 Found 99 hits of Enzyme Inhibition Constant Data
Found 99 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174708

(CHEMBL197375 | N-(2-(3-((2R,5S)-4-(4-fluorobenzyl)...)Show SMILES C[C@H]1CN([C@H](C)CN1Cc1ccc(F)cc1)C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C24H27ClFN3O2/c1-16-14-29(17(2)13-28(16)15-19-4-9-22(26)10-5-19)24(31)11-7-20-6-8-21(25)12-23(20)27-18(3)30/h4-12,16-17H,13-15H2,1-3H3,(H,27,30)/b11-7+/t16-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174703
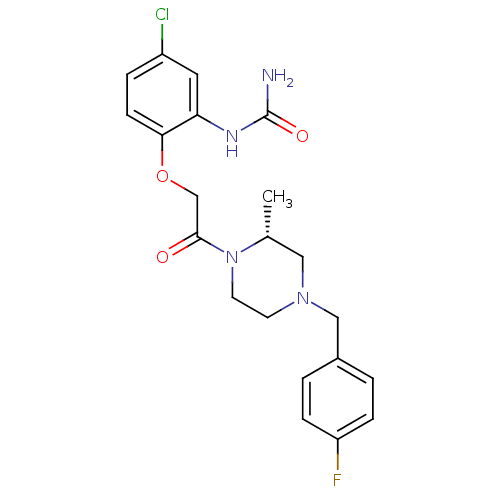
((R)-1-(2-(2-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)COc1ccc(Cl)cc1NC(N)=O Show InChI InChI=1S/C21H24ClFN4O3/c1-14-11-26(12-15-2-5-17(23)6-3-15)8-9-27(14)20(28)13-30-19-7-4-16(22)10-18(19)25-21(24)29/h2-7,10,14H,8-9,11-13H2,1H3,(H3,24,25,29)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174707

((R)-1-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(N)=O Show InChI InChI=1S/C22H24ClFN4O2/c1-15-13-27(14-16-2-7-19(24)8-3-16)10-11-28(15)21(29)9-5-17-4-6-18(23)12-20(17)26-22(25)30/h2-9,12,15H,10-11,13-14H2,1H3,(H3,25,26,30)/b9-5+/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174711
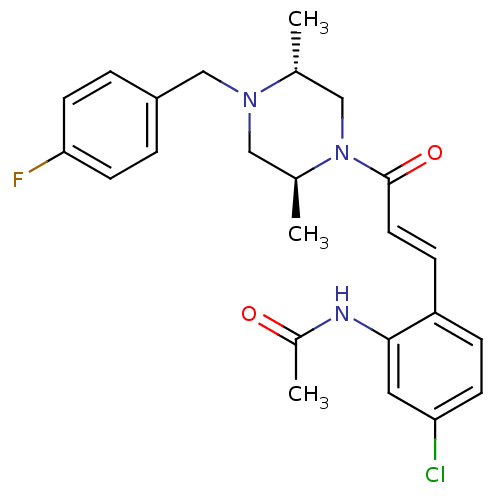
(CHEMBL197345 | N-(2-(3-((2S,5R)-4-(4-fluorobenzyl)...)Show SMILES C[C@@H]1CN([C@@H](C)CN1Cc1ccc(F)cc1)C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C24H27ClFN3O2/c1-16-14-29(17(2)13-28(16)15-19-4-9-22(26)10-5-19)24(31)11-7-20-6-8-21(25)12-23(20)27-18(3)30/h4-12,16-17H,13-15H2,1-3H3,(H,27,30)/b11-7+/t16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174709
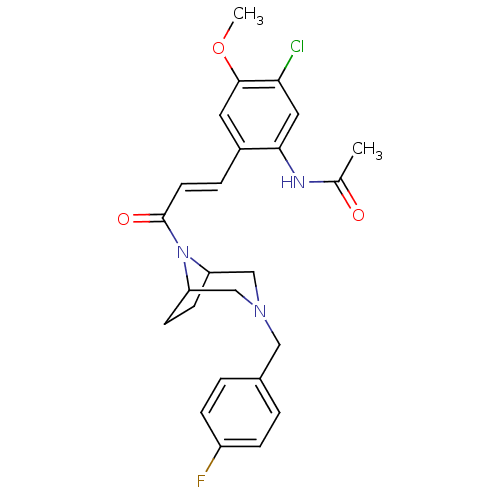
(CHEMBL372807 | N-(2-(3-(3-(4-fluorobenzyl)-3,8-dia...)Show SMILES COc1cc(\C=C\C(=O)N2C3CCC2CN(Cc2ccc(F)cc2)C3)c(NC(C)=O)cc1Cl Show InChI InChI=1S/C25H27ClFN3O3/c1-16(31)28-23-12-22(26)24(33-2)11-18(23)5-10-25(32)30-20-8-9-21(30)15-29(14-20)13-17-3-6-19(27)7-4-17/h3-7,10-12,20-21H,8-9,13-15H2,1-2H3,(H,28,31)/b10-5+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at rat CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174714
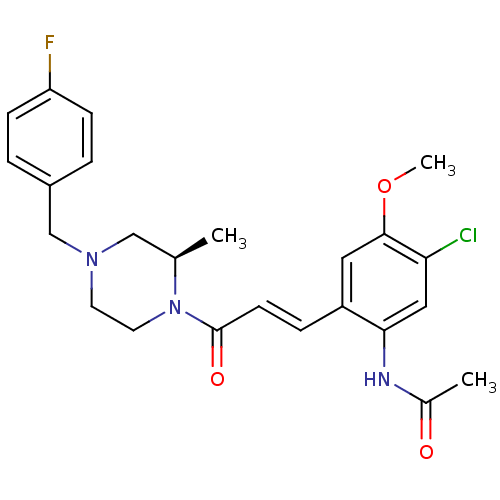
((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES COc1cc(\C=C\C(=O)N2CCN(Cc3ccc(F)cc3)C[C@H]2C)c(NC(C)=O)cc1Cl Show InChI InChI=1S/C24H27ClFN3O3/c1-16-14-28(15-18-4-7-20(26)8-5-18)10-11-29(16)24(31)9-6-19-12-23(32-3)21(25)13-22(19)27-17(2)30/h4-9,12-13,16H,10-11,14-15H2,1-3H3,(H,27,30)/b9-6+/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174720
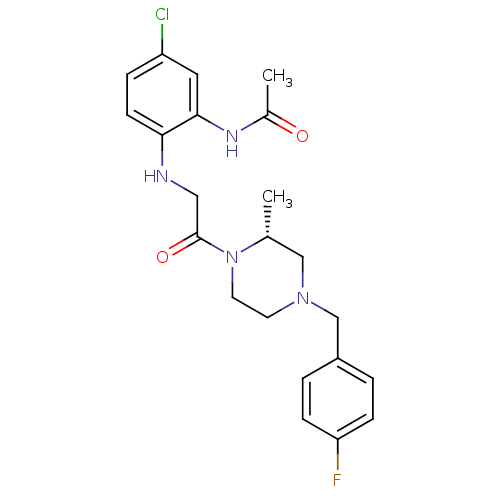
((R)-N-(2-(2-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)CNc1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C22H26ClFN4O2/c1-15-13-27(14-17-3-6-19(24)7-4-17)9-10-28(15)22(30)12-25-20-8-5-18(23)11-21(20)26-16(2)29/h3-8,11,15,25H,9-10,12-14H2,1-2H3,(H,26,29)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174703

