 Found 419 hits with Last Name = 'van de poël' and Initial = 'a'
Found 419 hits with Last Name = 'van de poël' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM50571476
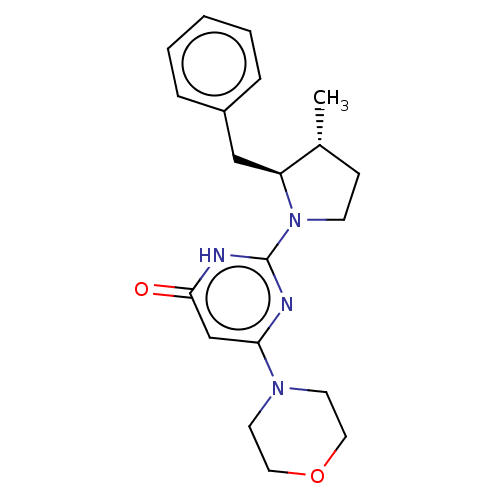
(CHEMBL4852400 | US11685734, Example 215)Show SMILES C[C@@H]1CCN([C@H]1Cc1ccccc1)c1nc(cc(=O)[nH]1)N1CCOCC1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24Q803G |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM50571471
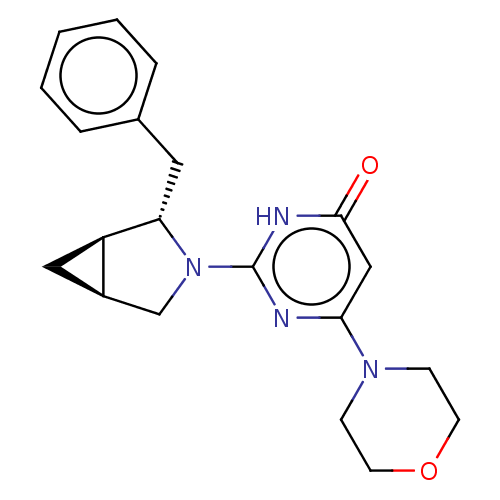
(CHEMBL4858310 | US11685734, Example 213)Show SMILES [H][C@]12C[C@@]1([H])[C@H](Cc1ccccc1)N(C2)c1nc(cc(=O)[nH]1)N1CCOCC1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24Q803G |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM606903
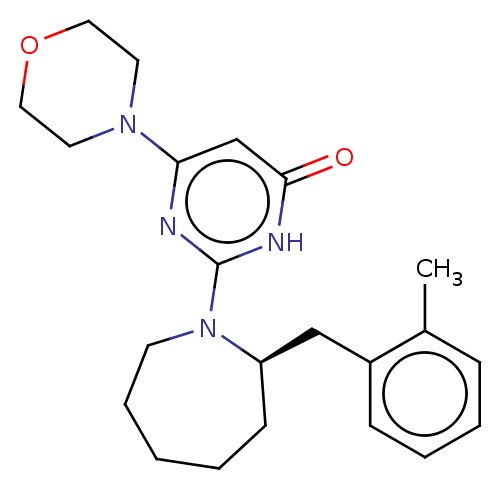
(US11685734, Example 181)Show SMILES Cc1ccccc1C[C@H]1CCCCCN1c1nc(cc(=O)[nH]1)N1CCOCC1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24Q803G |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM606927
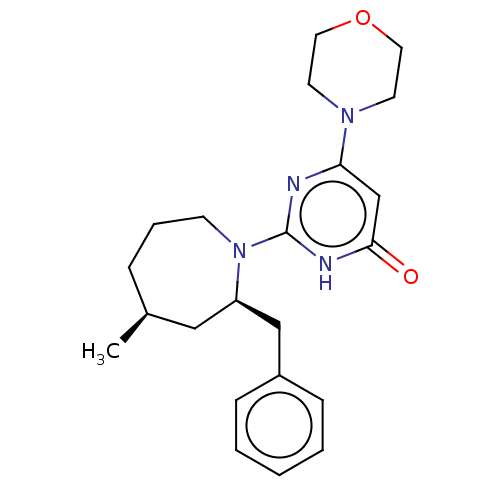
(US11685734, Example 233)Show SMILES C[C@H]1CCCN([C@@H](Cc2ccccc2)C1)c1nc(cc(=O)[nH]1)N1CCOCC1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24Q803G |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM606934
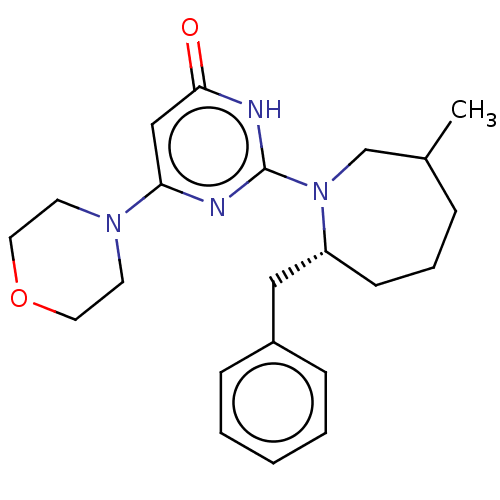
(US11685734, Example 246 | US11685734, Example 247)Show SMILES CC1CCC[C@H](Cc2ccccc2)N(C1)c1nc(cc(=O)[nH]1)N1CCOCC1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24Q803G |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM606943
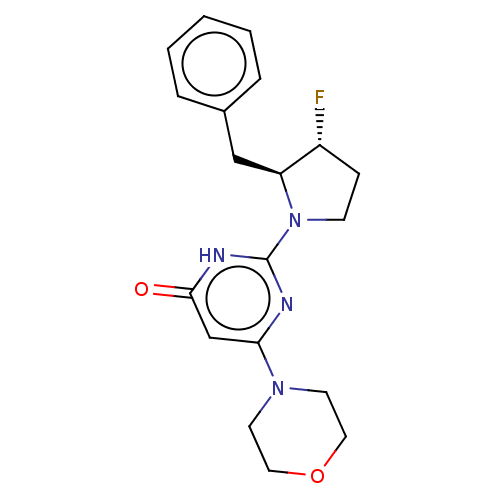
(US11685734, Example 259)Show SMILES F[C@@H]1CCN([C@H]1Cc1ccccc1)c1nc(cc(=O)[nH]1)N1CCOCC1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24Q803G |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM606913
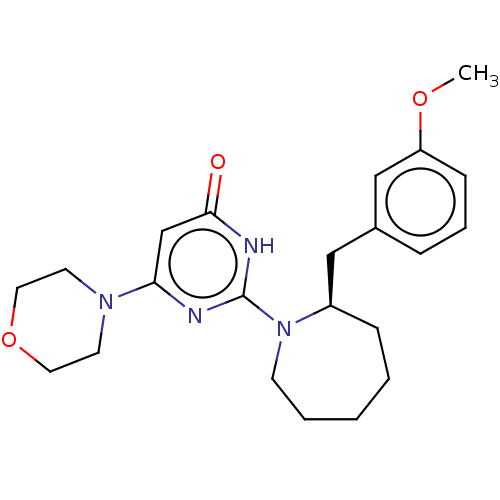
(US11685734, Example 203)Show SMILES COc1cccc(C[C@H]2CCCCCN2c2nc(cc(=O)[nH]2)N2CCOCC2)c1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24Q803G |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50195239
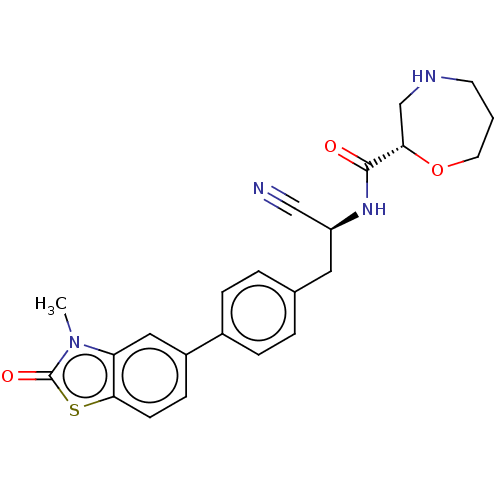
(CHEMBL3972563 | US10287258, Example 29 | US1066924...)Show SMILES Cn1c2cc(ccc2sc1=O)-c1ccc(C[C@H](NC(=O)[C@@H]2CNCCCO2)C#N)cc1 |r| Show InChI InChI=1S/C23H24N4O3S/c1-27-19-12-17(7-8-21(19)31-23(27)29)16-5-3-15(4-6-16)11-18(13-24)26-22(28)20-14-25-9-2-10-30-20/h3-8,12,18,20,25H,2,9-11,14H2,1H3,(H,26,28)/t18-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2F76HN2 |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM606875
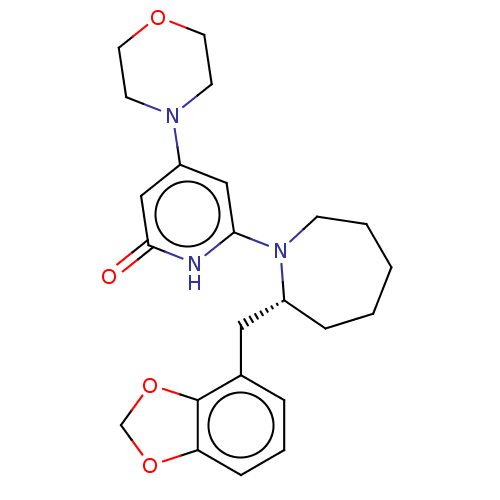
(US11685734, Example 70)Show SMILES O=c1cc(cc([nH]1)N1CCCCC[C@@H]1Cc1cccc2OCOc12)N1CCOCC1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24Q803G |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50195239

