

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50341023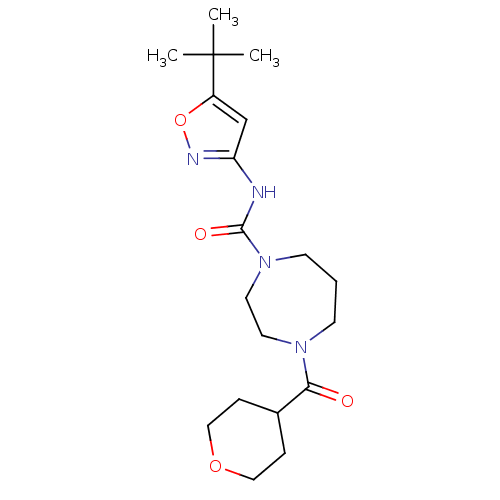 (CHEMBL1762293 | N-(5-tert-butylisoxazol-3-yl)-4-(t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of CYP2D6 flourescent probe 3-[2-(N,N-diethyl-N-methylamino)ethyl]-7-methoxy-4-methylcoumarin | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50340991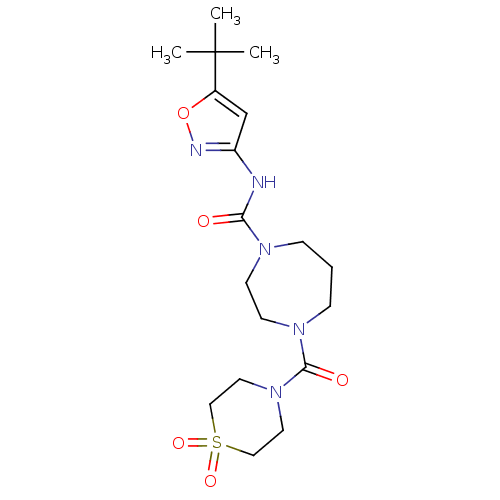 (4-(1,1-Dioxo-1lambda*6*-thiomorpholine-4-carbonyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of CYP3A4 using flourescent probe 7-benzyloxyquinoline and 7-benzyloxy-4-(trifluoromethyl)-coumarin) | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50341008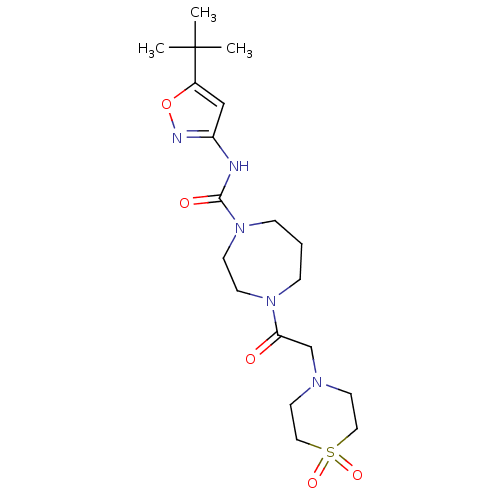 (4-[2-(1,1-Dioxo-1lambda*6*-thiomorpholin-4-yl)-ace...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of CYP2D6 flourescent probe 3-[2-(N,N-diethyl-N-methylamino)ethyl]-7-methoxy-4-methylcoumarin | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50341008 (4-[2-(1,1-Dioxo-1lambda*6*-thiomorpholin-4-yl)-ace...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of CYP3A4 using flourescent probe 7-benzyloxyquinoline and 7-benzyloxy-4-(trifluoromethyl)-coumarin) | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50340991 (4-(1,1-Dioxo-1lambda*6*-thiomorpholine-4-carbonyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of CYP2C9 using flourescent probe 7-methoxy-4-trifluoromethylcoumarin | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50340991 (4-(1,1-Dioxo-1lambda*6*-thiomorpholine-4-carbonyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of CYP2D6 flourescent probe 3-[2-(N,N-diethyl-N-methylamino)ethyl]-7-methoxy-4-methylcoumarin | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50341008 (4-[2-(1,1-Dioxo-1lambda*6*-thiomorpholin-4-yl)-ace...