((R)-1-(2-(2-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)COc1ccc(Cl)cc1NC(N)=O Show InChI InChI=1S/C21H24ClFN4O3/c1-14-11-26(12-15-2-5-17(23)6-3-15)8-9-27(14)20(28)13-30-19-7-4-16(22)10-18(19)25-21(24)29/h2-7,10,14H,8-9,11-13H2,1H3,(H3,24,25,29)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibitory effect on transwell chemotaxis induced by 1 nM MIP-1alpha in mouse pre-B cells transfected with human CCR1 |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Mus musculus) | BDBM50174709

(CHEMBL372807 | N-(2-(3-(3-(4-fluorobenzyl)-3,8-dia...)Show SMILES COc1cc(\C=C\C(=O)N2C3CCC2CN(Cc2ccc(F)cc2)C3)c(NC(C)=O)cc1Cl Show InChI InChI=1S/C25H27ClFN3O3/c1-16(31)28-23-12-22(26)24(33-2)11-18(23)5-10-25(32)30-20-8-9-21(30)15-29(14-20)13-17-3-6-19(27)7-4-17/h3-7,10-12,20-21H,8-9,13-15H2,1-2H3,(H,28,31)/b10-5+ | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at mouse CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174714

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES COc1cc(\C=C\C(=O)N2CCN(Cc3ccc(F)cc3)C[C@H]2C)c(NC(C)=O)cc1Cl Show InChI InChI=1S/C24H27ClFN3O3/c1-16-14-28(15-18-4-7-20(26)8-5-18)10-11-29(16)24(31)9-6-19-12-23(32-3)21(25)13-22(19)27-17(2)30/h4-9,12-13,16H,10-11,14-15H2,1-3H3,(H,27,30)/b9-6+/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174718

(CHEMBL200680 | N-(6-(3-(3-(4-fluorobenzyl)-3,8-dia...)Show SMILES CC(=O)Nc1cc2ncccc2cc1\C=C\C(=O)N1C2CCC1CN(Cc1ccc(F)cc1)C2 Show InChI InChI=1S/C27H27FN4O2/c1-18(33)30-26-14-25-20(3-2-12-29-25)13-21(26)6-11-27(34)32-23-9-10-24(32)17-31(16-23)15-19-4-7-22(28)8-5-19/h2-8,11-14,23-24H,9-10,15-17H2,1H3,(H,30,33)/b11-6+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at rat CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174709

(CHEMBL372807 | N-(2-(3-(3-(4-fluorobenzyl)-3,8-dia...)Show SMILES COc1cc(\C=C\C(=O)N2C3CCC2CN(Cc2ccc(F)cc2)C3)c(NC(C)=O)cc1Cl Show InChI InChI=1S/C25H27ClFN3O3/c1-16(31)28-23-12-22(26)24(33-2)11-18(23)5-10-25(32)30-20-8-9-21(30)15-29(14-20)13-17-3-6-19(27)7-4-17/h3-7,10-12,20-21H,8-9,13-15H2,1-2H3,(H,28,31)/b10-5+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174709