(CHEMBL3972563 | US10287258, Example 29 | US1066924...)Show SMILES Cn1c2cc(ccc2sc1=O)-c1ccc(C[C@H](NC(=O)[C@@H]2CNCCCO2)C#N)cc1 |r| Show InChI InChI=1S/C23H24N4O3S/c1-27-19-12-17(7-8-21(19)31-23(27)29)16-5-3-15(4-6-16)11-18(13-24)26-22(28)20-14-25-9-2-10-30-20/h3-8,12,18,20,25H,2,9-11,14H2,1H3,(H,26,28)/t18-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FB56W4 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50195239

(CHEMBL3972563 | US10287258, Example 29 | US1066924...)Show SMILES Cn1c2cc(ccc2sc1=O)-c1ccc(C[C@H](NC(=O)[C@@H]2CNCCCO2)C#N)cc1 |r| Show InChI InChI=1S/C23H24N4O3S/c1-27-19-12-17(7-8-21(19)31-23(27)29)16-5-3-15(4-6-16)11-18(13-24)26-22(28)20-14-25-9-2-10-30-20/h3-8,12,18,20,25H,2,9-11,14H2,1H3,(H,26,28)/t18-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
The activity of DPP1 was determined by measuring the enzymatic release of aminomethyl coumarin (AMC) from the peptide substrate (H-Gly-Arg-AMC), whic... |
J Med Chem 51: 7049-52 (2008)
BindingDB Entry DOI: 10.7270/Q2RJ4MTP |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50195239

(CHEMBL3972563 | US10287258, Example 29 | US1066924...)Show SMILES Cn1c2cc(ccc2sc1=O)-c1ccc(C[C@H](NC(=O)[C@@H]2CNCCCO2)C#N)cc1 |r| Show InChI InChI=1S/C23H24N4O3S/c1-27-19-12-17(7-8-21(19)31-23(27)29)16-5-3-15(4-6-16)11-18(13-24)26-22(28)20-14-25-9-2-10-30-20/h3-8,12,18,20,25H,2,9-11,14H2,1H3,(H,26,28)/t18-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6X5T |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50195239

