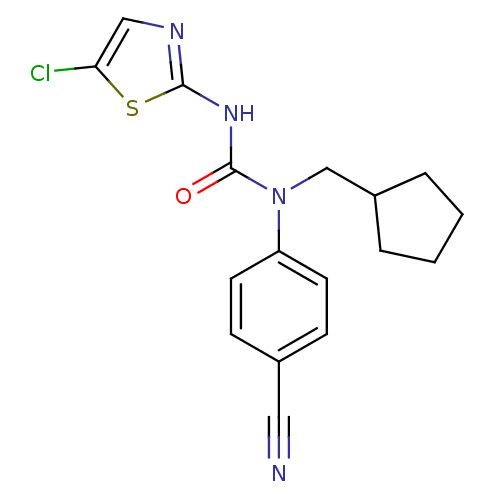 (3-(5-Chloro-thiazol-2-yl)-1-(4-cyano-phenyl)-1-cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161690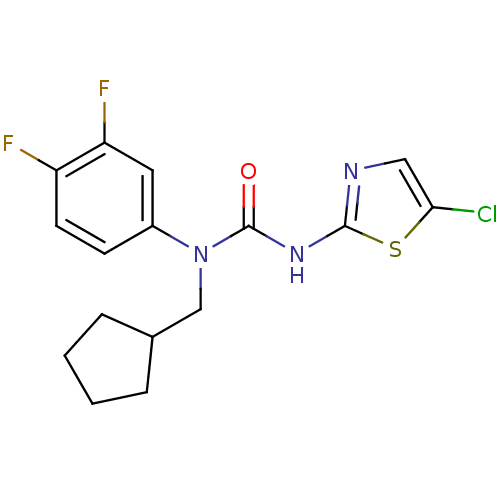 (3-(5-Chloro-thiazol-2-yl)-1-cyclopentylmethyl-1-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161678 (3-(5-Chloro-thiazol-2-yl)-1-cyclopentylmethyl-1-(6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161683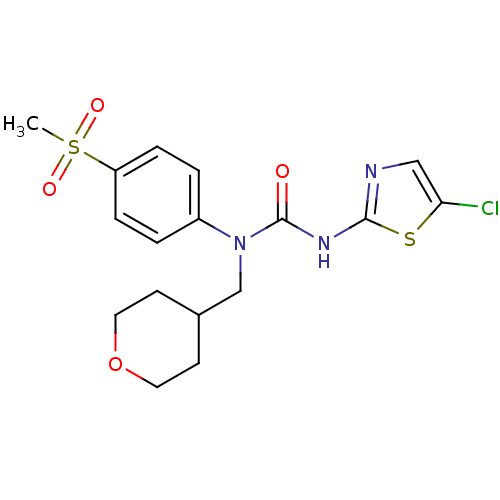 (3-(5-Chloro-thiazol-2-yl)-1-(4-methanesulfonyl-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161694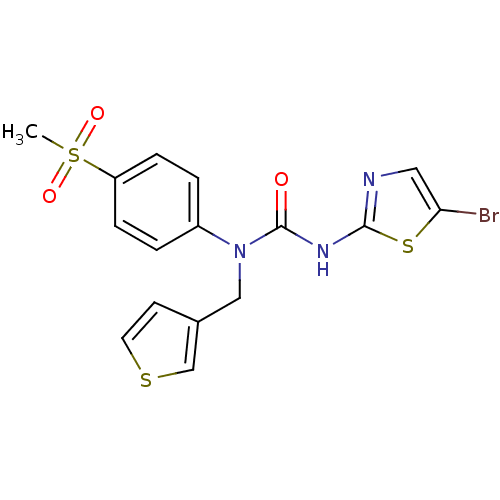 (3-(5-Bromo-thiazol-2-yl)-1-(4-methanesulfonyl-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Rattus norvegicus) | BDBM50161688 (3-(5-Chloro-thiazol-2-yl)-1-(4-methanesulfonyl-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for glucokinase activation with 5 mM glucose | Bioorg Med Chem Lett 15: 1501-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.083 BindingDB Entry DOI: 10.7270/Q2HT2NVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 66 total ) | Next | Last >> |