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of CYP2C9 using flourescent probe 7-methoxy-4-trifluoromethylcoumarin | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50341023 (CHEMBL1762293 | N-(5-tert-butylisoxazol-3-yl)-4-(t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of CYP2C9 using flourescent probe 7-methoxy-4-trifluoromethylcoumarin | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50341023 (CHEMBL1762293 | N-(5-tert-butylisoxazol-3-yl)-4-(t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of CYP3A4 using flourescent probe 7-benzyloxyquinoline and 7-benzyloxy-4-(trifluoromethyl)-coumarin) | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50340992 (CHEMBL1762298 | N-(5-tert-butylthiazol-2-yl)-4-(te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50340998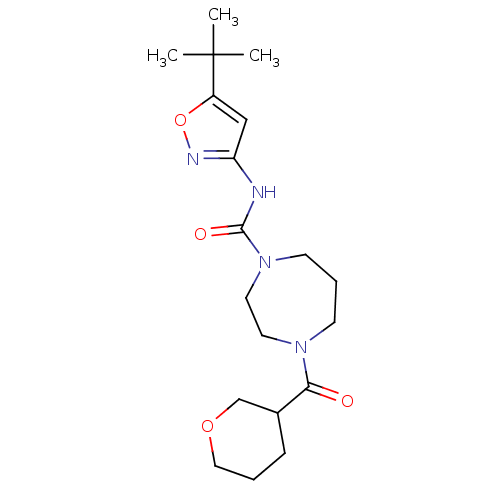 (CHEMBL1762417 | N-(5-tert-butylisoxazol-3-yl)-4-(t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.05E+3 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341008 (4-[2-(1,1-Dioxo-1lambda*6*-thiomorpholin-4-yl)-ace...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 107 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50340997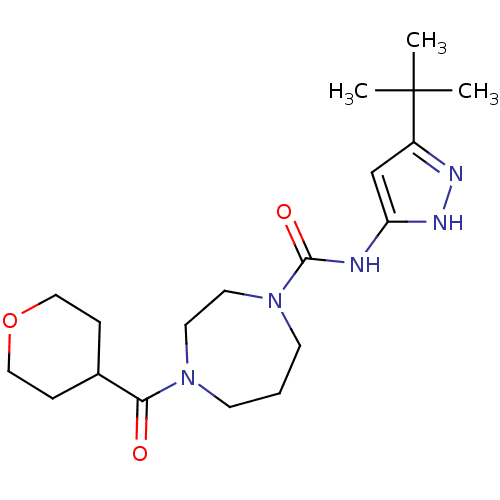 (CHEMBL1762416 | N-(3-tert-butyl-1H-pyrazol-5-yl)-4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341000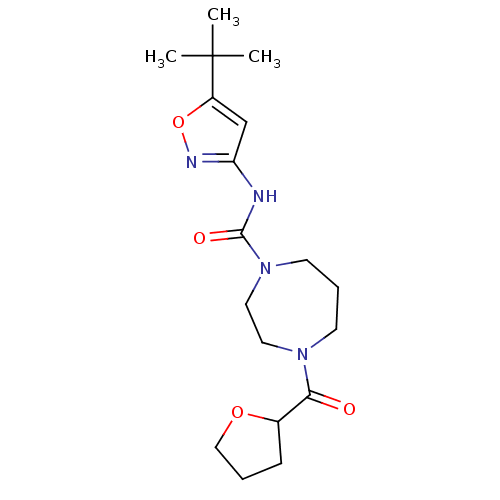 (CHEMBL1762419 | N-(5-tert-butylisoxazol-3-yl)-4-(t...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50340999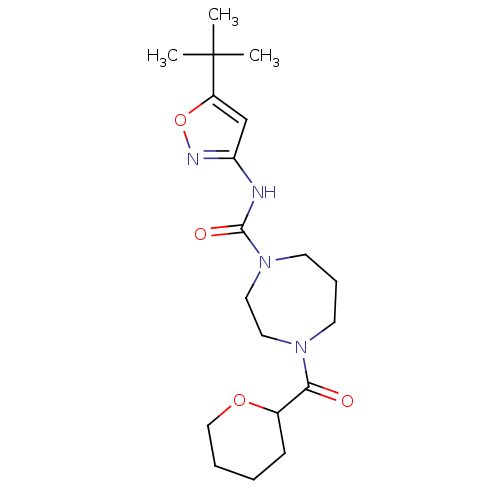 (CHEMBL1762418 | N-(5-tert-butylisoxazol-3-yl)-4-(t...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341012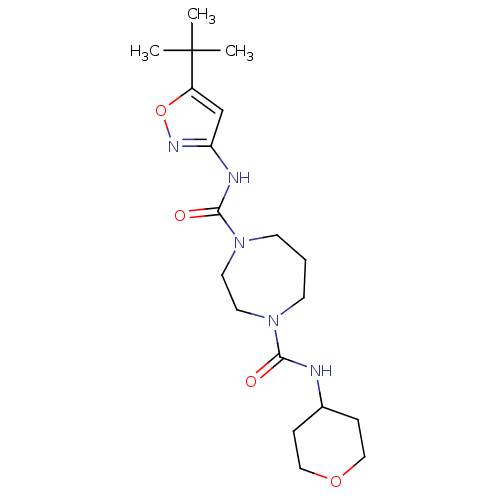 (CHEMBL1762432 | N1-(5-tert-butylisoxazol-3-yl)-N4-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341028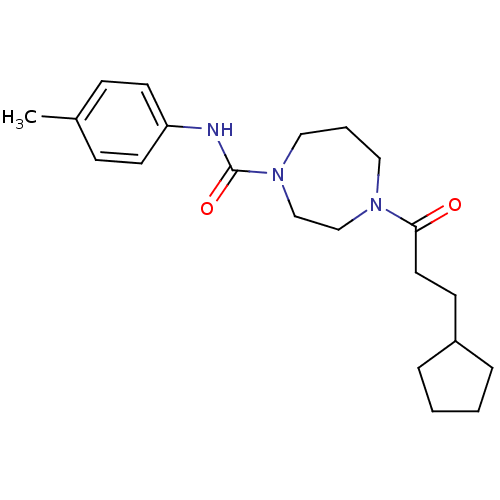 (4-(3-cyclopentylpropanoyl)-N-p-tolyl-1,4-diazepane...