(CHEMBL3972563 | US10287258, Example 29 | US1066924...)Show SMILES Cn1c2cc(ccc2sc1=O)-c1ccc(C[C@H](NC(=O)[C@@H]2CNCCCO2)C#N)cc1 |r| Show InChI InChI=1S/C23H24N4O3S/c1-27-19-12-17(7-8-21(19)31-23(27)29)16-5-3-15(4-6-16)11-18(13-24)26-22(28)20-14-25-9-2-10-30-20/h3-8,12,18,20,25H,2,9-11,14H2,1H3,(H,26,28)/t18-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB
US Patent
| Assay Description
The activity of DPP1 was determined by measuring the enzymatic release of aminomethyl coumarin (AMC) from the peptide substrate (H-Gly-Arg-AMC), whic... |
US Patent US10669245 (2020)
BindingDB Entry DOI: 10.7270/Q2862KG7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50195239

(CHEMBL3972563 | US10287258, Example 29 | US1066924...)Show SMILES Cn1c2cc(ccc2sc1=O)-c1ccc(C[C@H](NC(=O)[C@@H]2CNCCCO2)C#N)cc1 |r| Show InChI InChI=1S/C23H24N4O3S/c1-27-19-12-17(7-8-21(19)31-23(27)29)16-5-3-15(4-6-16)11-18(13-24)26-22(28)20-14-25-9-2-10-30-20/h3-8,12,18,20,25H,2,9-11,14H2,1H3,(H,26,28)/t18-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Examples 1-35: The activity of DPP1 was determined by measuring the enzymatic release of aminomethyl coumarin (AMC) from the peptide substrate (H-Gly... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q243DZ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM604873
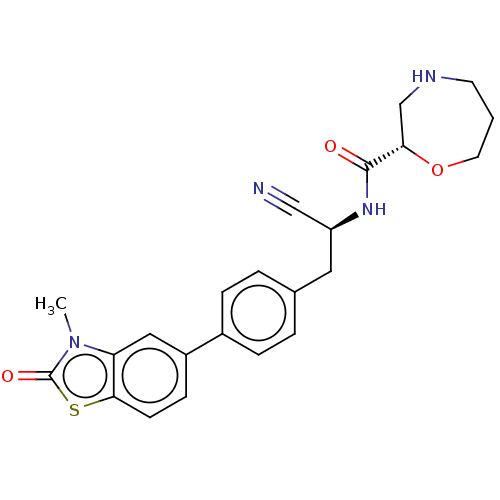
((2S)-N-[(1S)-1-Cyano-2-[4-(3-methyl-2-oxo-2,3-dihy...)Show SMILES Cn1c2cc(ccc2sc1=O)-c1ccc(C[C@H](NC(=O)[C@@H]2CNCCCO2)C#N)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2H99949 |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM606911
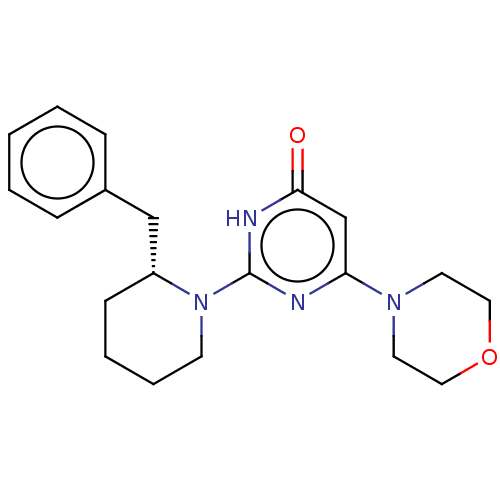
(US11685734, Example 199)Show SMILES O=c1cc(nc([nH]1)N1CCCC[C@@H]1Cc1ccccc1)N1CCOCC1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24Q803G |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM228164
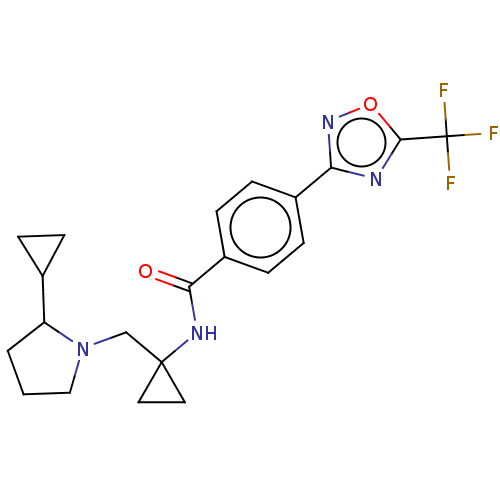
(US10047073, 6 | US10047073, 7)Show SMILES FC(F)(F)c1nc(no1)-c1ccc(cc1)C(=O)NC1(CN2CCCC2C2CC2)CC1 Show InChI InChI=1S/C21H23F3N4O2/c22-21(23,24)19-25-17(27-30-19)14-5-7-15(8-6-14)18(29)26-20(9-10-20)12-28-11-1-2-16(28)13-3-4-13/h5-8,13,16H,1-4,9-12H2,(H,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC
US Patent
| Assay Description
The Class I HDAC activity of Class IIa Histone Deacetylase (HDAC) inhibitors was quantified by measuring the cellular histone deacetylase enzymatic a... |
US Patent US10047073 (2018)
BindingDB Entry DOI: 10.7270/Q2PG1TQC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4 [648-1032]
(Homo sapiens (Human)) | BDBM243173
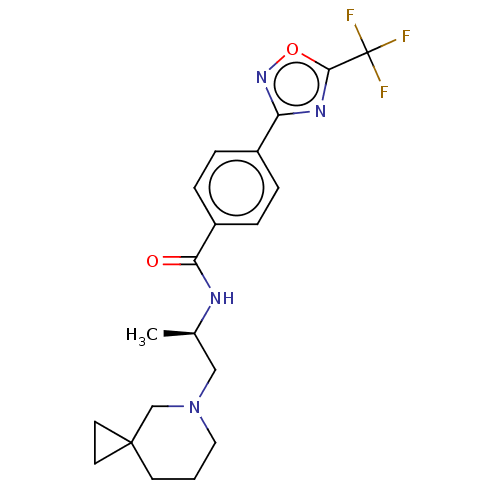
((R)-N-(1-(5-Azaspiro[2.5]octan-5- yl)propan-2-yl)-...)Show SMILES C[C@H](CN1CCCC2(CC2)C1)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C20H23F3N4O2/c1-13(11-27-10-2-7-19(12-27)8-9-19)24-17(28)15-5-3-14(4-6-15)16-25-18(29-26-16)20(21,22)23/h3-6,13H,2,7-12H2,1H3,(H,24,28)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC.
US Patent
| Assay Description
5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th... |
US Patent US10053434 (2018)
BindingDB Entry DOI: 10.7270/Q2MG7RJZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4 [648-1032]
(Homo sapiens (Human)) | BDBM243152
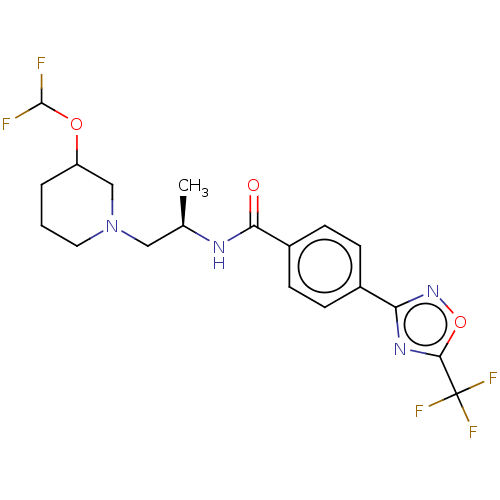
(D1: N-((R)-1-((abs)-3- (Difluoromethoxy)piperidin-...)Show SMILES C[C@H](CN1CCCC(C1)OC(F)F)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C19H21F5N4O3/c1-11(9-28-8-2-3-14(10-28)30-18(20)21)25-16(29)13-6-4-12(5-7-13)15-26-17(31-27-15)19(22,23)24/h4-7,11,14,18H,2-3,8-10H2,1H3,(H,25,29)/t11-,14?/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC.
US Patent
| Assay Description
5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th... |
US Patent US10053434 (2018)
BindingDB Entry DOI: 10.7270/Q2MG7RJZ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM385058
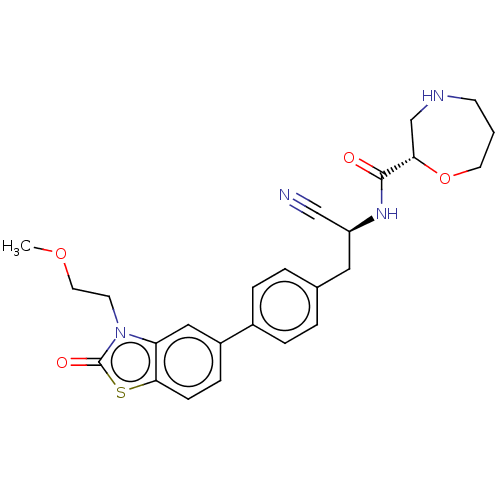
((2S)-N-[(1S)-1-Cyano-2-{4-[3-(2-methoxyethyl)-2-ox...)Show SMILES COCCn1c2cc(ccc2sc1=O)-c1ccc(C[C@H](NC(=O)[C@@H]2CNCCCO2)C#N)cc1 Show InChI InChI=1S/C25H28N4O4S/c1-32-12-10-29-21-14-19(7-8-23(21)34-25(29)31)18-5-3-17(4-6-18)13-20(15-26)28-24(30)22-16-27-9-2-11-33-22/h3-8,14,20,22,27H,2,9-13,16H2,1H3,(H,28,30)/t20-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2F76HN2 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM385058