(CHEMBL372807 | N-(2-(3-(3-(4-fluorobenzyl)-3,8-dia...)Show SMILES COc1cc(\C=C\C(=O)N2C3CCC2CN(Cc2ccc(F)cc2)C3)c(NC(C)=O)cc1Cl Show InChI InChI=1S/C25H27ClFN3O3/c1-16(31)28-23-12-22(26)24(33-2)11-18(23)5-10-25(32)30-20-8-9-21(30)15-29(14-20)13-17-3-6-19(27)7-4-17/h3-7,10-12,20-21H,8-9,13-15H2,1-2H3,(H,28,31)/b10-5+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibitory effect on transwell chemotaxis induced by 1 nM MIP-1alpha in mouse pre-B cells transfected with human CCR1 |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174710
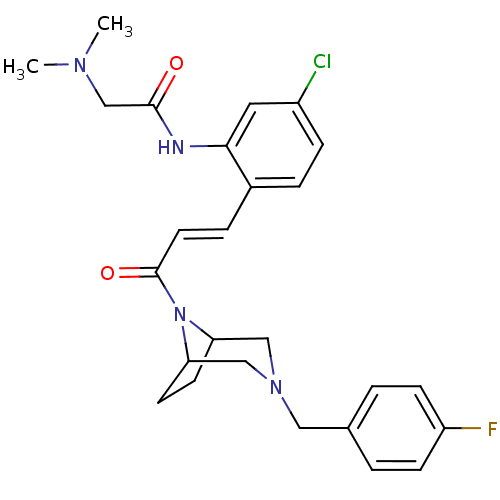
(CHEMBL200242 | N-(2-(3-(3-(4-fluorobenzyl)-3,8-dia...)Show SMILES CN(C)CC(=O)Nc1cc(Cl)ccc1\C=C\C(=O)N1C2CCC1CN(Cc1ccc(F)cc1)C2 Show InChI InChI=1S/C26H30ClFN4O2/c1-30(2)17-25(33)29-24-13-20(27)7-5-19(24)6-12-26(34)32-22-10-11-23(32)16-31(15-22)14-18-3-8-21(28)9-4-18/h3-9,12-13,22-23H,10-11,14-17H2,1-2H3,(H,29,33)/b12-6+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174708

(CHEMBL197375 | N-(2-(3-((2R,5S)-4-(4-fluorobenzyl)...)Show SMILES C[C@H]1CN([C@H](C)CN1Cc1ccc(F)cc1)C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C24H27ClFN3O2/c1-16-14-29(17(2)13-28(16)15-19-4-9-22(26)10-5-19)24(31)11-7-20-6-8-21(25)12-23(20)27-18(3)30/h4-12,16-17H,13-15H2,1-3H3,(H,27,30)/b11-7+/t16-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174704

((R)-N-(2-(1-(4-fluorobenzyl)-3-methylpiperazine-4-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)c1cc2cc(Cl)cc(NC(C)=O)c2[nH]1 Show InChI InChI=1S/C23H24ClFN4O2/c1-14-12-28(13-16-3-5-19(25)6-4-16)7-8-29(14)23(31)21-10-17-9-18(24)11-20(22(17)27-21)26-15(2)30/h3-6,9-11,14,27H,7-8,12-13H2,1-2H3,(H,26,30)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174710

(CHEMBL200242 | N-(2-(3-(3-(4-fluorobenzyl)-3,8-dia...)Show SMILES CN(C)CC(=O)Nc1cc(Cl)ccc1\C=C\C(=O)N1C2CCC1CN(Cc1ccc(F)cc1)C2 Show InChI InChI=1S/C26H30ClFN4O2/c1-30(2)17-25(33)29-24-13-20(27)7-5-19(24)6-12-26(34)32-22-10-11-23(32)16-31(15-22)14-18-3-8-21(28)9-4-18/h3-9,12-13,22-23H,10-11,14-17H2,1-2H3,(H,29,33)/b12-6+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at rat CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibitory effect on transwell chemotaxis induced by 1 nM MIP-1alpha in mouse pre-B cells transfected with human CCR1 |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174706
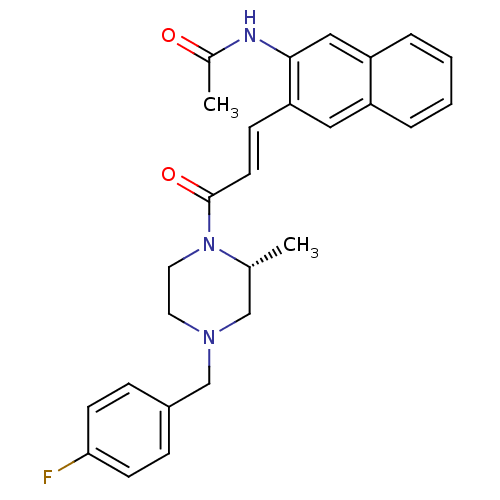
((R)-N-(3-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1cc2ccccc2cc1NC(C)=O Show InChI InChI=1S/C27H28FN3O2/c1-19-17-30(18-21-7-10-25(28)11-8-21)13-14-31(19)27(33)12-9-24-15-22-5-3-4-6-23(22)16-26(24)29-20(2)32/h3-12,15-16,19H,13-14,17-18H2,1-2H3,(H,29,32)/b12-9+/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174707

((R)-1-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(N)=O Show InChI InChI=1S/C22H24ClFN4O2/c1-15-13-27(14-16-2-7-19(24)8-3-16)10-11-28(15)21(29)9-5-17-4-6-18(23)12-20(17)26-22(25)30/h2-9,12,15H,10-11,13-14H2,1H3,(H3,25,26,30)/b9-5+/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174705
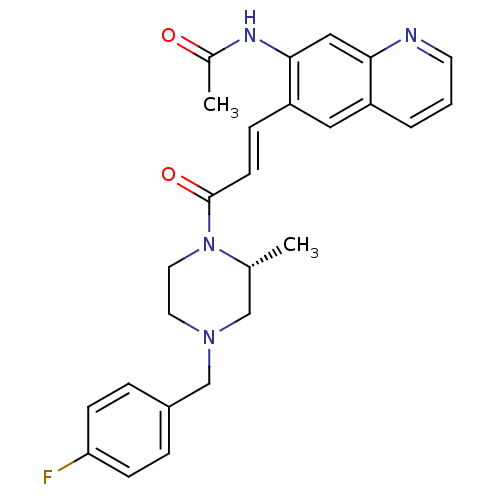
((R)-N-(6-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1cc2cccnc2cc1NC(C)=O Show InChI InChI=1S/C26H27FN4O2/c1-18-16-30(17-20-5-8-23(27)9-6-20)12-13-31(18)26(33)10-7-22-14-21-4-3-11-28-24(21)15-25(22)29-19(2)32/h3-11,14-15,18H,12-13,16-17H2,1-2H3,(H,29,32)/b10-7+/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174714

