

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50088982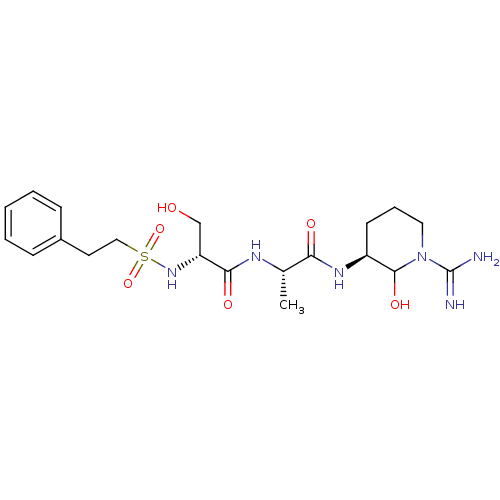 (CHEMBL160253 | CHEMBL367004 | N-[1-(1-Carbamimidoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50088978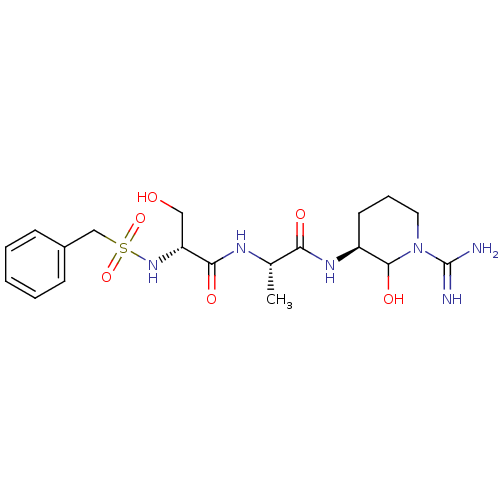 (CHEMBL176515 | N-[1-(1-Carbamimidoyl-2-hydroxy-pip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50088984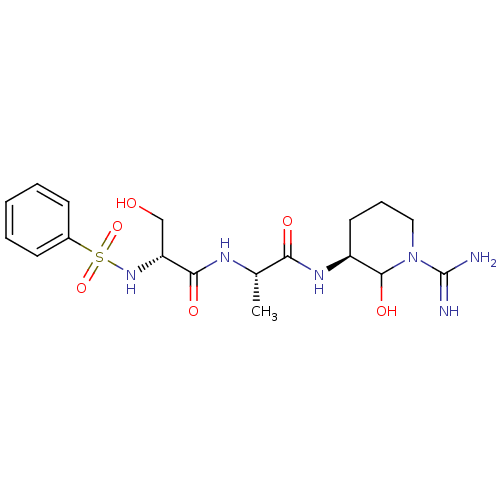 (2-Benzenesulfonylamino-N-[1-(1-carbamimidoyl-2-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50088979 (CHEMBL366666 | {(R)-2-[2-((S)-1-Carbamimidoyl-2-hy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50088985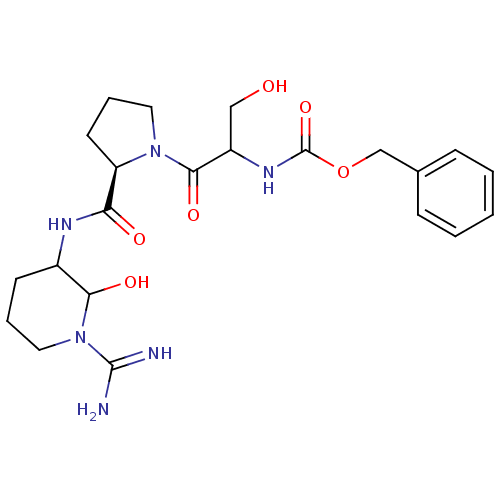 (CHEMBL369042 | {2-[2-(1-Carbamimidoyl-2-hydroxy-pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50088977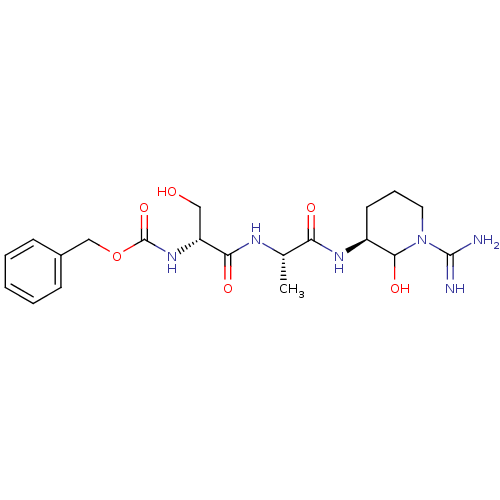 (CHEMBL177557 | {1-[1-(1-Carbamimidoyl-2-hydroxy-pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50088987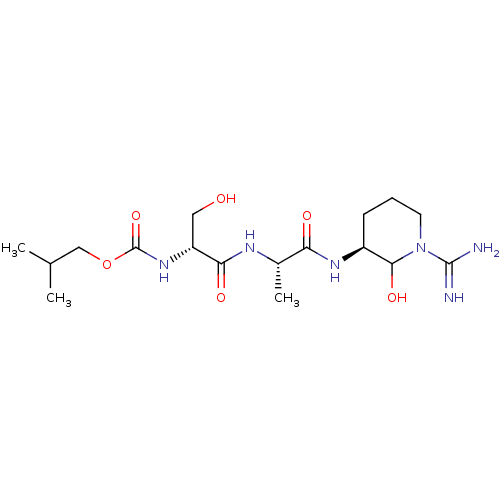 (CHEMBL174813 | {1-[1-(1-Carbamimidoyl-2-hydroxy-pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50088986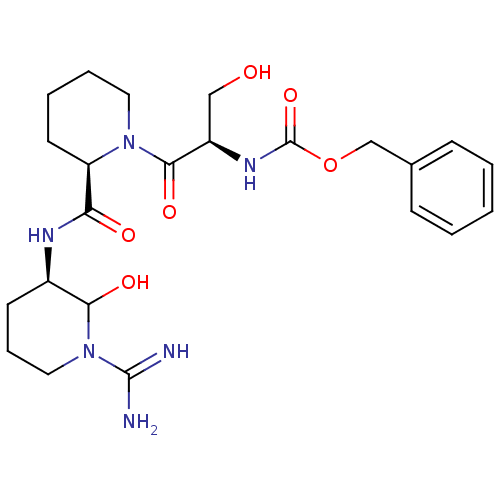 (CHEMBL433809 | {2-[2-(1-Carbamimidoyl-2-hydroxy-pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50088985 (CHEMBL369042 | {2-[2-(1-Carbamimidoyl-2-hydroxy-pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human Plasmin enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50088981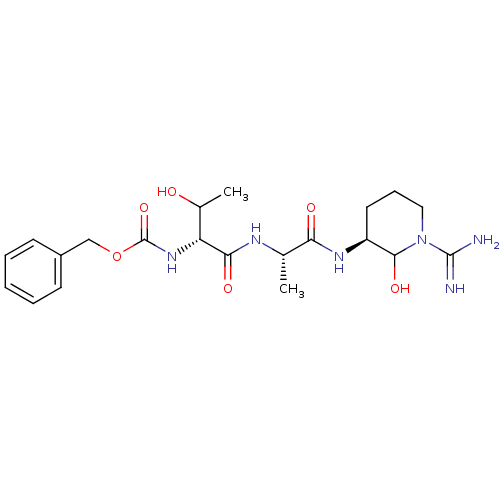 (CHEMBL425850 | {1-[1-(1-Carbamimidoyl-2-hydroxy-pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 261 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50088978 (CHEMBL176515 | N-[1-(1-Carbamimidoyl-2-hydroxy-pip...