

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50395732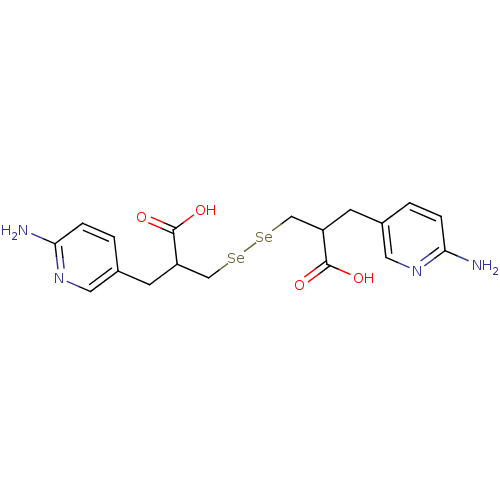 (CHEMBL2164462) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human activated TAFI using Hip-Arg as substrate incubated for 10 mins prior to substrate addition measured after 30 mins by spectrophot... | J Med Chem 55: 7696-705 (2012) Article DOI: 10.1021/jm300735t BindingDB Entry DOI: 10.7270/Q2N58NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50395730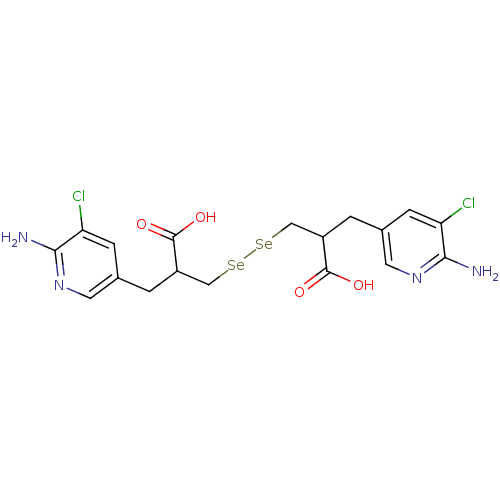 (CHEMBL2164450) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human activated TAFI using Hip-Arg as substrate incubated for 10 mins prior to substrate addition measured after 30 mins by spectrophot... | J Med Chem 55: 7696-705 (2012) Article DOI: 10.1021/jm300735t BindingDB Entry DOI: 10.7270/Q2N58NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089758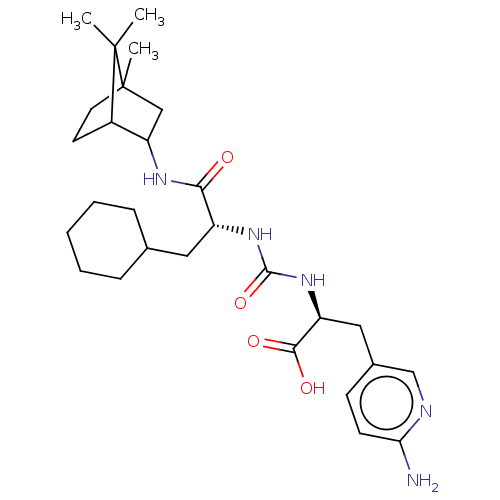 (CHEMBL3577442) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50144342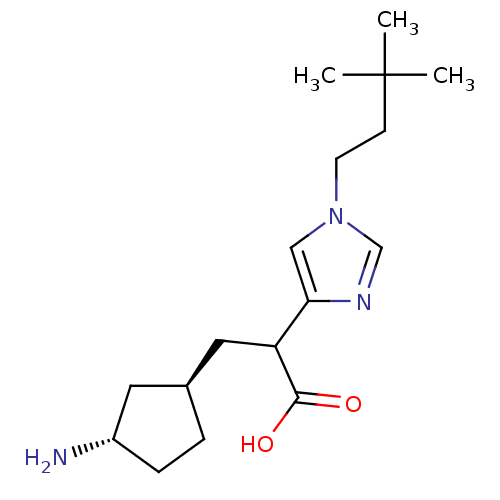 (3-((1R,3S)-3-Amino-cyclopentyl)-2-[1-(3,3-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) | Bioorg Med Chem Lett 14: 2141-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.033 BindingDB Entry DOI: 10.7270/Q2N8797S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575776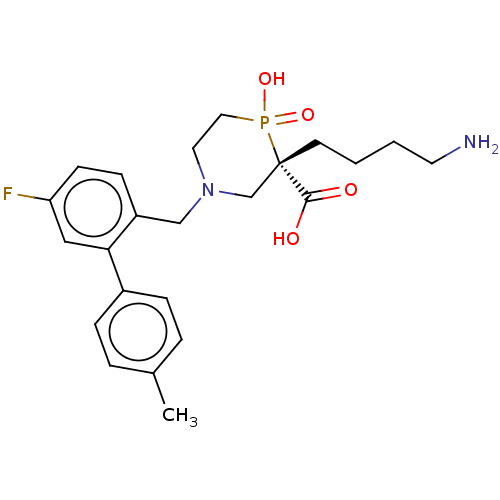 (CHEMBL4868605) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50395735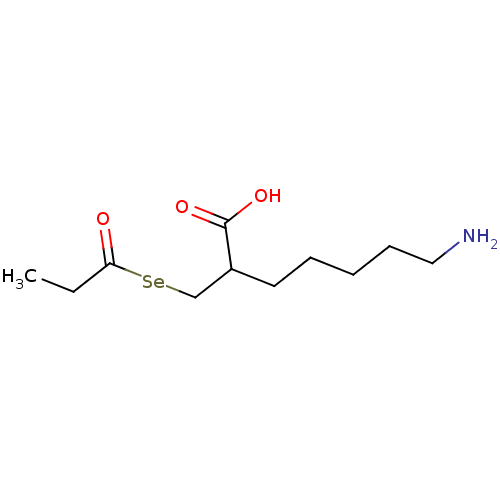 (CHEMBL2164459) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human activated TAFI using Hip-Arg as substrate incubated for 10 mins prior to substrate addition measured after 30 mins by spectrophot... | J Med Chem 55: 7696-705 (2012) Article DOI: 10.1021/jm300735t BindingDB Entry DOI: 