((2S)-N-[(1S)-1-Cyano-2-{4-[3-(2-methoxyethyl)-2-ox...)Show SMILES COCCn1c2cc(ccc2sc1=O)-c1ccc(C[C@H](NC(=O)[C@@H]2CNCCCO2)C#N)cc1 Show InChI InChI=1S/C25H28N4O4S/c1-32-12-10-29-21-14-19(7-8-23(21)34-25(29)31)18-5-3-17(4-6-18)13-20(15-26)28-24(30)22-16-27-9-2-11-33-22/h3-8,14,20,22,27H,2,9-13,16H2,1H3,(H,28,30)/t20-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
The activity of DPP1 was determined by measuring the enzymatic release of aminomethyl coumarin (AMC) from the peptide substrate (H-Gly-Arg-AMC), whic... |
J Med Chem 51: 7049-52 (2008)
BindingDB Entry DOI: 10.7270/Q2RJ4MTP |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM385058

((2S)-N-[(1S)-1-Cyano-2-{4-[3-(2-methoxyethyl)-2-ox...)Show SMILES COCCn1c2cc(ccc2sc1=O)-c1ccc(C[C@H](NC(=O)[C@@H]2CNCCCO2)C#N)cc1 Show InChI InChI=1S/C25H28N4O4S/c1-32-12-10-29-21-14-19(7-8-23(21)34-25(29)31)18-5-3-17(4-6-18)13-20(15-26)28-24(30)22-16-27-9-2-11-33-22/h3-8,14,20,22,27H,2,9-13,16H2,1H3,(H,28,30)/t20-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2H99949 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM385058