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES COc1cc(\C=C\C(=O)N2CCN(Cc3ccc(F)cc3)C[C@H]2C)c(NC(C)=O)cc1Cl Show InChI InChI=1S/C24H27ClFN3O3/c1-16-14-28(15-18-4-7-20(26)8-5-18)10-11-29(16)24(31)9-6-19-12-23(32-3)21(25)13-22(19)27-17(2)30/h4-9,12-13,16H,10-11,14-15H2,1-3H3,(H,27,30)/b9-6+/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at rat CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174717

((R)-N-(2-(1-(4-fluorobenzyl)-3-methylpiperazine-4-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)c1cc2cc(Cl)cc(NC(C)=O)c2o1 Show InChI InChI=1S/C23H23ClFN3O3/c1-14-12-27(13-16-3-5-19(25)6-4-16)7-8-28(14)23(30)21-10-17-9-18(24)11-20(22(17)31-21)26-15(2)29/h3-6,9-11,14H,7-8,12-13H2,1-2H3,(H,26,29)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174705

((R)-N-(6-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1cc2cccnc2cc1NC(C)=O Show InChI InChI=1S/C26H27FN4O2/c1-18-16-30(17-20-5-8-23(27)9-6-20)12-13-31(18)26(33)10-7-22-14-21-4-3-11-28-24(21)15-25(22)29-19(2)32/h3-11,14-15,18H,12-13,16-17H2,1-2H3,(H,29,32)/b10-7+/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174710

(CHEMBL200242 | N-(2-(3-(3-(4-fluorobenzyl)-3,8-dia...)Show SMILES CN(C)CC(=O)Nc1cc(Cl)ccc1\C=C\C(=O)N1C2CCC1CN(Cc1ccc(F)cc1)C2 Show InChI InChI=1S/C26H30ClFN4O2/c1-30(2)17-25(33)29-24-13-20(27)7-5-19(24)6-12-26(34)32-22-10-11-23(32)16-31(15-22)14-18-3-8-21(28)9-4-18/h3-9,12-13,22-23H,10-11,14-17H2,1-2H3,(H,29,33)/b12-6+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174707

((R)-1-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(N)=O Show InChI InChI=1S/C22H24ClFN4O2/c1-15-13-27(14-16-2-7-19(24)8-3-16)10-11-28(15)21(29)9-5-17-4-6-18(23)12-20(17)26-22(25)30/h2-9,12,15H,10-11,13-14H2,1H3,(H3,25,26,30)/b9-5+/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at rat CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174703

((R)-1-(2-(2-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)COc1ccc(Cl)cc1NC(N)=O Show InChI InChI=1S/C21H24ClFN4O3/c1-14-11-26(12-15-2-5-17(23)6-3-15)8-9-27(14)20(28)13-30-19-7-4-16(22)10-18(19)25-21(24)29/h2-7,10,14H,8-9,11-13H2,1H3,(H3,24,25,29)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Mus musculus) | BDBM50174710

(CHEMBL200242 | N-(2-(3-(3-(4-fluorobenzyl)-3,8-dia...)Show SMILES CN(C)CC(=O)Nc1cc(Cl)ccc1\C=C\C(=O)N1C2CCC1CN(Cc1ccc(F)cc1)C2 Show InChI InChI=1S/C26H30ClFN4O2/c1-30(2)17-25(33)29-24-13-20(27)7-5-19(24)6-12-26(34)32-22-10-11-23(32)16-31(15-22)14-18-3-8-21(28)9-4-18/h3-9,12-13,22-23H,10-11,14-17H2,1-2H3,(H,29,33)/b12-6+ | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at mouse CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174710

(CHEMBL200242 | N-(2-(3-(3-(4-fluorobenzyl)-3,8-dia...)Show SMILES CN(C)CC(=O)Nc1cc(Cl)ccc1\C=C\C(=O)N1C2CCC1CN(Cc1ccc(F)cc1)C2 Show InChI InChI=1S/C26H30ClFN4O2/c1-30(2)17-25(33)29-24-13-20(27)7-5-19(24)6-12-26(34)32-22-10-11-23(32)16-31(15-22)14-18-3-8-21(28)9-4-18/h3-9,12-13,22-23H,10-11,14-17H2,1-2H3,(H,29,33)/b12-6+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibitory effect on transwell chemotaxis induced by 1 nM MIP-1alpha in mouse pre-B cells transfected with human CCR1 |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174707

((R)-1-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(N)=O Show InChI InChI=1S/C22H24ClFN4O2/c1-15-13-27(14-16-2-7-19(24)8-3-16)10-11-28(15)21(29)9-5-17-4-6-18(23)12-20(17)26-22(25)30/h2-9,12,15H,10-11,13-14H2,1H3,(H3,25,26,30)/b9-5+/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibitory effect on transwell chemotaxis induced by 1 nM MIP-1alpha in mouse pre-B cells transfected with human CCR1 |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174720