10.7270/Q2N58NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089688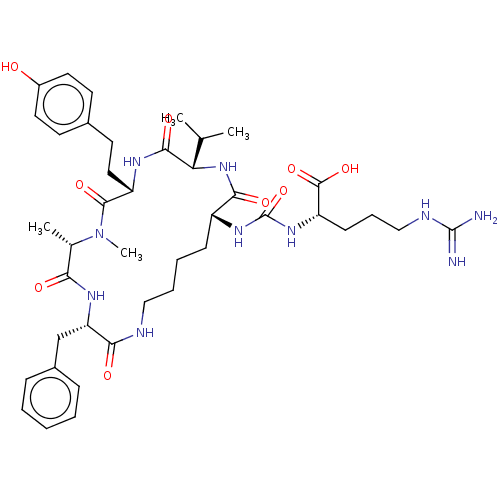 (ANABAENOPEPTIN B) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089687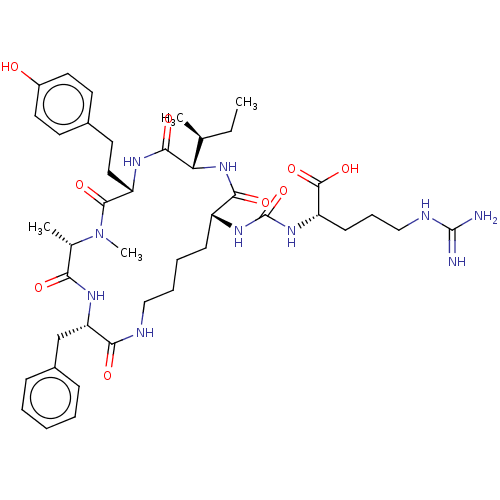 (Anabaenopeptin F) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50275212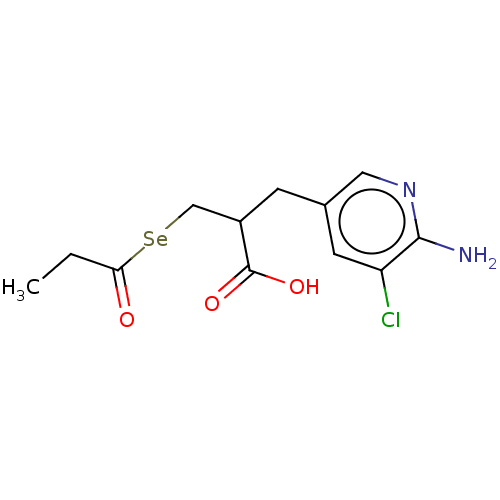 (CHEMBL4127473) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human plasma activated thrombin-activatable fibrinolysis inhibitor after 10 mins in presence of DTT | Bioorg Med Chem Lett 28: 2256-2260 (2018) Article DOI: 10.1016/j.bmcl.2018.05.042 BindingDB Entry DOI: 10.7270/Q25M686N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089686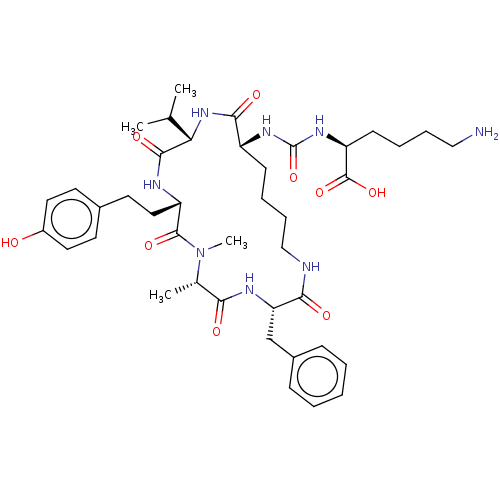 (CHEMBL3577334) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575768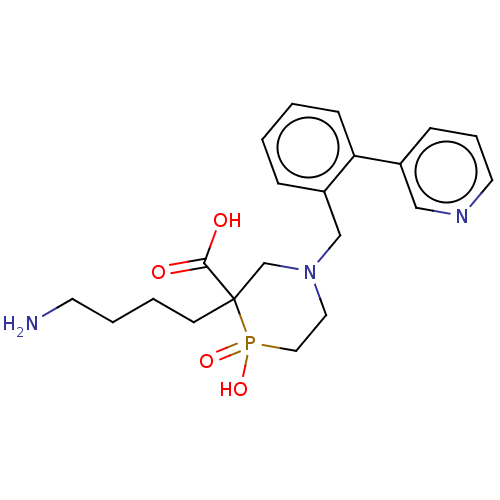 (CHEMBL4846664) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50395737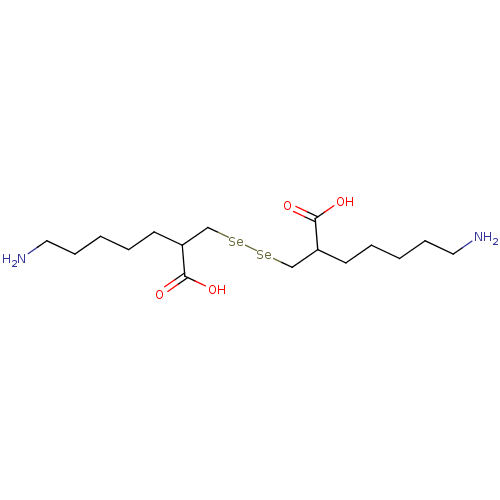 (CHEMBL2164457) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human activated TAFI using Hip-Arg as substrate incubated for 10 mins prior to substrate addition measured after 30 mins by spectrophot... | J Med Chem 55: 7696-705 (2012) Article DOI: 10.1021/jm300735t BindingDB Entry DOI: 10.7270/Q2N58NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50144337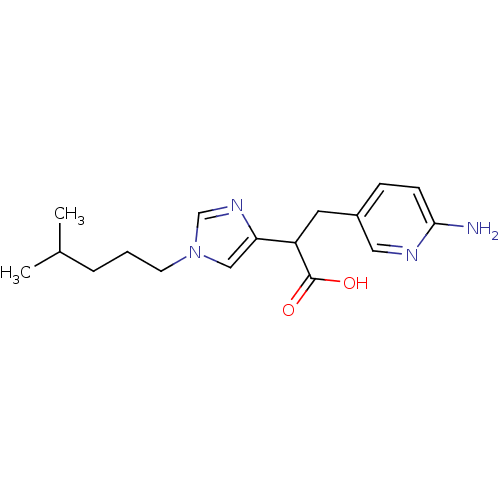 (3-(6-Amino-pyridin-3-yl)-2-[1-(4-methyl-pentyl)-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) | Bioorg Med Chem Lett 14: 2141-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.033 BindingDB Entry DOI: 10.7270/Q2N8797S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM109264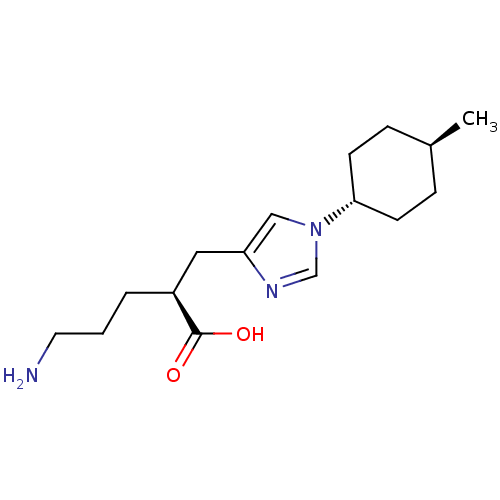 (US8609710, 40) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Company, Limited US Patent | Assay Description Enzyme inhibition assay using TAFIa. | US