((2S)-N-[(1S)-1-Cyano-2-{4-[3-(2-methoxyethyl)-2-ox...)Show SMILES COCCn1c2cc(ccc2sc1=O)-c1ccc(C[C@H](NC(=O)[C@@H]2CNCCCO2)C#N)cc1 Show InChI InChI=1S/C25H28N4O4S/c1-32-12-10-29-21-14-19(7-8-23(21)34-25(29)31)18-5-3-17(4-6-18)13-20(15-26)28-24(30)22-16-27-9-2-11-33-22/h3-8,14,20,22,27H,2,9-13,16H2,1H3,(H,28,30)/t20-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Examples 1-35: The activity of DPP1 was determined by measuring the enzymatic release of aminomethyl coumarin (AMC) from the peptide substrate (H-Gly... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q243DZ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM385058

((2S)-N-[(1S)-1-Cyano-2-{4-[3-(2-methoxyethyl)-2-ox...)Show SMILES COCCn1c2cc(ccc2sc1=O)-c1ccc(C[C@H](NC(=O)[C@@H]2CNCCCO2)C#N)cc1 Show InChI InChI=1S/C25H28N4O4S/c1-32-12-10-29-21-14-19(7-8-23(21)34-25(29)31)18-5-3-17(4-6-18)13-20(15-26)28-24(30)22-16-27-9-2-11-33-22/h3-8,14,20,22,27H,2,9-13,16H2,1H3,(H,28,30)/t20-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB
US Patent
| Assay Description
The activity of DPP1 was determined by measuring the enzymatic release of aminomethyl coumarin (AMC) from the peptide substrate (H-Gly-Arg-AMC), whic... |
US Patent US10669245 (2020)
BindingDB Entry DOI: 10.7270/Q2862KG7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM385058

((2S)-N-[(1S)-1-Cyano-2-{4-[3-(2-methoxyethyl)-2-ox...)Show SMILES COCCn1c2cc(ccc2sc1=O)-c1ccc(C[C@H](NC(=O)[C@@H]2CNCCCO2)C#N)cc1 Show InChI InChI=1S/C25H28N4O4S/c1-32-12-10-29-21-14-19(7-8-23(21)34-25(29)31)18-5-3-17(4-6-18)13-20(15-26)28-24(30)22-16-27-9-2-11-33-22/h3-8,14,20,22,27H,2,9-13,16H2,1H3,(H,28,30)/t20-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6X5T |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM385058

((2S)-N-[(1S)-1-Cyano-2-{4-[3-(2-methoxyethyl)-2-ox...)Show SMILES COCCn1c2cc(ccc2sc1=O)-c1ccc(C[C@H](NC(=O)[C@@H]2CNCCCO2)C#N)cc1 Show InChI InChI=1S/C25H28N4O4S/c1-32-12-10-29-21-14-19(7-8-23(21)34-25(29)31)18-5-3-17(4-6-18)13-20(15-26)28-24(30)22-16-27-9-2-11-33-22/h3-8,14,20,22,27H,2,9-13,16H2,1H3,(H,28,30)/t20-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FB56W4 |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM606890
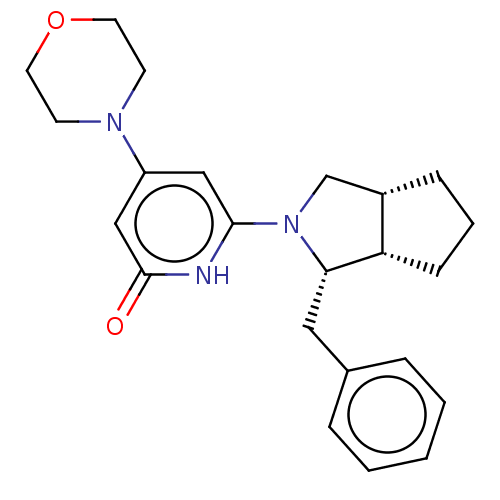
(US11685734, Example 124)Show SMILES O=c1cc(cc([nH]1)N1C[C@@H]2CCC[C@@H]2[C@@H]1Cc1ccccc1)N1CCOCC1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24Q803G |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM50571474
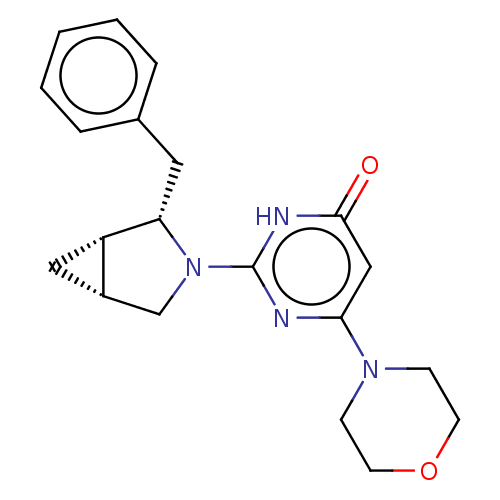
(CHEMBL4857803 | US11685734, Example 211)Show SMILES [H][C@@]12C[C@]1([H])[C@H](Cc1ccccc1)N(C2)c1nc(cc(=O)[nH]1)N1CCOCC1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24Q803G |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM606923
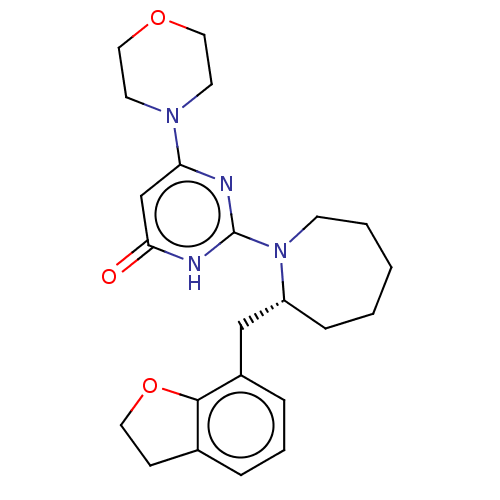
(US11685734, Example 225)Show SMILES O=c1cc(nc([nH]1)N1CCCCC[C@@H]1Cc1cccc2CCOc12)N1CCOCC1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24Q803G |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM606936
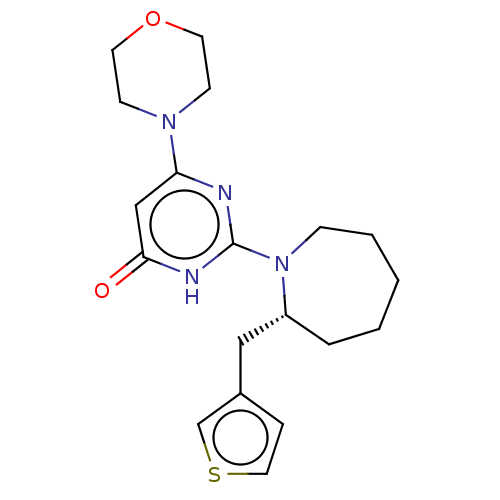
(US11685734, Example 248)Show SMILES O=c1cc(nc([nH]1)N1CCCCC[C@@H]1Cc1ccsc1)N1CCOCC1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24Q803G |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM606941
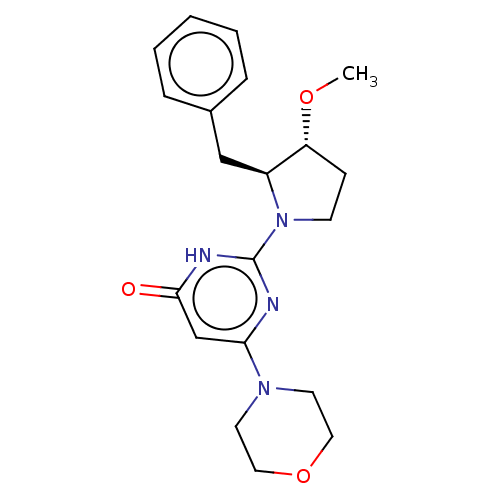
(US11685734, Example 257)Show SMILES CO[C@@H]1CCN([C@H]1Cc1ccccc1)c1nc(cc(=O)[nH]1)N1CCOCC1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24Q803G |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4 [648-1032]
(Homo sapiens (Human)) | BDBM243192