((R)-N-(2-(2-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)CNc1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C22H26ClFN4O2/c1-15-13-27(14-17-3-6-19(24)7-4-17)9-10-28(15)22(30)12-25-20-8-5-18(23)11-21(20)26-16(2)29/h3-8,11,15,25H,9-10,12-14H2,1-2H3,(H,26,29)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Mus musculus) | BDBM50174718

(CHEMBL200680 | N-(6-(3-(3-(4-fluorobenzyl)-3,8-dia...)Show SMILES CC(=O)Nc1cc2ncccc2cc1\C=C\C(=O)N1C2CCC1CN(Cc1ccc(F)cc1)C2 Show InChI InChI=1S/C27H27FN4O2/c1-18(33)30-26-14-25-20(3-2-12-29-25)13-21(26)6-11-27(34)32-23-9-10-24(32)17-31(16-23)15-19-4-7-22(28)8-5-19/h2-8,11-14,23-24H,9-10,15-17H2,1H3,(H,30,33)/b11-6+ | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at mouse CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Mus musculus) | BDBM50174714

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES COc1cc(\C=C\C(=O)N2CCN(Cc3ccc(F)cc3)C[C@H]2C)c(NC(C)=O)cc1Cl Show InChI InChI=1S/C24H27ClFN3O3/c1-16-14-28(15-18-4-7-20(26)8-5-18)10-11-29(16)24(31)9-6-19-12-23(32-3)21(25)13-22(19)27-17(2)30/h4-9,12-13,16H,10-11,14-15H2,1-3H3,(H,27,30)/b9-6+/t16-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at mouse CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174713
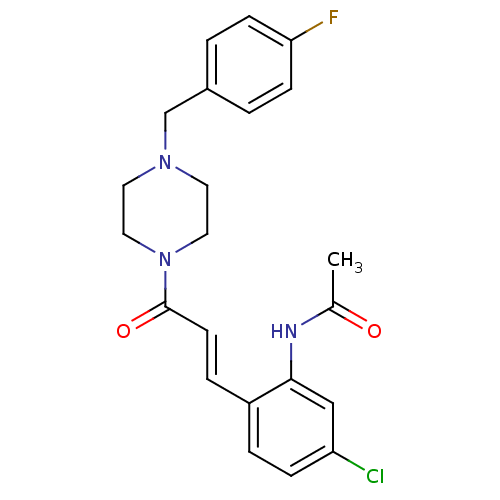
(CHEMBL198852 | N-(2-(3-(4-(4-fluorobenzyl)piperazi...)Show SMILES CC(=O)Nc1cc(Cl)ccc1\C=C\C(=O)N1CCN(Cc2ccc(F)cc2)CC1 Show InChI InChI=1S/C22H23ClFN3O2/c1-16(28)25-21-14-19(23)6-4-18(21)5-9-22(29)27-12-10-26(11-13-27)15-17-2-7-20(24)8-3-17/h2-9,14H,10-13,15H2,1H3,(H,25,28)/b9-5+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 by inhibition of MIP-1alpha induced calcium mobilization in THP1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174714

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES COc1cc(\C=C\C(=O)N2CCN(Cc3ccc(F)cc3)C[C@H]2C)c(NC(C)=O)cc1Cl Show InChI InChI=1S/C24H27ClFN3O3/c1-16-14-28(15-18-4-7-20(26)8-5-18)10-11-29(16)24(31)9-6-19-12-23(32-3)21(25)13-22(19)27-17(2)30/h4-9,12-13,16H,10-11,14-15H2,1-3H3,(H,27,30)/b9-6+/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibitory effect on transwell chemotaxis induced by 1 nM MIP-1alpha in mouse pre-B cells transfected with human CCR1 |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174706

((R)-N-(3-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1cc2ccccc2cc1NC(C)=O Show InChI InChI=1S/C27H28FN3O2/c1-19-17-30(18-21-7-10-25(28)11-8-21)13-14-31(19)27(33)12-9-24-15-22-5-3-4-6-23(22)16-26(24)29-20(2)32/h3-12,15-16,19H,13-14,17-18H2,1-2H3,(H,29,32)/b12-9+/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174711

(CHEMBL197345 | N-(2-(3-((2S,5R)-4-(4-fluorobenzyl)...)Show SMILES C[C@@H]1CN([C@@H](C)CN1Cc1ccc(F)cc1)C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C24H27ClFN3O2/c1-16-14-29(17(2)13-28(16)15-19-4-9-22(26)10-5-19)24(31)11-7-20-6-8-21(25)12-23(20)27-18(3)30/h4-12,16-17H,13-15H2,1-3H3,(H,27,30)/b11-7+/t16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174717

((R)-N-(2-(1-(4-fluorobenzyl)-3-methylpiperazine-4-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)c1cc2cc(Cl)cc(NC(C)=O)c2o1 Show InChI InChI=1S/C23H23ClFN3O3/c1-14-12-27(13-16-3-5-19(25)6-4-16)7-8-28(14)23(30)21-10-17-9-18(24)11-20(22(17)31-21)26-15(2)29/h3-6,9-11,14H,7-8,12-13H2,1-2H3,(H,26,29)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174713

(CHEMBL198852 | N-(2-(3-(4-(4-fluorobenzyl)piperazi...)Show SMILES CC(=O)Nc1cc(Cl)ccc1\C=C\C(=O)N1CCN(Cc2ccc(F)cc2)CC1 Show InChI InChI=1S/C22H23ClFN3O2/c1-16(28)25-21-14-19(23)6-4-18(21)5-9-22(29)27-12-10-26(11-13-27)15-17-2-7-20(24)8-3-17/h2-9,14H,10-13,15H2,1H3,(H,25,28)/b9-5+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at rat CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174718