Patent US8609710 (2013) BindingDB Entry DOI: 10.7270/Q2KW5DPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50135934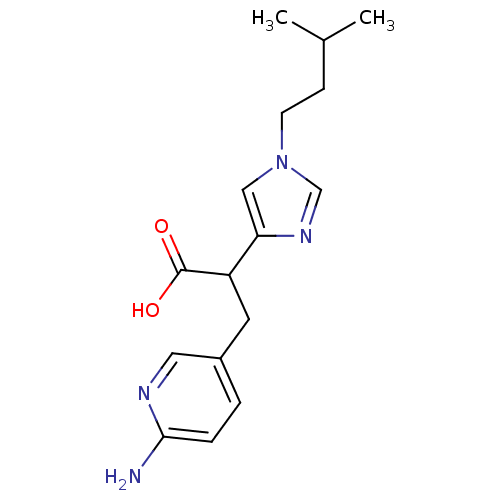 (3-(6-Amino-pyridin-3-yl)-2-[1-(3-methyl-butyl)-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of purified Carboxypeptidase B (CPB) by clot lysis assay in human plasma | J Med Chem 46: 5294-7 (2003) Article DOI: 10.1021/jm034141y BindingDB Entry DOI: 10.7270/Q27H1J0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50197573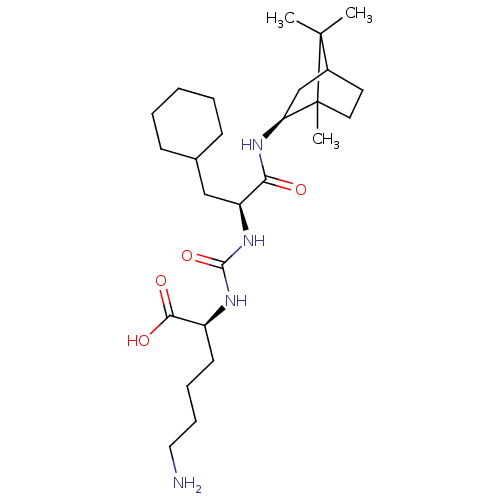 (CHEMBL3912764) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Inhibition of activated human plasma TAFI incubated for 15 mins by chromogenic assay | J Med Chem 59: 9567-9573 (2016) Article DOI: 10.1021/acs.jmedchem.6b01276 BindingDB Entry DOI: 10.7270/Q2MG7RG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089691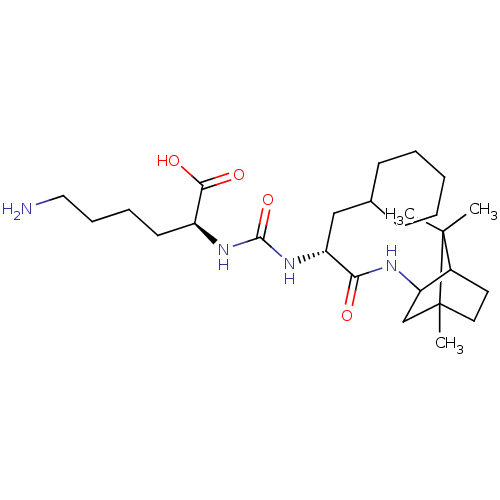 (CHEMBL3577425) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089755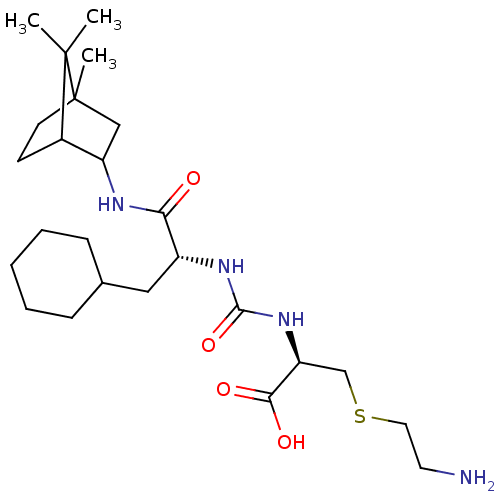 (CHEMBL3577439) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089751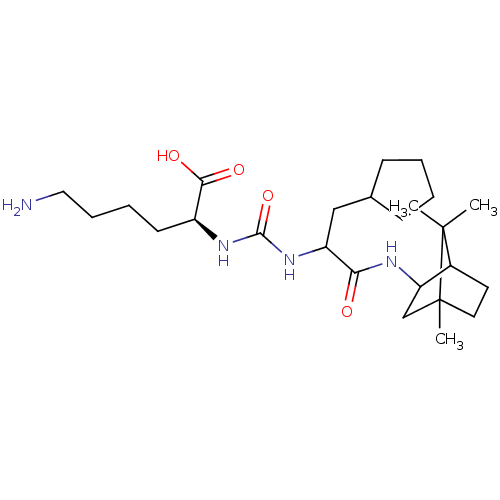 (CHEMBL3577435) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089756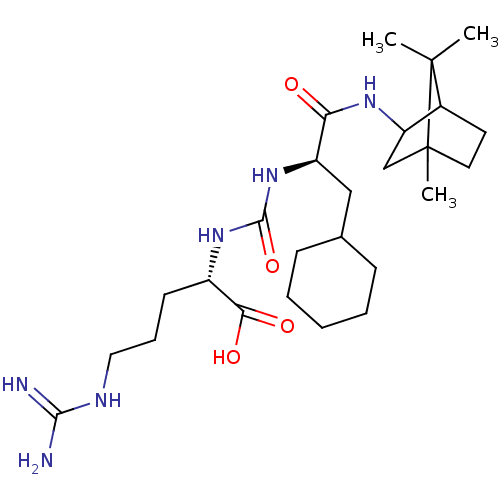 (CHEMBL3577440) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50395734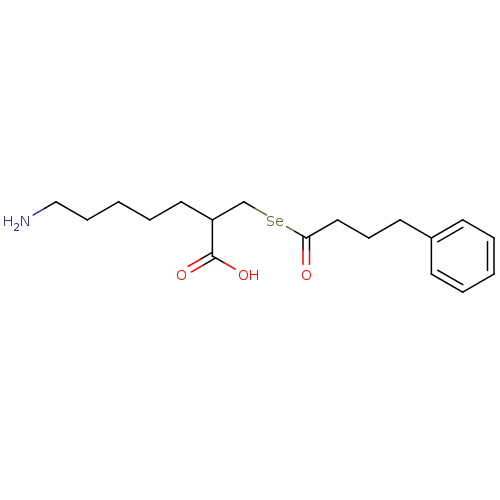 (CHEMBL2164460) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human activated TAFI using Hip-Arg as substrate incubated for 10 mins prior to substrate addition measured after 30 mins by spectrophot... | J Med Chem 55: 7696-705 (2012) Article DOI: 10.1021/jm300735t BindingDB Entry DOI: 10.7270/Q2N58NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM326336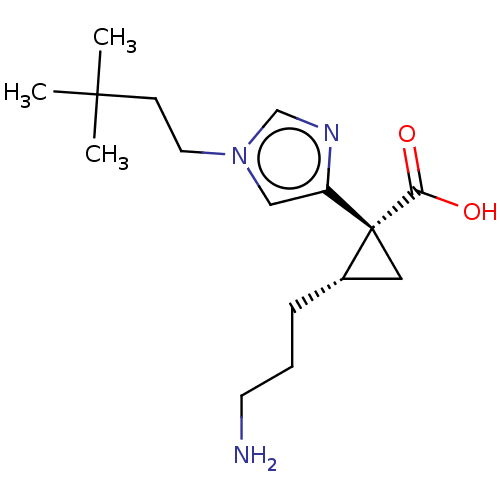 ((1R*,2S*)-2-(3-Aminopropyl)-1-[1-(3,3-dimethylbuty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