(N-((2R)-1-(3-Azabicyclo[3.2.0]heptan- 3-yl)propan-...)Show SMILES C[C@H](CN1CC2CCC2C1)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C19H21F3N4O2/c1-11(8-26-9-14-6-7-15(14)10-26)23-17(27)13-4-2-12(3-5-13)16-24-18(28-25-16)19(20,21)22/h2-5,11,14-15H,6-10H2,1H3,(H,23,27)/t11-,14?,15?/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC.
US Patent
| Assay Description
5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th... |
US Patent US10053434 (2018)
BindingDB Entry DOI: 10.7270/Q2MG7RJZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4 [648-1032]
(Homo sapiens (Human)) | BDBM243193
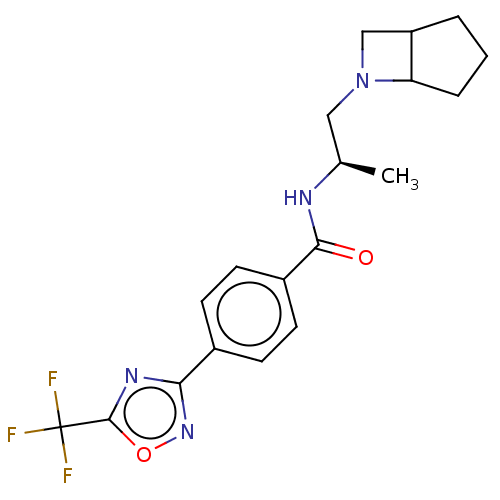
(D1: N-((R)-1-((abs-1,5-cis)-6- Azabicyclo[3.2.0]he...)Show SMILES C[C@H](CN1CC2CCCC12)NC(=O)c1ccc(cc1)-c1noc(n1)C(F)(F)F |r| Show InChI InChI=1S/C19H21F3N4O2/c1-11(9-26-10-14-3-2-4-15(14)26)23-17(27)13-7-5-12(6-8-13)16-24-18(28-25-16)19(20,21)22/h5-8,11,14-15H,2-4,9-10H2,1H3,(H,23,27)/t11-,14?,15?/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC.
US Patent
| Assay Description
5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th... |
US Patent US10053434 (2018)
BindingDB Entry DOI: 10.7270/Q2MG7RJZ |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM606906

(US11685734, Example 190)Show SMILES Clc1ccc(C[C@H]2CCCN2c2nc(cc(=O)[nH]2)N2CCOCC2)cc1Cl |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24Q803G |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM606915
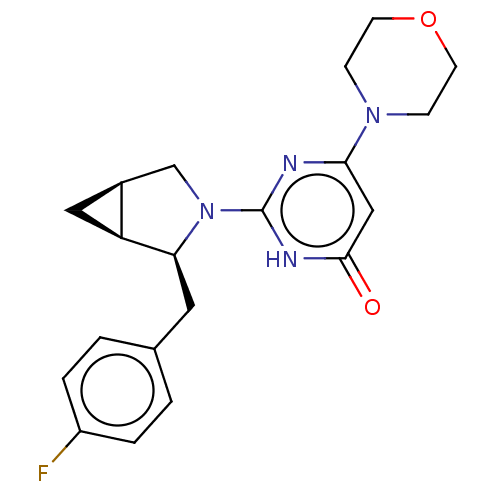
(US11685734, Example 207)Show SMILES Fc1ccc(C[C@H]2[C@H]3C[C@H]3CN2c2nc(cc(=O)[nH]2)N2CCOCC2)cc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24Q803G |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50195235
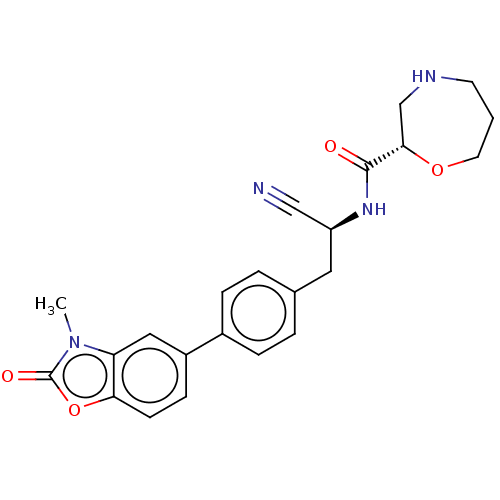
(CHEMBL3900409 | US10287258, Example 2 | US10669245...)Show SMILES Cn1c2cc(ccc2oc1=O)-c1ccc(C[C@H](NC(=O)[C@@H]2CNCCCO2)C#N)cc1 |r| Show InChI InChI=1S/C23H24N4O4/c1-27-19-12-17(7-8-20(19)31-23(27)29)16-5-3-15(4-6-16)11-18(13-24)26-22(28)21-14-25-9-2-10-30-21/h3-8,12,18,21,25H,2,9-11,14H2,1H3,(H,26,28)/t18-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 4.47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6X5T |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50195235