(CHEMBL200680 | N-(6-(3-(3-(4-fluorobenzyl)-3,8-dia...)Show SMILES CC(=O)Nc1cc2ncccc2cc1\C=C\C(=O)N1C2CCC1CN(Cc1ccc(F)cc1)C2 Show InChI InChI=1S/C27H27FN4O2/c1-18(33)30-26-14-25-20(3-2-12-29-25)13-21(26)6-11-27(34)32-23-9-10-24(32)17-31(16-23)15-19-4-7-22(28)8-5-19/h2-8,11-14,23-24H,9-10,15-17H2,1H3,(H,30,33)/b11-6+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174713

(CHEMBL198852 | N-(2-(3-(4-(4-fluorobenzyl)piperazi...)Show SMILES CC(=O)Nc1cc(Cl)ccc1\C=C\C(=O)N1CCN(Cc2ccc(F)cc2)CC1 Show InChI InChI=1S/C22H23ClFN3O2/c1-16(28)25-21-14-19(23)6-4-18(21)5-9-22(29)27-12-10-26(11-13-27)15-17-2-7-20(24)8-3-17/h2-9,14H,10-13,15H2,1H3,(H,25,28)/b9-5+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174715

(CHEMBL380568 | N-(2-(3-(3-(4-fluorobenzyl)-3,8-dia...)Show SMILES CC(=O)Nc1cc(Cl)ccc1\C=C\C(=O)N1C2CCC1CN(Cc1ccc(F)cc1)C2 Show InChI InChI=1S/C24H25ClFN3O2/c1-16(30)27-23-12-19(25)6-4-18(23)5-11-24(31)29-21-9-10-22(29)15-28(14-21)13-17-2-7-20(26)8-3-17/h2-8,11-12,21-22H,9-10,13-15H2,1H3,(H,27,30)/b11-5+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Mus musculus) | BDBM50174707

((R)-1-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(N)=O Show InChI InChI=1S/C22H24ClFN4O2/c1-15-13-27(14-16-2-7-19(24)8-3-16)10-11-28(15)21(29)9-5-17-4-6-18(23)12-20(17)26-22(25)30/h2-9,12,15H,10-11,13-14H2,1H3,(H3,25,26,30)/b9-5+/t15-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at mouse CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174705

((R)-N-(6-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1cc2cccnc2cc1NC(C)=O Show InChI InChI=1S/C26H27FN4O2/c1-18-16-30(17-20-5-8-23(27)9-6-20)12-13-31(18)26(33)10-7-22-14-21-4-3-11-28-24(21)15-25(22)29-19(2)32/h3-11,14-15,18H,12-13,16-17H2,1-2H3,(H,29,32)/b10-7+/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at rat CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174721

(CHEMBL371248 | N-(2-(3-(8-(4-fluorobenzyl)-3,8-dia...)Show SMILES CC(=O)Nc1cc(Cl)ccc1\C=C\C(=O)N1CC2CCC(C1)N2Cc1ccc(F)cc1 Show InChI InChI=1S/C24H25ClFN3O2/c1-16(30)27-23-12-19(25)6-4-18(23)5-11-24(31)28-14-21-9-10-22(15-28)29(21)13-17-2-7-20(26)8-3-17/h2-8,11-12,21-22H,9-10,13-15H2,1H3,(H,27,30)/b11-5+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at rat CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174722
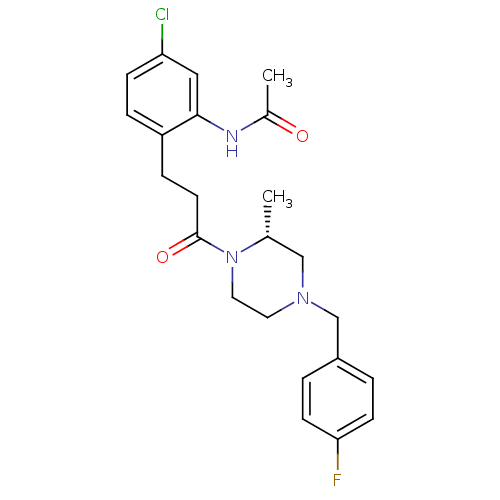
((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)CCc1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H27ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-5,7-9,13,16H,6,10-12,14-15H2,1-2H3,(H,26,29)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Mus musculus) | BDBM50174713

(CHEMBL198852 | N-(2-(3-(4-(4-fluorobenzyl)piperazi...)Show SMILES CC(=O)Nc1cc(Cl)ccc1\C=C\C(=O)N1CCN(Cc2ccc(F)cc2)CC1 Show InChI InChI=1S/C22H23ClFN3O2/c1-16(28)25-21-14-19(23)6-4-18(21)5-9-22(29)27-12-10-26(11-13-27)15-17-2-7-20(24)8-3-17/h2-9,14H,10-13,15H2,1H3,(H,25,28)/b9-5+ | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 448 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at mouse CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Mus musculus) | BDBM50174706

((R)-N-(3-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1cc2ccccc2cc1NC(C)=O Show InChI InChI=1S/C27H28FN3O2/c1-19-17-30(18-21-7-10-25(28)11-8-21)13-14-31(19)27(33)12-9-24-15-22-5-3-4-6-23(22)16-26(24)29-20(2)32/h3-12,15-16,19H,13-14,17-18H2,1-2H3,(H,29,32)/b12-9+/t19-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at mouse CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174703

((R)-1-(2-(2-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)COc1ccc(Cl)cc1NC(N)=O Show InChI InChI=1S/C21H24ClFN4O3/c1-14-11-26(12-15-2-5-17(23)6-3-15)8-9-27(14)20(28)13-30-19-7-4-16(22)10-18(19)25-21(24)29/h2-7,10,14H,8-9,11-13H2,1H3,(H3,24,25,29)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at rat CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174704