DAIICHI SANKYO COMPANY, LIMITED US Patent | Assay Description HEPES buffered saline (20 mM HEPES, 150 mM NaCl, pH 7.4; hereinafter, referred to as HBS) was used in the preparation of a reaction solution. To 12 &... | US Patent US9662310 (2017) BindingDB Entry DOI: 10.7270/Q2W09829 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575770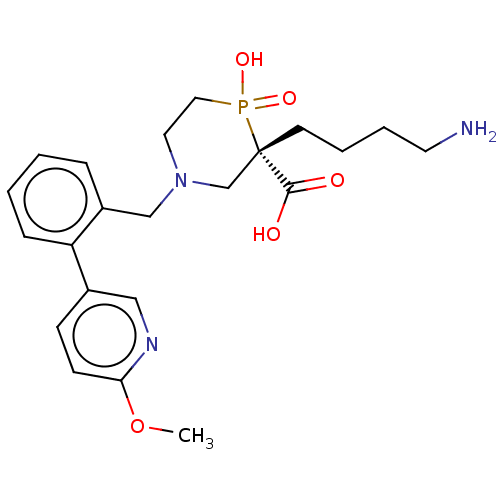 (CHEMBL4858095) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50135934 (3-(6-Amino-pyridin-3-yl)-2-[1-(3-methyl-butyl)-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) | Bioorg Med Chem Lett 14: 2141-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.033 BindingDB Entry DOI: 10.7270/Q2N8797S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50201427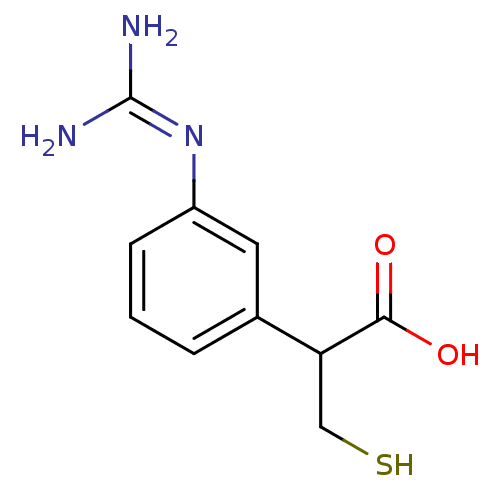 (2-(3-guanidinophenyl)-3-mercaptopropanoic acid | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibition of human activated thrombin activatable fibrinolysis inhibitor | Bioorg Med Chem Lett 17: 1349-54 (2007) Article DOI: 10.1016/j.bmcl.2006.11.078 BindingDB Entry DOI: 10.7270/Q2RJ4J5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575775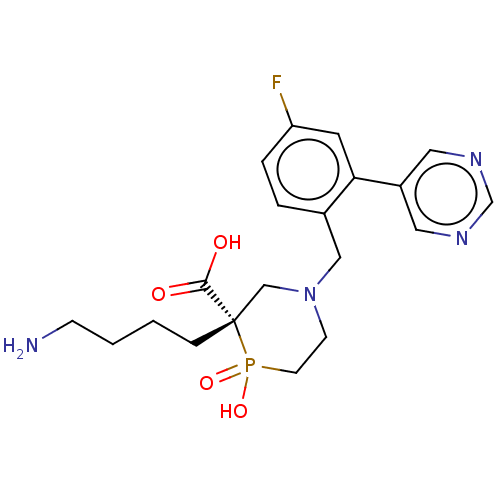 (CHEMBL4861532) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM109265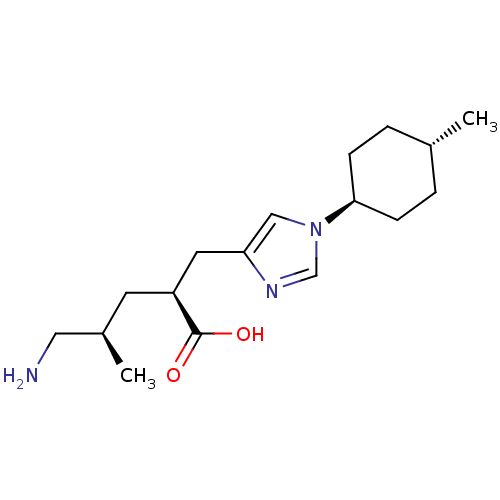 (US8609710, 35 (2S,4R form)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Company, Limited US Patent | Assay Description Enzyme inhibition assay using TAFIa. | US Patent US8609710 (2013) BindingDB Entry DOI: 10.7270/Q2KW5DPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50395733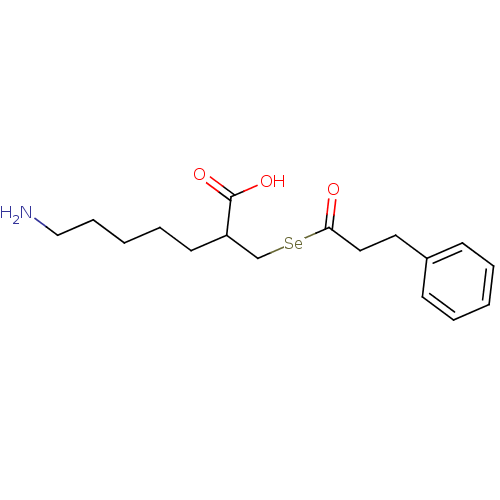 (CHEMBL2164461) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human activated TAFI using Hip-Arg as substrate incubated for 10 mins