(CHEMBL3900409 | US10287258, Example 2 | US10669245...)Show SMILES Cn1c2cc(ccc2oc1=O)-c1ccc(C[C@H](NC(=O)[C@@H]2CNCCCO2)C#N)cc1 |r| Show InChI InChI=1S/C23H24N4O4/c1-27-19-12-17(7-8-20(19)31-23(27)29)16-5-3-15(4-6-16)11-18(13-24)26-22(28)21-14-25-9-2-10-30-21/h3-8,12,18,21,25H,2,9-11,14H2,1H3,(H,26,28)/t18-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4.47 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB
US Patent
| Assay Description
The activity of DPP1 was determined by measuring the enzymatic release of aminomethyl coumarin (AMC) from the peptide substrate (H-Gly-Arg-AMC), whic... |
US Patent US10669245 (2020)
BindingDB Entry DOI: 10.7270/Q2862KG7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50195235

(CHEMBL3900409 | US10287258, Example 2 | US10669245...)Show SMILES Cn1c2cc(ccc2oc1=O)-c1ccc(C[C@H](NC(=O)[C@@H]2CNCCCO2)C#N)cc1 |r| Show InChI InChI=1S/C23H24N4O4/c1-27-19-12-17(7-8-20(19)31-23(27)29)16-5-3-15(4-6-16)11-18(13-24)26-22(28)21-14-25-9-2-10-30-21/h3-8,12,18,21,25H,2,9-11,14H2,1H3,(H,26,28)/t18-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4.47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Examples 1-35: The activity of DPP1 was determined by measuring the enzymatic release of aminomethyl coumarin (AMC) from the peptide substrate (H-Gly... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q243DZ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50195235

(CHEMBL3900409 | US10287258, Example 2 | US10669245...)Show SMILES Cn1c2cc(ccc2oc1=O)-c1ccc(C[C@H](NC(=O)[C@@H]2CNCCCO2)C#N)cc1 |r| Show InChI InChI=1S/C23H24N4O4/c1-27-19-12-17(7-8-20(19)31-23(27)29)16-5-3-15(4-6-16)11-18(13-24)26-22(28)21-14-25-9-2-10-30-21/h3-8,12,18,21,25H,2,9-11,14H2,1H3,(H,26,28)/t18-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 4.47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2H99949 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50195235

(CHEMBL3900409 | US10287258, Example 2 | US10669245...)Show SMILES Cn1c2cc(ccc2oc1=O)-c1ccc(C[C@H](NC(=O)[C@@H]2CNCCCO2)C#N)cc1 |r| Show InChI InChI=1S/C23H24N4O4/c1-27-19-12-17(7-8-20(19)31-23(27)29)16-5-3-15(4-6-16)11-18(13-24)26-22(28)21-14-25-9-2-10-30-21/h3-8,12,18,21,25H,2,9-11,14H2,1H3,(H,26,28)/t18-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4.47 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
The activity of DPP1 was determined by measuring the enzymatic release of aminomethyl coumarin (AMC) from the peptide substrate (H-Gly-Arg-AMC), whic... |
J Med Chem 51: 7049-52 (2008)
BindingDB Entry DOI: 10.7270/Q2RJ4MTP |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50195235

(CHEMBL3900409 | US10287258, Example 2 | US10669245...)Show SMILES Cn1c2cc(ccc2oc1=O)-c1ccc(C[C@H](NC(=O)[C@@H]2CNCCCO2)C#N)cc1 |r| Show InChI InChI=1S/C23H24N4O4/c1-27-19-12-17(7-8-20(19)31-23(27)29)16-5-3-15(4-6-16)11-18(13-24)26-22(28)21-14-25-9-2-10-30-21/h3-8,12,18,21,25H,2,9-11,14H2,1H3,(H,26,28)/t18-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 4.47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2F76HN2 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50195235