((R)-N-(2-(1-(4-fluorobenzyl)-3-methylpiperazine-4-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)c1cc2cc(Cl)cc(NC(C)=O)c2[nH]1 Show InChI InChI=1S/C23H24ClFN4O2/c1-14-12-28(13-16-3-5-19(25)6-4-16)7-8-29(14)23(31)21-10-17-9-18(24)11-20(22(17)27-21)26-15(2)30/h3-6,9-11,14,27H,7-8,12-13H2,1-2H3,(H,26,30)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Mus musculus) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at mouse CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Mus musculus) | BDBM50174708

(CHEMBL197375 | N-(2-(3-((2R,5S)-4-(4-fluorobenzyl)...)Show SMILES C[C@H]1CN([C@H](C)CN1Cc1ccc(F)cc1)C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C24H27ClFN3O2/c1-16-14-29(17(2)13-28(16)15-19-4-9-22(26)10-5-19)24(31)11-7-20-6-8-21(25)12-23(20)27-18(3)30/h4-12,16-17H,13-15H2,1-3H3,(H,27,30)/b11-7+/t16-,17+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at mouse CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174716
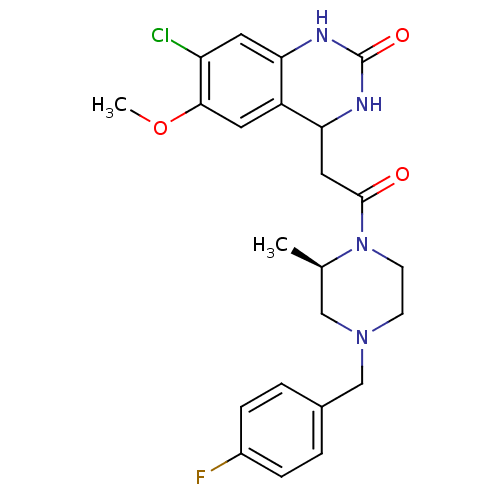
(4-(2-((R)-4-(4-fluorobenzyl)-2-methylpiperazin-1-y...)Show SMILES COc1cc2C(CC(=O)N3CCN(Cc4ccc(F)cc4)C[C@H]3C)NC(=O)Nc2cc1Cl Show InChI InChI=1S/C23H26ClFN4O3/c1-14-12-28(13-15-3-5-16(25)6-4-15)7-8-29(14)22(30)11-20-17-9-21(32-2)18(24)10-19(17)26-23(31)27-20/h3-6,9-10,14,20H,7-8,11-13H2,1-2H3,(H2,26,27,31)/t14-,20?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine receptor D3 |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human adrenergic alpha-2A receptor |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine receptor D4.4 |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174717

((R)-N-(2-(1-(4-fluorobenzyl)-3-methylpiperazine-4-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)c1cc2cc(Cl)cc(NC(C)=O)c2o1 Show InChI InChI=1S/C23H23ClFN3O3/c1-14-12-27(13-16-3-5-19(25)6-4-16)7-8-28(14)23(30)21-10-17-9-18(24)11-20(22(17)31-21)26-15(2)29/h3-6,9-11,14H,7-8,12-13H2,1-2H3,(H,26,29)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at rat CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human muscarinic receptor M4 |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human 5HT1B receptor |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human muscarinic receptor M3 |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human kappa opioid receptor |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human adrenergic alpha-2C receptor |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human delta opioid receptor |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human adrenergic alpha-2B receptor |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174704

((R)-N-(2-(1-(4-fluorobenzyl)-3-methylpiperazine-4-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)c1cc2cc(Cl)cc(NC(C)=O)c2[nH]1 Show InChI InChI=1S/C23H24ClFN4O2/c1-14-12-28(13-16-3-5-19(25)6-4-16)7-8-29(14)23(31)21-10-17-9-18(24)11-20(22(17)27-21)26-15(2)30/h3-6,9-11,14,27H,7-8,12-13H2,1-2H3,(H,26,30)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at rat CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Mus musculus) | BDBM50174705

((R)-N-(6-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1cc2cccnc2cc1NC(C)=O Show InChI InChI=1S/C26H27FN4O2/c1-18-16-30(17-20-5-8-23(27)9-6-20)12-13-31(18)26(33)10-7-22-14-21-4-3-11-28-24(21)15-25(22)29-19(2)32/h3-11,14-15,18H,12-13,16-17H2,1-2H3,(H,29,32)/b10-7+/t18-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at mouse CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human 5HT2C receptor |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human muscarinic receptor M1 |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human 5HT2B receptor |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human muscarinic receptor M2 |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human 5HT2A receptor |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human adrenergic beta-2 receptor |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine receptor D2 |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human adrenergic beta-1 receptor |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human 5HT7 receptor |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human mu opioid receptor |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine receptor D1 |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human 5HT3 receptor |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human muscarinic receptor M5 |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human 5HT1A receptor |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human 5HT6 receptor |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174708

(CHEMBL197375 | N-(2-(3-((2R,5S)-4-(4-fluorobenzyl)...)Show SMILES C[C@H]1CN([C@H](C)CN1Cc1ccc(F)cc1)C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C24H27ClFN3O2/c1-16-14-29(17(2)13-28(16)15-19-4-9-22(26)10-5-19)24(31)11-7-20-6-8-21(25)12-23(20)27-18(3)30/h4-12,16-17H,13-15H2,1-3H3,(H,27,30)/b11-7+/t16-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at rat CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174706