prior to substrate addition measured after 30 mins by spectrophot... | J Med Chem 55: 7696-705 (2012) Article DOI: 10.1021/jm300735t BindingDB Entry DOI: 10.7270/Q2N58NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM109258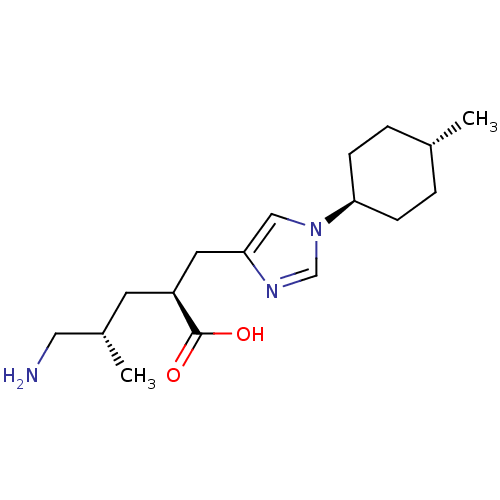 (US8609710, 34 (2S,4S form)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Company, Limited US Patent | Assay Description Enzyme inhibition assay using TAFIa. | US Patent US8609710 (2013) BindingDB Entry DOI: 10.7270/Q2KW5DPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50395728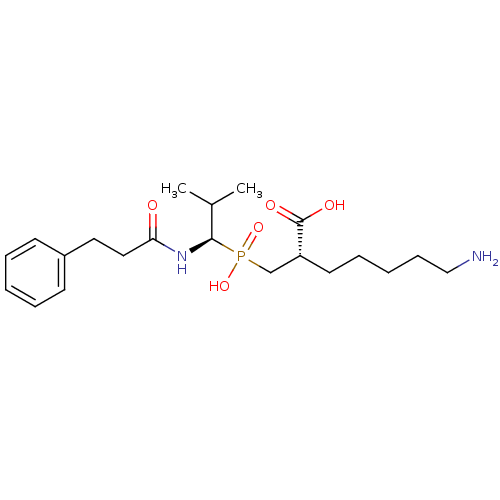 (CHEMBL2164463) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human activated TAFI using Hip-Arg as substrate incubated for 10 mins prior to substrate addition measured after 30 mins by spectrophot... | J Med Chem 55: 7696-705 (2012) Article DOI: 10.1021/jm300735t BindingDB Entry DOI: 10.7270/Q2N58NHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM173886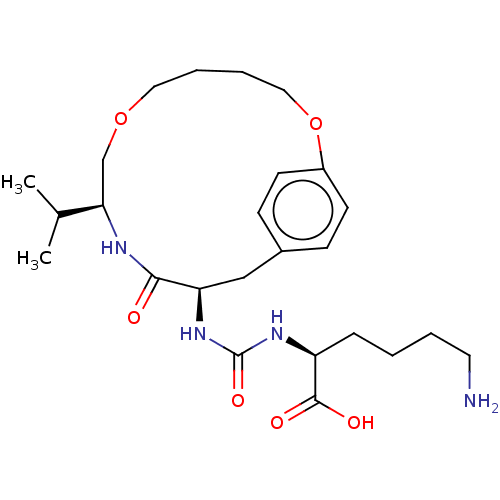 (US9688645, 1-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 25 |
SANOFI US Patent | Assay Description The prepared substances were tested for TAFIa inhibition using the Actichrome plasma TAFI Activity Kit from American Diagnostica (Pr. No. 874). This ... | US Patent US9688645 (2017) BindingDB Entry DOI: 10.7270/Q2PV6HJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575780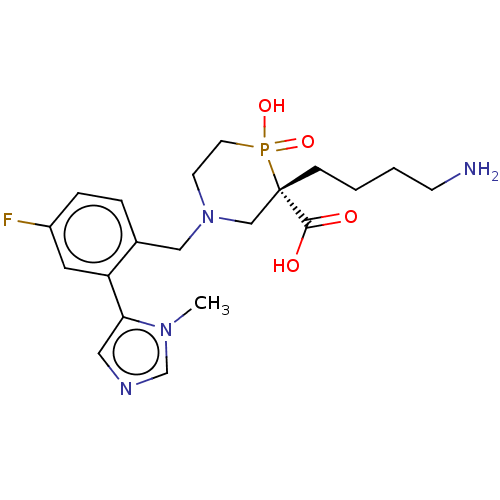 (CHEMBL4878039) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50135936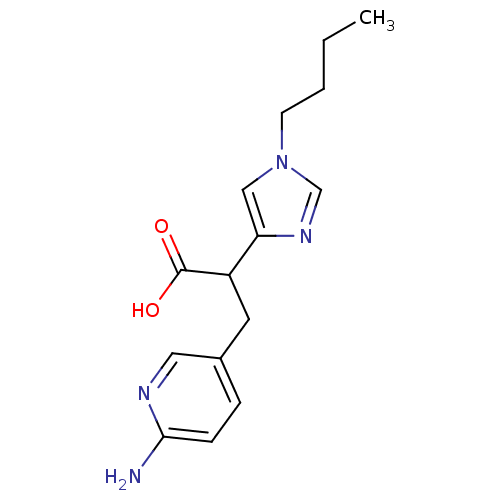 (3-(6-Amino-pyridin-3-yl)-2-(1-butyl-1H-imidazol-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of purified Carboxypeptidase B (CPB) by clot lysis assay in human plasma | J Med Chem 46: 5294-7 (2003) Article DOI: 10.1021/jm034141y BindingDB Entry DOI: 