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341016 (CHEMBL1762287 | N-(4-tert-butylphenyl)-4-(4-chloro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341025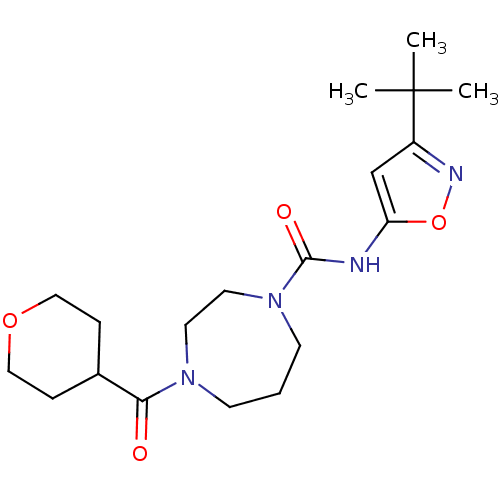 (CHEMBL1762294 | N-(3-tert-butylisoxazol-5-yl)-4-(t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50340993 (CHEMBL1762299 | N-(5-phenylthiazol-2-yl)-4-(tetrah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 135 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341016 (CHEMBL1762287 | N-(4-tert-butylphenyl)-4-(4-chloro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.47E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341020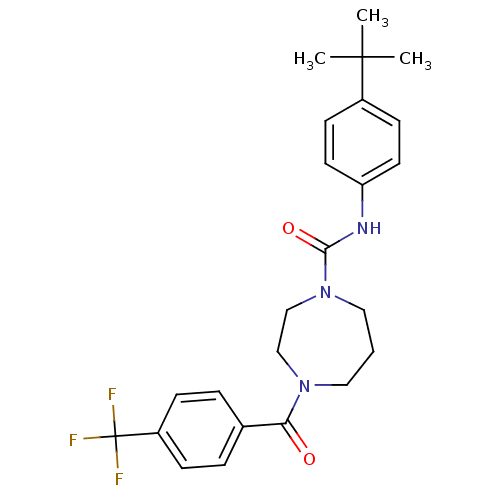 (CHEMBL1762290 | N-(4-tert-butylphenyl)-4-(4-(trifl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341023 (CHEMBL1762293 | N-(5-tert-butylisoxazol-3-yl)-4-(t...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341024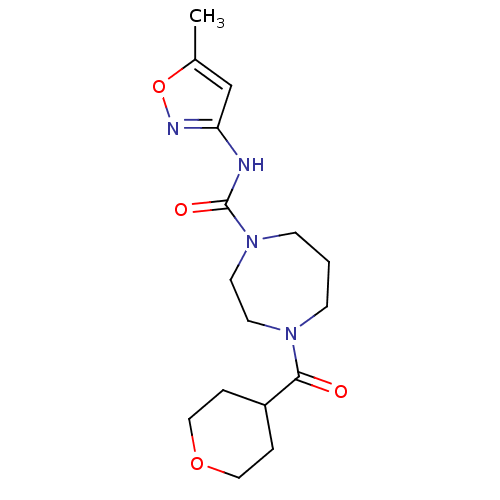 (CHEMBL1762295 | N-(5-methylisoxazol-3-yl)-4-(tetra...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50340993 (CHEMBL1762299 | N-(5-phenylthiazol-2-yl)-4-(tetrah...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50340994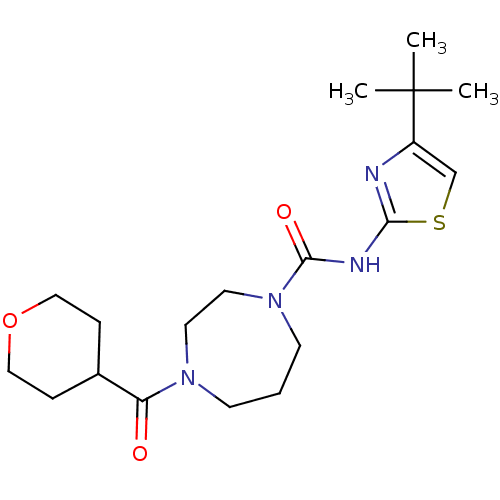 (CHEMBL1762300 | N-(4-tert-butylthiazol-2-yl)-4-(te...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341001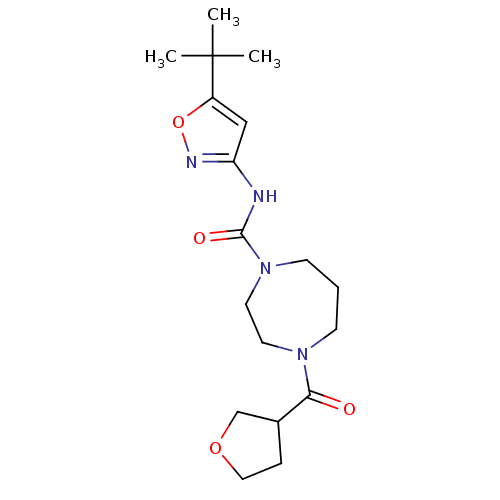 (CHEMBL1762420 | N-(5-tert-butylisoxazol-3-yl)-4-(t...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341004 (4-(1-acetylpiperidine-4-carbonyl)-N-(5-tert-butyli...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341029 (4-(3-cyclopentylpropanoyl)-N-phenyl-1,4-diazepane-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341032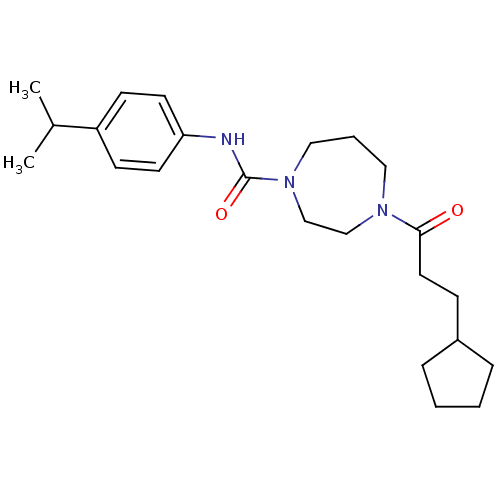 (4-(3-cyclopentylpropanoyl)-N-(4-isopropylphenyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 123 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341031 (4-(3-cyclopentylpropanoyl)-N-(4-ethylphenyl)-1,4-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341018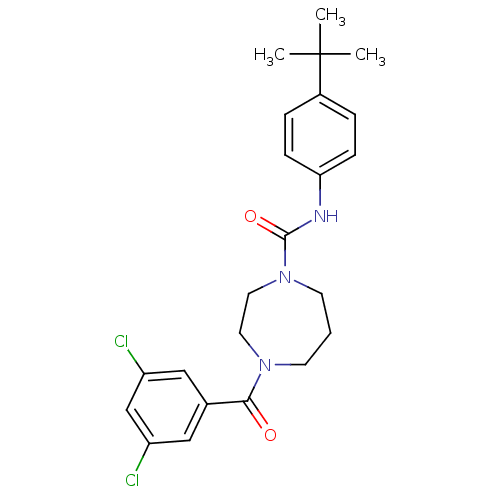 (CHEMBL1762288 | N-(4-tert-butylphenyl)-4-(3,5-dich...