

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398759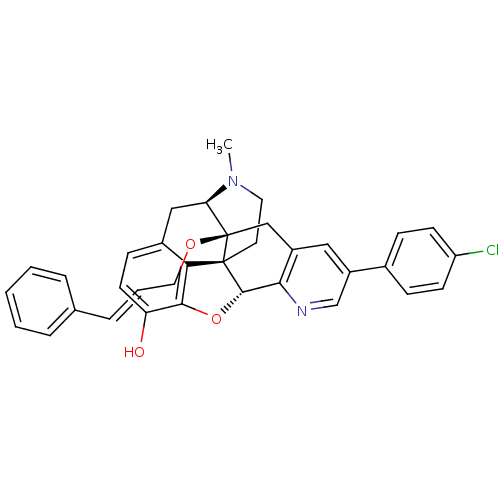 (CHEMBL2179655) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398758 (CHEMBL2179656) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398760 (CHEMBL2179652) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398750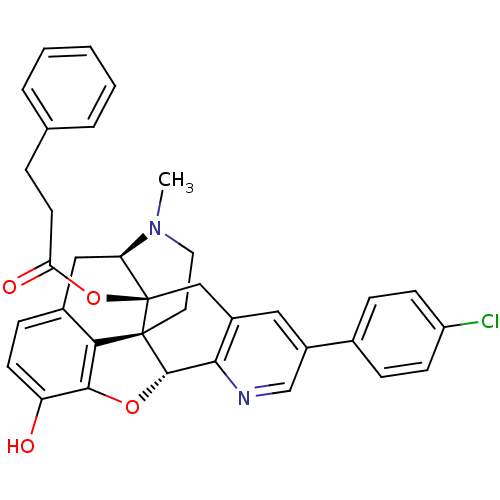 (CHEMBL2179662) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398750 (CHEMBL2179662) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398751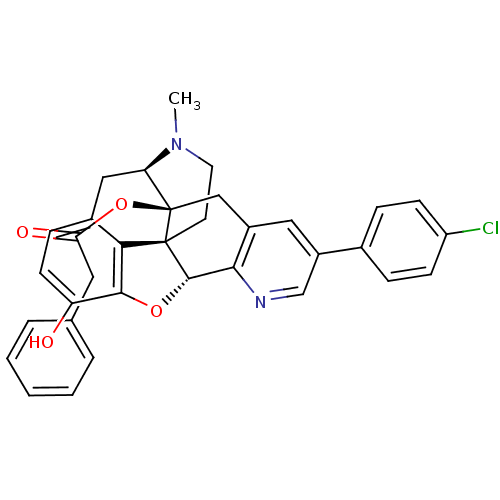 (CHEMBL2179661) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398753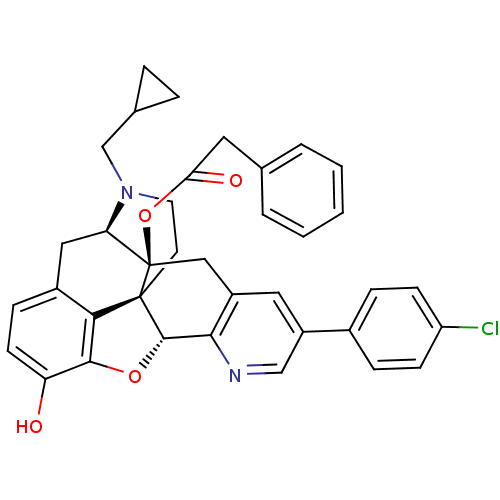 (CHEMBL2179658) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398758 (CHEMBL2179656) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398759 (CHEMBL2179655) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398760 (CHEMBL2179652) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398753 (CHEMBL2179658) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398751 (CHEMBL2179661) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398758 (CHEMBL2179656) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398759 (CHEMBL2179655) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398755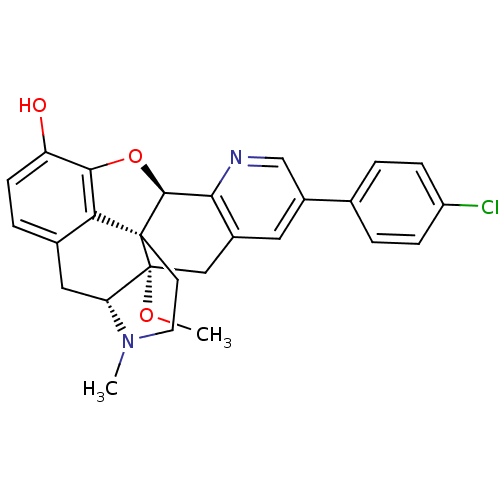 (CHEMBL2179653) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398754 (CHEMBL2179654) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398760 (CHEMBL2179652) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398756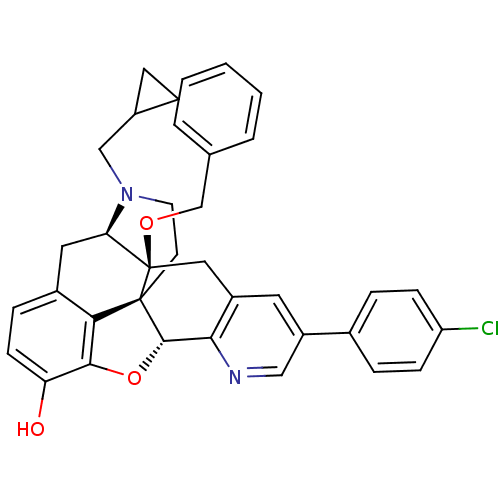 (CHEMBL2179650) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398765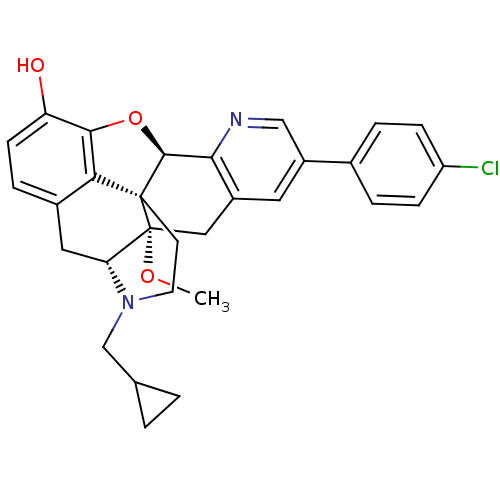 (CHEMBL2179649) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50080467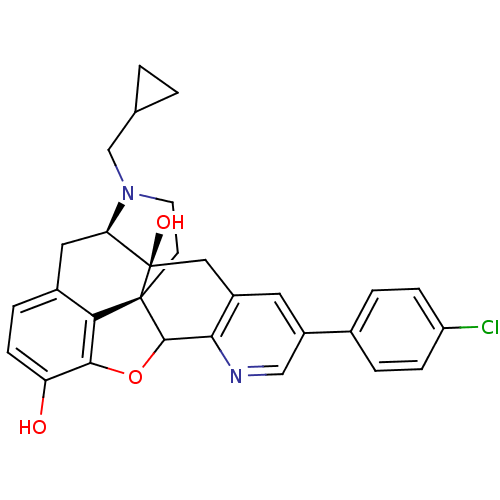 (5'-(Chlorophenyl)-17-(cyclopropylmethyl) -6,7-dide...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity to delta opioid receptor by radioligand binding assay | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398752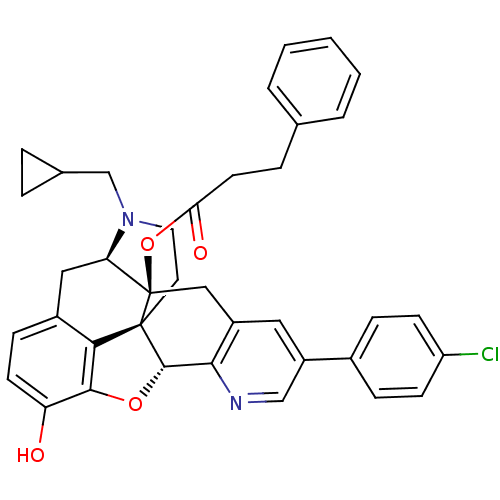 (CHEMBL2179659) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398762 (CHEMBL2179663) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398764 (CHEMBL2179651) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398753 (CHEMBL2179658) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398764 (CHEMBL2179651) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398764 (CHEMBL2179651) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398761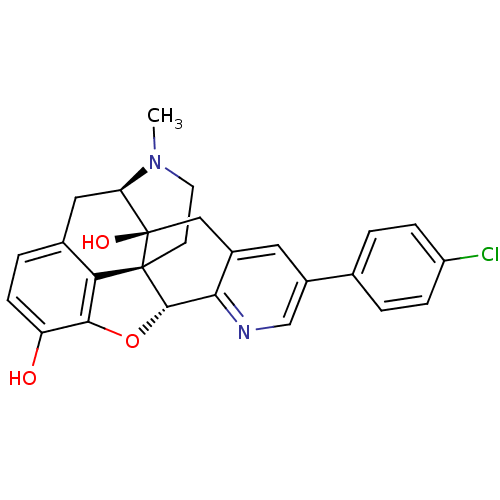 (CHEMBL611438) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity to delta opioid receptor by radioligand binding assay | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398752 (CHEMBL2179659) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398750 (CHEMBL2179662) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398754 (CHEMBL2179654) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398756 (CHEMBL2179650) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398765 (CHEMBL2179649) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398762 (CHEMBL2179663) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398755 (CHEMBL2179653) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398756 (CHEMBL2179650) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398752 (CHEMBL2179659) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398762 (CHEMBL2179663) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398757 (CHEMBL2179660) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398751 (CHEMBL2179661) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50080467 (5'-(Chlorophenyl)-17-(cyclopropylmethyl) -6,7-dide...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity to kappa opioid receptor by radioligand binding assay | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398755 (CHEMBL2179653) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398765 (CHEMBL2179649) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398754 (CHEMBL2179654) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50080467 (5'-(Chlorophenyl)-17-(cyclopropylmethyl) -6,7-dide...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity to mu opioid receptor by radioligand binding assay | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398757 (CHEMBL2179660) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398763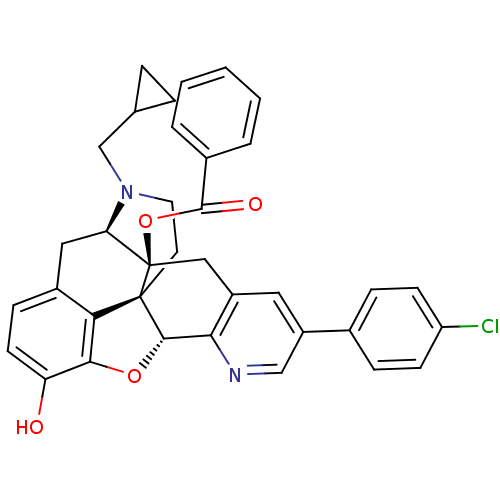 (CHEMBL2179657) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398757 (CHEMBL2179660) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398763 (CHEMBL2179657) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398763 (CHEMBL2179657) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 192 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398761 (CHEMBL611438) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity to mu opioid receptor by radioligand binding assay | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398761 (CHEMBL611438) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 468 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity to kappa opioid receptor by radioligand binding assay | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398750 (CHEMBL2179662) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at delta opioid receptor expressed in rat/mouse NG108-15 cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398758 (CHEMBL2179656) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398751 (CHEMBL2179661) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 109 | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398751 (CHEMBL2179661) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 87 | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398754 (CHEMBL2179654) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 301 | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398751 (CHEMBL2179661) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at delta opioid receptor expressed in rat/mouse NG108-15 cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398758 (CHEMBL2179656) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at delta opioid receptor expressed in rat/mouse NG108-15 cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398754 (CHEMBL2179654) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at delta opioid receptor expressed in rat/mouse NG108-15 cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398757 (CHEMBL2179660) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.62E+3 | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398750 (CHEMBL2179662) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 136 | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398756 (CHEMBL2179650) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398755 (CHEMBL2179653) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 633 | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398754 (CHEMBL2179654) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 215 | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398753 (CHEMBL2179658) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398759 (CHEMBL2179655) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398750 (CHEMBL2179662) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 48 | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398752 (CHEMBL2179659) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398760 (CHEMBL2179652) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398759 (CHEMBL2179655) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at delta opioid receptor expressed in rat/mouse NG108-15 cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398755 (CHEMBL2179653) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 379 | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||