10.7270/Q27H1J0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575769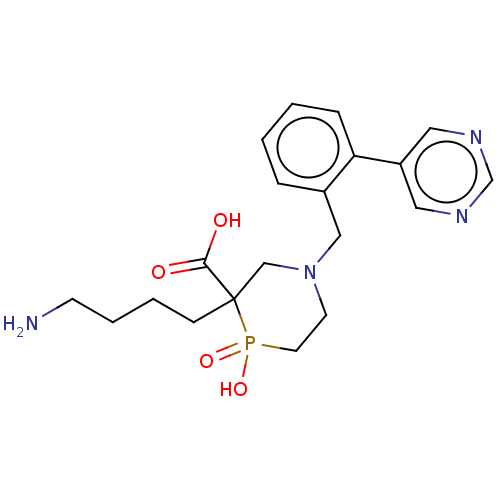 (CHEMBL4853133) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM109254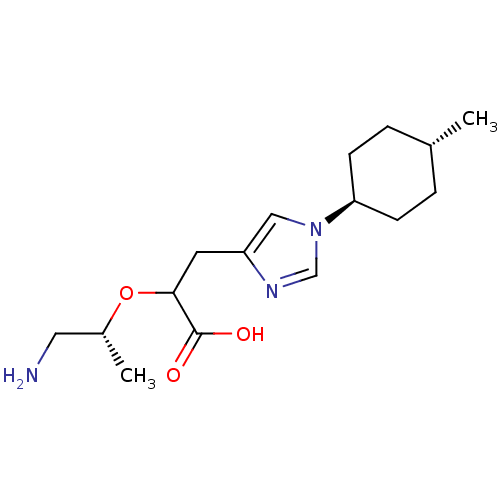 (US8609710, 25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Company, Limited US Patent | Assay Description Enzyme inhibition assay using TAFIa. | US Patent US8609710 (2013) BindingDB Entry DOI: 10.7270/Q2KW5DPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089691 (CHEMBL3577425) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins in presence of 1% human serum albumin by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089749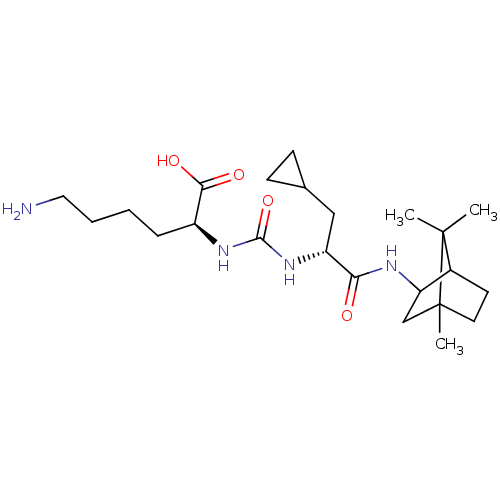 (CHEMBL3577433) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM109256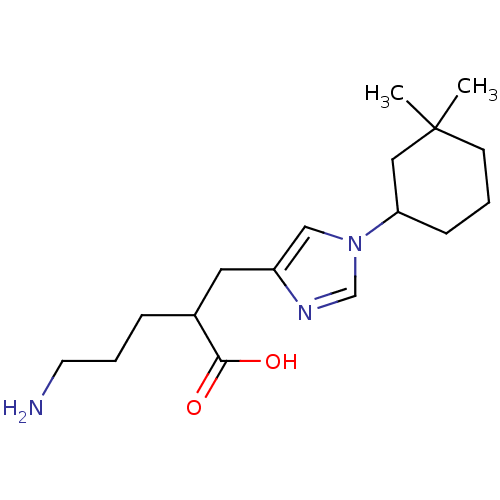 (US8609710, 33) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Company, Limited US Patent | Assay Description Enzyme inhibition assay using TAFIa. | US Patent US8609710 (2013) BindingDB Entry DOI: 10.7270/Q2KW5DPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM173906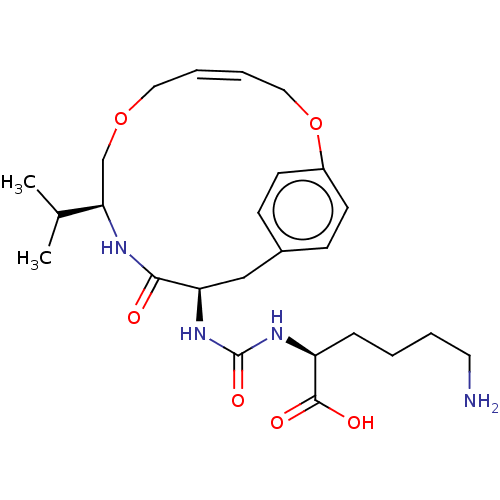 (US9688645, 3-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | 7.4 | 25 |
SANOFI US Patent | Assay Description The prepared substances were tested for TAFIa inhibition using the Actichrome plasma TAFI Activity Kit from American Diagnostica (Pr. No. 874). This ... | US Patent US9688645 (2017) BindingDB Entry DOI: 10.7270/Q2PV6HJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM109252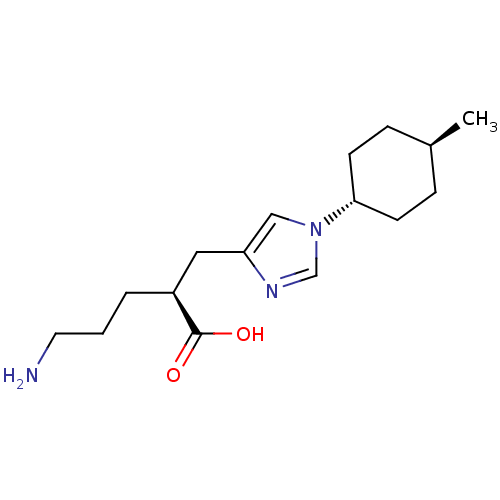 (US8609710, 15 (2S form)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Company, Limited US Patent | Assay Description Enzyme inhibition assay using TAFIa. | US Patent US8609710 (2013) BindingDB Entry DOI: 10.7270/Q2KW5DPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM326397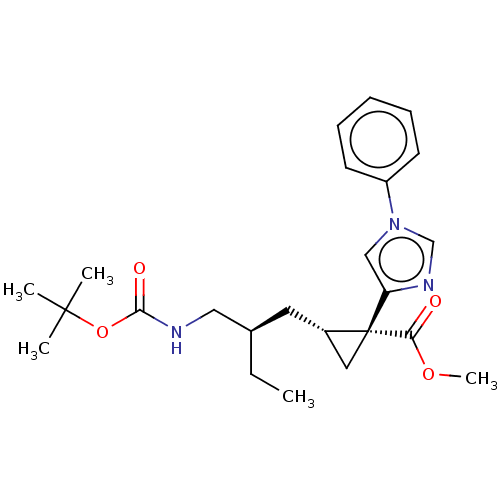 ((1R,2S)-2-[(2R)-2-(Aminomethyl)butyl]-1-(1-phenyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