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 215 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341021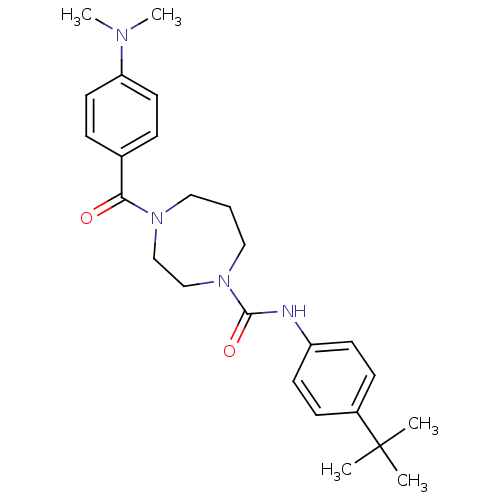 (CHEMBL1762291 | N-(4-tert-butylphenyl)-4-(4-(dimet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 49 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341023 (CHEMBL1762293 | N-(5-tert-butylisoxazol-3-yl)-4-(t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 67 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341026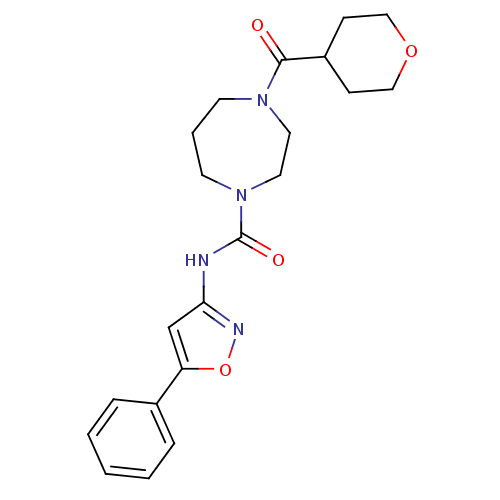 (CHEMBL1762297 | N-(5-phenylisoxazol-3-yl)-4-(tetra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.94E+3 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50340996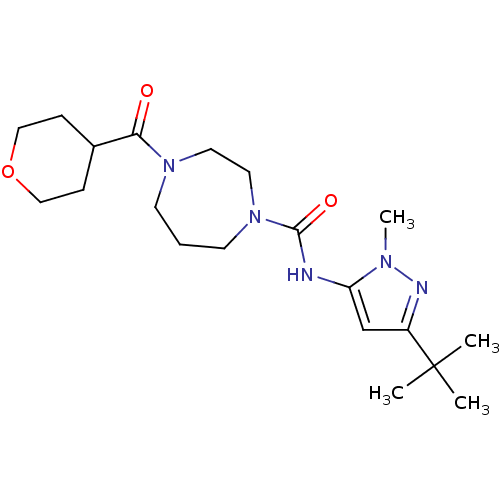 (CHEMBL1762302 | N-(3-tert-butyl-1-methyl-1H-pyrazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.49E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341007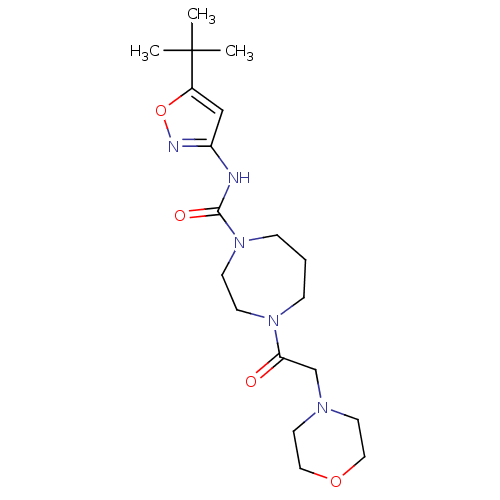 (CHEMBL1762426 | N-(5-tert-butylisoxazol-3-yl)-4-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 157 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50340998 (CHEMBL1762417 | N-(5-tert-butylisoxazol-3-yl)-4-(t...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341003 (CHEMBL1762422 | Trans-N-(5-tert-butylisoxazol-3-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341005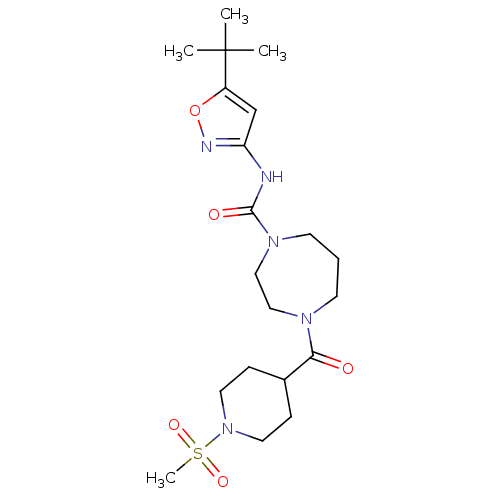 (CHEMBL1762424 | N-(5-tert-butylisoxazol-3-yl)-4-(1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341028 (4-(3-cyclopentylpropanoyl)-N-p-tolyl-1,4-diazepane...