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human Plasmin enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50088982 (CHEMBL160253 | CHEMBL367004 | N-[1-(1-Carbamimidoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 367 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human Plasmin enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50088979 (CHEMBL366666 | {(R)-2-[2-((S)-1-Carbamimidoyl-2-hy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 482 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human Plasmin enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50088981 (CHEMBL425850 | {1-[1-(1-Carbamimidoyl-2-hydroxy-pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 699 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human Plasmin enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50088977 (CHEMBL177557 | {1-[1-(1-Carbamimidoyl-2-hydroxy-pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 904 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human Plasmin enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50088984 (2-Benzenesulfonylamino-N-[1-(1-carbamimidoyl-2-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human Plasmin enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50088987 (CHEMBL174813 | {1-[1-(1-Carbamimidoyl-2-hydroxy-pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human Plasmin enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50088983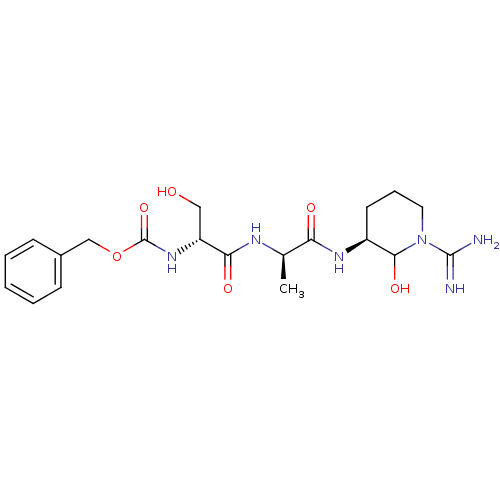 (CHEMBL177251 | {1-[1-(1-Carbamimidoyl-2-hydroxy-pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human t-PA enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50088984 (2-Benzenesulfonylamino-N-[1-(1-carbamimidoyl-2-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human t-PA enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50088979 (CHEMBL366666 | {(R)-2-[2-((S)-1-Carbamimidoyl-2-hy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human t-PA enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50088987 (CHEMBL174813 | {1-[1-(1-Carbamimidoyl-2-hydroxy-pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human t-PA enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50088983 (CHEMBL177251 | {1-[1-(1-Carbamimidoyl-2-hydroxy-pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50088980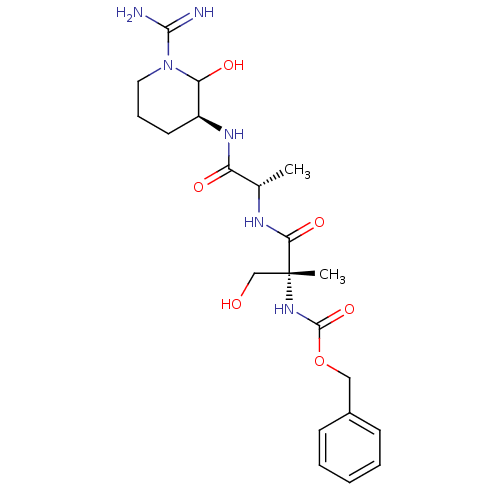 (CHEMBL177745 | {(R)-1-[(S)-1-((S)-1-Carbamimidoyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human t-PA enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50088986 (CHEMBL433809 | {2-[2-(1-Carbamimidoyl-2-hydroxy-pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human t-PA enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50088980 (CHEMBL177745 | {(R)-1-[(S)-1-((S)-1-Carbamimidoyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human Plasmin enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50088985 (CHEMBL369042 | {2-[2-(1-Carbamimidoyl-2-hydroxy-pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human t-PA enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50088983 (CHEMBL177251 | {1-[1-(1-Carbamimidoyl-2-hydroxy-pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human Plasmin enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50088982 (CHEMBL160253 | CHEMBL367004 | N-[1-(1-Carbamimidoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human t-PA enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50088981 (CHEMBL425850 | {1-[1-(1-Carbamimidoyl-2-hydroxy-pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human t-PA enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50088978 (CHEMBL176515 | N-[1-(1-Carbamimidoyl-2-hydroxy-pip...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human t-PA enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50088977 (CHEMBL177557 | {1-[1-(1-Carbamimidoyl-2-hydroxy-pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human t-PA enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50088980 (CHEMBL177745 | {(R)-1-[(S)-1-((S)-1-Carbamimidoyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||