DAIICHI SANKYO COMPANY, LIMITED US Patent | Assay Description HEPES buffered saline (20 mM HEPES, 150 mM NaCl, pH 7.4; hereinafter, referred to as HBS) was used in the preparation of a reaction solution. To 12 &... | US Patent US9662310 (2017) BindingDB Entry DOI: 10.7270/Q2W09829 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50201438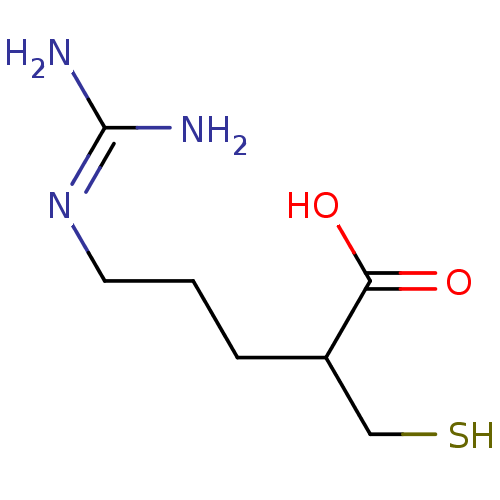 ((+/-)-5-guanidino-2-(mercaptomethyl)pentanoic acid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibition of human activated thrombin activatable fibrinolysis inhibitor | Bioorg Med Chem Lett 17: 1349-54 (2007) Article DOI: 10.1016/j.bmcl.2006.11.078 BindingDB Entry DOI: 10.7270/Q2RJ4J5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50008271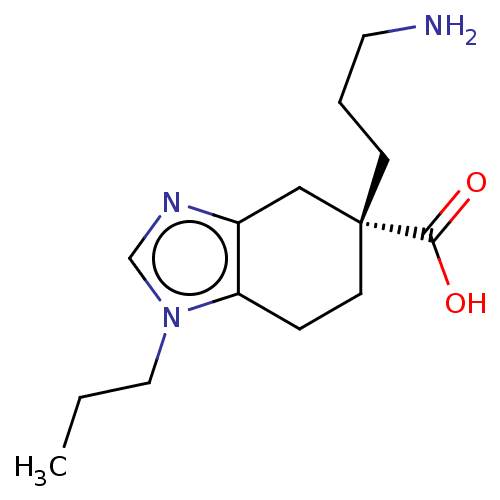 (CHEMBL3235132) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human TAF1a using hippuryl-L-arginine/hippuryl-L-lysine as substrate by liquid chromatographic analysis | Bioorg Med Chem 22: 2261-8 (2014) Article DOI: 10.1016/j.bmc.2014.02.010 BindingDB Entry DOI: 10.7270/Q2DZ09TG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575779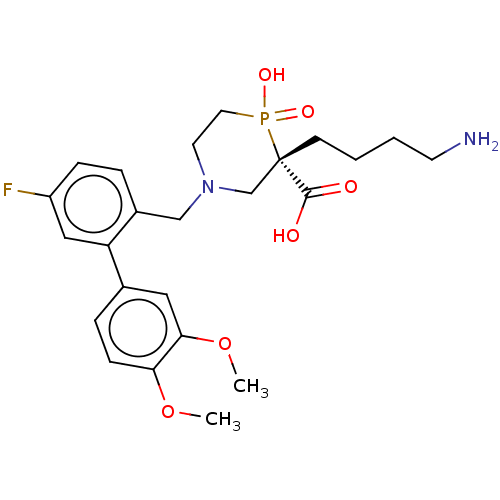 (CHEMBL4858909) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50575777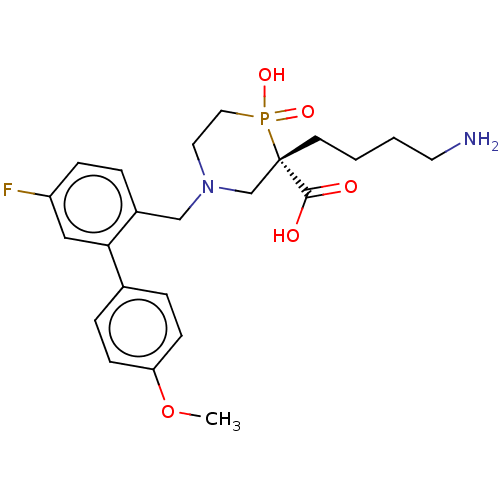 (CHEMBL4852722) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02072 BindingDB Entry DOI: 10.7270/Q2SB49JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM109253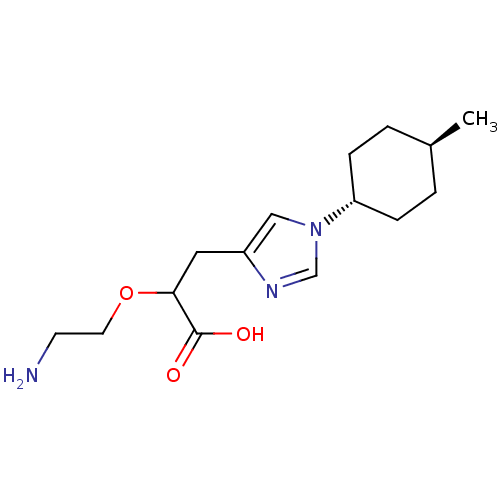 (US8609710, 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Company, Limited US Patent | Assay Description Enzyme inhibition assay using