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341006 (CHEMBL1762425 | N-(5-tert-butylisoxazol-3-yl)-4-(p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341011 (CHEMBL1762431 | N-(5-tert-butylisoxazol-3-yl)-4-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 426 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341012 (CHEMBL1762432 | N1-(5-tert-butylisoxazol-3-yl)-N4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 814 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341013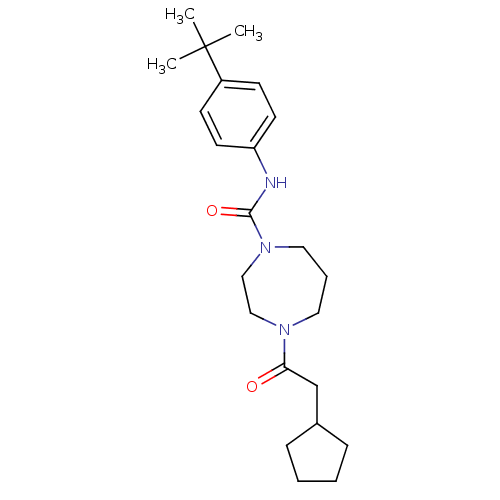 (CHEMBL1762283 | N-(4-tert-butylphenyl)-4-(2-cyclop...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341032 (4-(3-cyclopentylpropanoyl)-N-(4-isopropylphenyl)-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341033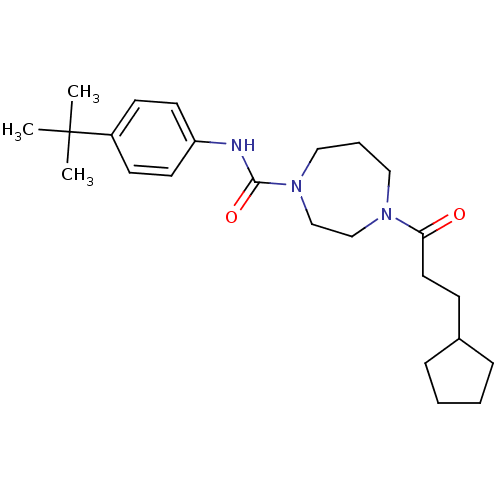 (CHEMBL1762282 | N-(4-tert-butylphenyl)-4-(3-cyclop...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.93E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341014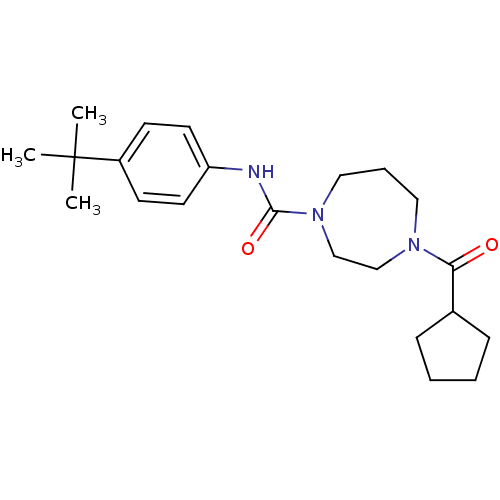 (CHEMBL1762284 | N-(4-tert-butylphenyl)-4-(cyclopen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 168 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341019 (CHEMBL1762289 | N-(4-tert-butylphenyl)-4-(2,5-dich...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 87 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341022 (CHEMBL1762292 | N-(4-tert-butylphenyl)-4-(tetrahyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 111 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50340994 (CHEMBL1762300 | N-(4-tert-butylthiazol-2-yl)-4-(te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341000 (CHEMBL1762419 | N-(5-tert-butylisoxazol-3-yl)-4-(t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.03E+3 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341001 (CHEMBL1762420 | N-(5-tert-butylisoxazol-3-yl)-4-(t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341003 (CHEMBL1762422 | Trans-N-(5-tert-butylisoxazol-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50340991 (4-(1,1-Dioxo-1lambda*6*-thiomorpholine-4-carbonyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 135 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341009 (CHEMBL1762428 | N-(5-tert-butylisoxazol-3-yl)-4-(m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 177 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341014 (CHEMBL1762284 | N-(4-tert-butylphenyl)-4-(cyclopen...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341017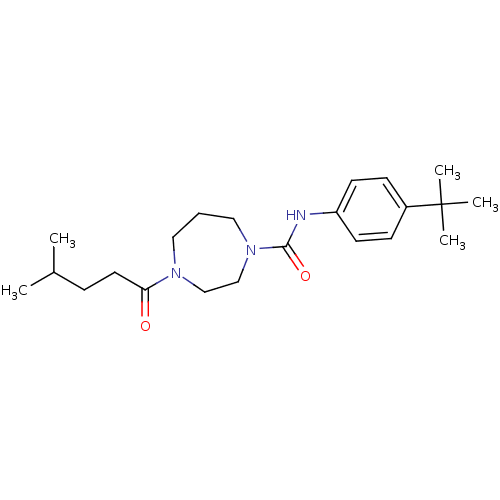 (CHEMBL1762286 | N-(4-tert-butylphenyl)-4-(4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.98E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341021 (CHEMBL1762291 | N-(4-tert-butylphenyl)-4-(4-(dimet...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341026 (CHEMBL1762297 | N-(5-phenylisoxazol-3-yl)-4-(tetra...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50340992 (CHEMBL1762298 | N-(5-tert-butylthiazol-2-yl)-4-(te...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50340995 (CHEMBL1762301 | N-(4-phenylthiazol-2-yl)-4-(tetrah...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341009 (CHEMBL1762428 | N-(5-tert-butylisoxazol-3-yl)-4-(m...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341030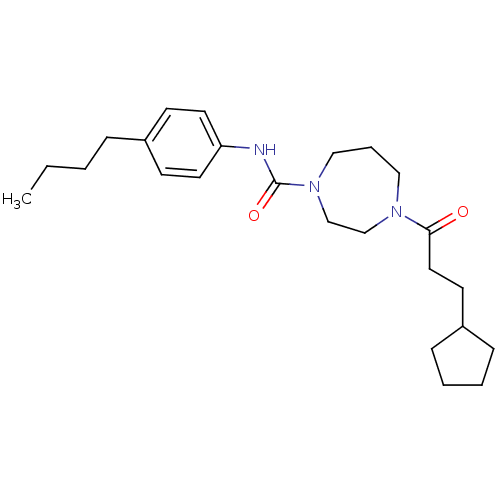 (CHEMBL1762277 | N-(4-butylphenyl)-4-(3-cyclopentyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341020 (CHEMBL1762290 | N-(4-tert-butylphenyl)-4-(4-(trifl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341024 (CHEMBL1762295 | N-(5-methylisoxazol-3-yl)-4-(tetra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341027 (CHEMBL1762296 | N-(4,5-dimethylisoxazol-3-yl)-4-(t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50340999 (CHEMBL1762418 | N-(5-tert-butylisoxazol-3-yl)-4-(t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 750 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341005 (CHEMBL1762424 | N-(5-tert-butylisoxazol-3-yl)-4-(1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.69E+3 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341015 (CHEMBL1762285 | N-(4-tert-butylphenyl)-4-(cyclohex...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341027 (CHEMBL1762296 | N-(4,5-dimethylisoxazol-3-yl)-4-(t...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341006 (CHEMBL1762425 | N-(5-tert-butylisoxazol-3-yl)-4-(p...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341008 (4-[2-(1,1-Dioxo-1lambda*6*-thiomorpholin-4-yl)-ace...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50340997 (CHEMBL1762416 | N-(3-tert-butyl-1H-pyrazol-5-yl)-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341018 (CHEMBL1762288 | N-(4-tert-butylphenyl)-4-(3,5-dich...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341019 (CHEMBL1762289 | N-(4-tert-butylphenyl)-4-(2,5-dich...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341022 (CHEMBL1762292 | N-(4-tert-butylphenyl)-4-(tetrahyd...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341002 (CHEMBL1762421 | cis-N-(5-tert-butylisoxazol-3-yl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341007 (CHEMBL1762426 | N-(5-tert-butylisoxazol-3-yl)-4-(2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50340991 (4-(1,1-Dioxo-1lambda*6*-thiomorpholine-4-carbonyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341031 (4-(3-cyclopentylpropanoyl)-N-(4-ethylphenyl)-1,4-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 226 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50340995 (CHEMBL1762301 | N-(4-phenylthiazol-2-yl)-4-(tetrah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 450 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341002 (CHEMBL1762421 | cis-N-(5-tert-butylisoxazol-3-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341004 (4-(1-acetylpiperidine-4-carbonyl)-N-(5-tert-butyli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341010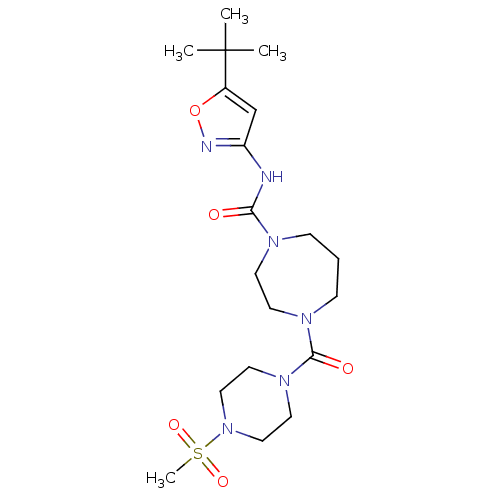 (CHEMBL1762430 | N-(5-tert-butylisoxazol-3-yl)-4-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 2.87E+3 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341025 (CHEMBL1762294 | N-(3-tert-butylisoxazol-5-yl)-4-(t...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50340996 (CHEMBL1762302 | N-(3-tert-butyl-1-methyl-1H-pyrazo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341010 (CHEMBL1762430 | N-(5-tert-butylisoxazol-3-yl)-4-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341011 (CHEMBL1762431 | N-(5-tert-butylisoxazol-3-yl)-4-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341030 (CHEMBL1762277 | N-(4-butylphenyl)-4-(3-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 136 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341033 (CHEMBL1762282 | N-(4-tert-butylphenyl)-4-(3-cyclop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 85 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50341029 (4-(3-cyclopentylpropanoyl)-N-phenyl-1,4-diazepane-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341013 (CHEMBL1762283 | N-(4-tert-butylphenyl)-4-(2-cyclop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341015 (CHEMBL1762285 | N-(4-tert-butylphenyl)-4-(cyclohex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341017 (CHEMBL1762286 | N-(4-tert-butylphenyl)-4-(4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 96 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 21: 2011-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.017 BindingDB Entry DOI: 10.7270/Q2KK9C2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||