((R)-N-(3-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1cc2ccccc2cc1NC(C)=O Show InChI InChI=1S/C27H28FN3O2/c1-19-17-30(18-21-7-10-25(28)11-8-21)13-14-31(19)27(33)12-9-24-15-22-5-3-4-6-23(22)16-26(24)29-20(2)32/h3-12,15-16,19H,13-14,17-18H2,1-2H3,(H,29,32)/b12-9+/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at rat CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Mus musculus) | BDBM50174703

((R)-1-(2-(2-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)COc1ccc(Cl)cc1NC(N)=O Show InChI InChI=1S/C21H24ClFN4O3/c1-14-11-26(12-15-2-5-17(23)6-3-15)8-9-27(14)20(28)13-30-19-7-4-16(22)10-18(19)25-21(24)29/h2-7,10,14H,8-9,11-13H2,1H3,(H3,24,25,29)/t14-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at mouse CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174712
((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methyl-1,4-diaze...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C24H27ClFN3O2/c1-17-15-28(16-19-4-9-22(26)10-5-19)12-3-13-29(17)24(31)11-7-20-6-8-21(25)14-23(20)27-18(2)30/h4-11,14,17H,3,12-13,15-16H2,1-2H3,(H,27,30)/b11-7+/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174719
(CHEMBL196908 | N-(2-(3-((1R,4R)-5-(4-fluorobenzyl)...)Show SMILES CC(=O)Nc1cc(Cl)ccc1\C=C\C(=O)N1C[C@H]2C[C@@H]1CN2Cc1ccc(F)cc1 Show InChI InChI=1S/C23H23ClFN3O2/c1-15(29)26-22-10-18(24)6-4-17(22)5-9-23(30)28-14-20-11-21(28)13-27(20)12-16-2-7-19(25)8-3-16/h2-10,20-21H,11-14H2,1H3,(H,26,29)/b9-5+/t20-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at human CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174720

((R)-N-(2-(2-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)CNc1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C22H26ClFN4O2/c1-15-13-27(14-17-3-6-19(24)7-4-17)9-10-28(15)22(30)12-25-20-8-5-18(23)11-21(20)26-16(2)29/h3-8,11,15,25H,9-10,12-14H2,1-2H3,(H,26,29)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at rat CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Mus musculus) | BDBM50174704

((R)-N-(2-(1-(4-fluorobenzyl)-3-methylpiperazine-4-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)c1cc2cc(Cl)cc(NC(C)=O)c2[nH]1 Show InChI InChI=1S/C23H24ClFN4O2/c1-14-12-28(13-16-3-5-19(25)6-4-16)7-8-29(14)23(31)21-10-17-9-18(24)11-20(22(17)27-21)26-15(2)30/h3-6,9-11,14,27H,7-8,12-13H2,1-2H3,(H,26,30)/t14-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at mouse CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Mus musculus) | BDBM50174720

((R)-N-(2-(2-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)CNc1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C22H26ClFN4O2/c1-15-13-27(14-17-3-6-19(24)7-4-17)9-10-28(15)22(30)12-25-20-8-5-18(23)11-21(20)26-16(2)29/h3-8,11,15,25H,9-10,12-14H2,1-2H3,(H,26,29)/t15-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at mouse CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Mus musculus) | BDBM50174717

((R)-N-(2-(1-(4-fluorobenzyl)-3-methylpiperazine-4-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)c1cc2cc(Cl)cc(NC(C)=O)c2o1 Show InChI InChI=1S/C23H23ClFN3O3/c1-14-12-27(13-16-3-5-19(25)6-4-16)7-8-28(14)23(30)21-10-17-9-18(24)11-20(22(17)31-21)26-15(2)29/h3-6,9-11,14H,7-8,12-13H2,1-2H3,(H,26,29)/t14-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at mouse CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2b |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CCR3 |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Mus musculus) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of mouse CCR5 |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50174711

(CHEMBL197345 | N-(2-(3-((2S,5R)-4-(4-fluorobenzyl)...)Show SMILES C[C@@H]1CN([C@@H](C)CN1Cc1ccc(F)cc1)C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C24H27ClFN3O2/c1-16-14-29(17(2)13-28(16)15-19-4-9-22(26)10-5-19)24(31)11-7-20-6-8-21(25)12-23(20)27-18(3)30/h4-12,16-17H,13-15H2,1-3H3,(H,27,30)/b11-7+/t16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at rat CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Mus musculus) | BDBM50174711

(CHEMBL197345 | N-(2-(3-((2S,5R)-4-(4-fluorobenzyl)...)Show SMILES C[C@@H]1CN([C@@H](C)CN1Cc1ccc(F)cc1)C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C24H27ClFN3O2/c1-16-14-29(17(2)13-28(16)15-19-4-9-22(26)10-5-19)24(31)11-7-20-6-8-21(25)12-23(20)27-18(3)30/h4-12,16-17H,13-15H2,1-3H3,(H,27,30)/b11-7+/t16-,17+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity at mouse CCR1 in CHO-K1 cells |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 6
(Homo sapiens (Human)) | BDBM50174702

((R)-N-(2-(3-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)\C=C\c1ccc(Cl)cc1NC(C)=O Show InChI InChI=1S/C23H25ClFN3O2/c1-16-14-27(15-18-3-8-21(25)9-4-18)11-12-28(16)23(30)10-6-19-5-7-20(24)13-22(19)26-17(2)29/h3-10,13,16H,11-12,14-15H2,1-2H3,(H,26,29)/b10-6+/t16-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CCR6 |
Bioorg Med Chem Lett 15: 5160-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.057
BindingDB Entry DOI: 10.7270/Q2C53MMD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data