TAFIa. | US Patent US8609710 (2013) BindingDB Entry DOI: 10.7270/Q2KW5DPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50135939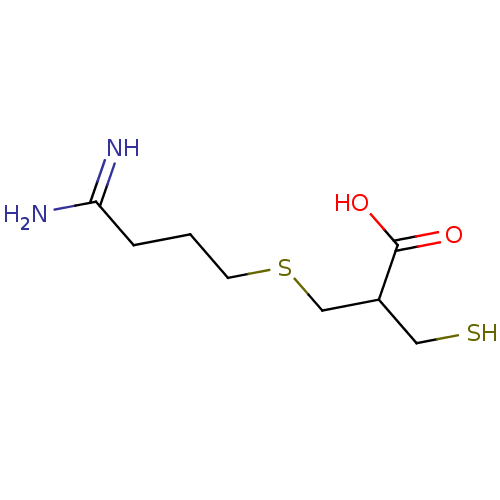 (3-(4,4-Diamino-but-3-enylsulfanyl)-2-mercaptomethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of purified Carboxypeptidase B (CPB) by clot lysis assay in human plasma | J Med Chem 46: 5294-7 (2003) Article DOI: 10.1021/jm034141y BindingDB Entry DOI: 10.7270/Q27H1J0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM326395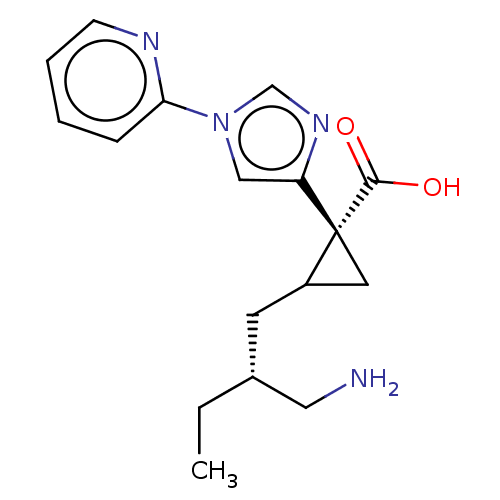 ((1R,2S)-2-[(2R)-2-(Aminomethyl)butyl]-1-(1-pyridin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
DAIICHI SANKYO COMPANY, LIMITED US Patent | Assay Description HEPES buffered saline (20 mM HEPES, 150 mM NaCl, pH 7.4; hereinafter, referred to as HBS) was used in the preparation of a reaction solution. To 12 &... | US Patent US9662310 (2017) BindingDB Entry DOI: 10.7270/Q2W09829 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM109238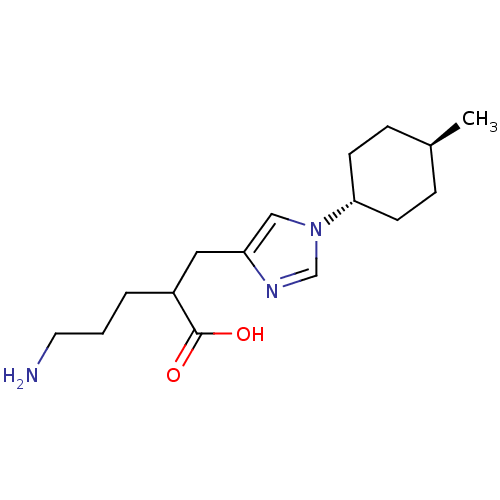 (US8609710, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Company, Limited US Patent | Assay Description Enzyme inhibition assay using TAFIa. | US Patent US8609710 (2013) BindingDB Entry DOI: 10.7270/Q2KW5DPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM326393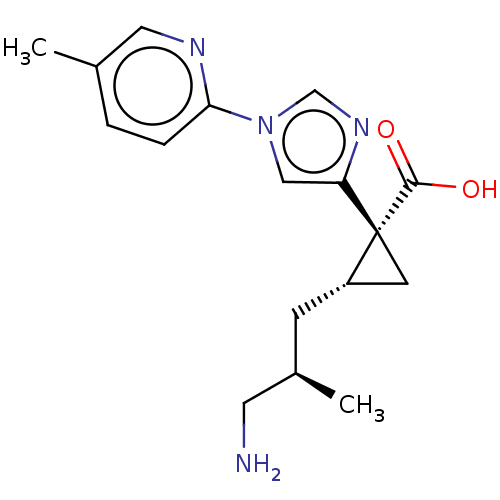 ((1R,2S)-2-[(2R)-3-Amino-2-methylpropyl]-1-[1-(5-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
DAIICHI SANKYO COMPANY, LIMITED US Patent | Assay Description HEPES buffered saline (20 mM HEPES, 150 mM NaCl, pH 7.4; hereinafter, referred to as HBS) was used in the preparation of a reaction solution. To 12 &... | US Patent US9662310 (2017) BindingDB Entry DOI: 10.7270/Q2W09829 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 387 total ) | Next | Last >> |