(CHEMBL3900409 | US10287258, Example 2 | US10669245...)Show SMILES Cn1c2cc(ccc2oc1=O)-c1ccc(C[C@H](NC(=O)[C@@H]2CNCCCO2)C#N)cc1 |r| Show InChI InChI=1S/C23H24N4O4/c1-27-19-12-17(7-8-20(19)31-23(27)29)16-5-3-15(4-6-16)11-18(13-24)26-22(28)21-14-25-9-2-10-30-21/h3-8,12,18,21,25H,2,9-11,14H2,1H3,(H,26,28)/t18-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 4.47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FB56W4 |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM606937
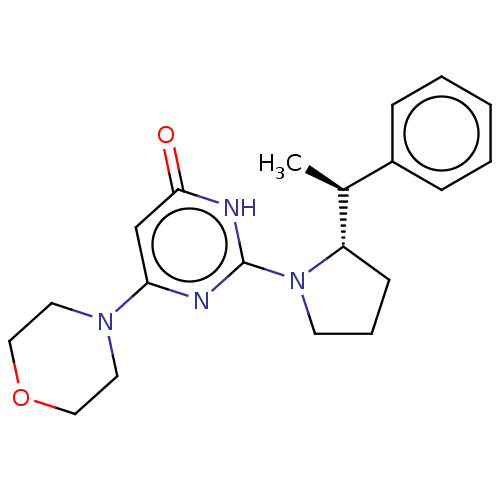
(US11685734, Example 250)Show SMILES C[C@H]([C@@H]1CCCN1c1nc(cc(=O)[nH]1)N1CCOCC1)c1ccccc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24Q803G |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM385076
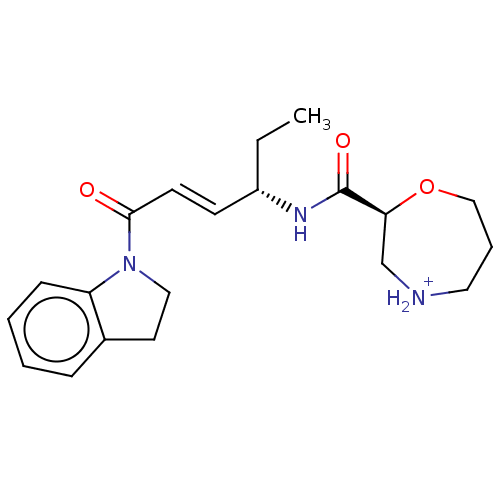
(BDBM445984 | US10287258, Example 36 | US11667615, ...)Show SMILES CC[C@H](NC(=O)[C@@H]1CNCCCO1)\C=C\C(=O)N1CCc2ccccc12 |r| Show InChI InChI=1S/C20H27N3O3/c1-2-16(22-20(25)18-14-21-11-5-13-26-18)8-9-19(24)23-12-10-15-6-3-4-7-17(15)23/h3-4,6-9,16,18,21H,2,5,10-14H2,1H3,(H,22,25)/b9-8+/t16-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FB56W4 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM385076

(BDBM445984 | US10287258, Example 36 | US11667615, ...)Show SMILES CC[C@H](NC(=O)[C@@H]1CNCCCO1)\C=C\C(=O)N1CCc2ccccc12 |r| Show InChI InChI=1S/C20H27N3O3/c1-2-16(22-20(25)18-14-21-11-5-13-26-18)8-9-19(24)23-12-10-15-6-3-4-7-17(15)23/h3-4,6-9,16,18,21H,2,5,10-14H2,1H3,(H,22,25)/b9-8+/t16-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2F76HN2 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM385076

(BDBM445984 | US10287258, Example 36 | US11667615, ...)Show SMILES CC[C@H](NC(=O)[C@@H]1CNCCCO1)\C=C\C(=O)N1CCc2ccccc12 |r| Show InChI InChI=1S/C20H27N3O3/c1-2-16(22-20(25)18-14-21-11-5-13-26-18)8-9-19(24)23-12-10-15-6-3-4-7-17(15)23/h3-4,6-9,16,18,21H,2,5,10-14H2,1H3,(H,22,25)/b9-8+/t16-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6X5T |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM385076

(BDBM445984 | US10287258, Example 36 | US11667615, ...)Show SMILES CC[C@H](NC(=O)[C@@H]1CNCCCO1)\C=C\C(=O)N1CCc2ccccc12 |r| Show InChI InChI=1S/C20H27N3O3/c1-2-16(22-20(25)18-14-21-11-5-13-26-18)8-9-19(24)23-12-10-15-6-3-4-7-17(15)23/h3-4,6-9,16,18,21H,2,5,10-14H2,1H3,(H,22,25)/b9-8+/t16-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2H99949 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM385076

(BDBM445984 | US10287258, Example 36 | US11667615, ...)Show SMILES CC[C@H](NC(=O)[C@@H]1CNCCCO1)\C=C\C(=O)N1CCc2ccccc12 |r| Show InChI InChI=1S/C20H27N3O3/c1-2-16(22-20(25)18-14-21-11-5-13-26-18)8-9-19(24)23-12-10-15-6-3-4-7-17(15)23/h3-4,6-9,16,18,21H,2,5,10-14H2,1H3,(H,22,25)/b9-8+/t16-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Examples 36-37: The activity of DPP1 was determined by measuring the enzymatic release of aminomethyl coumarin (AMC) from the peptide substrate (H-Gl... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q243DZ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM385076

(BDBM445984 | US10287258, Example 36 | US11667615, ...)Show SMILES CC[C@H](NC(=O)[C@@H]1CNCCCO1)\C=C\C(=O)N1CCc2ccccc12 |r| Show InChI InChI=1S/C20H27N3O3/c1-2-16(22-20(25)18-14-21-11-5-13-26-18)8-9-19(24)23-12-10-15-6-3-4-7-17(15)23/h3-4,6-9,16,18,21H,2,5,10-14H2,1H3,(H,22,25)/b9-8+/t16-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB
US Patent
| Assay Description
The activity of DPP1 was determined by measuring the enzymatic release of aminomethyl coumarin (AMC) from the peptide substrate (H-Gly-Arg-AMC), whic... |
US Patent US10669245 (2020)
BindingDB Entry DOI: 10.7270/Q2862KG7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM385076

(BDBM445984 | US10287258, Example 36 | US11667615, ...)Show SMILES CC[C@H](NC(=O)[C@@H]1CNCCCO1)\C=C\C(=O)N1CCc2ccccc12 |r| Show InChI InChI=1S/C20H27N3O3/c1-2-16(22-20(25)18-14-21-11-5-13-26-18)8-9-19(24)23-12-10-15-6-3-4-7-17(15)23/h3-4,6-9,16,18,21H,2,5,10-14H2,1H3,(H,22,25)/b9-8+/t16-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
The activity of DPP1 was determined by measuring the enzymatic release of aminomethyl coumarin (AMC) from the peptide substrate (H-Gly-Arg-AMC), whic... |
J Med Chem 51: 7049-52 (2008)
BindingDB Entry DOI: 10.7270/Q2RJ4MTP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data