

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50543677 (CHEMBL4635823) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Binding affinity to recombinant human RORgammat transfected in human HEK293T cells incubated for 24 hrs by dual-glo luciferase reporter gene assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127174 BindingDB Entry DOI: 10.7270/Q2862M1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402386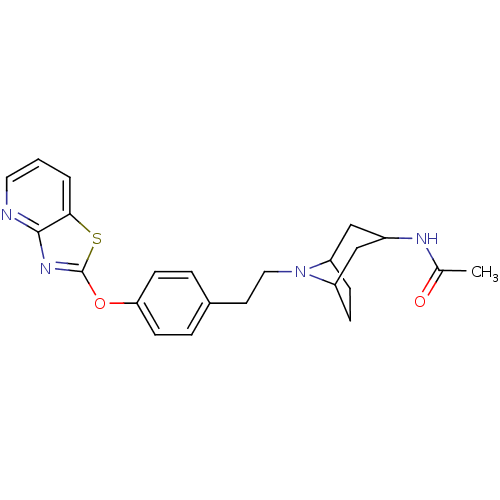 (CHEMBL2207747) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402382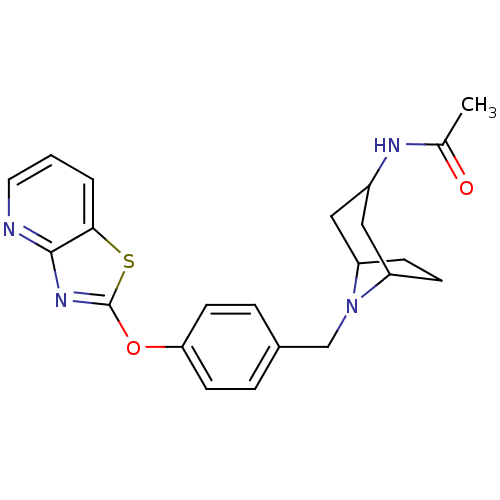 (CHEMBL2207751) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM285736 (US10080744, Example 3/4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Cells used in this assay were transiently co-transfected with three different plasmids, one expressing the GAL4-DNA binding domain (DBD)-RORγt f... | US Patent US10080744 (2018) BindingDB Entry DOI: 10.7270/Q21J9CVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50425166 (CHEMBL2313573) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H expressed in Sf9 cells using LTA4 as substrate incubated for 10 mins prior to substrate addition measured after... | Bioorg Med Chem Lett 23: 811-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.074 BindingDB Entry DOI: 10.7270/Q27H1KW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50543664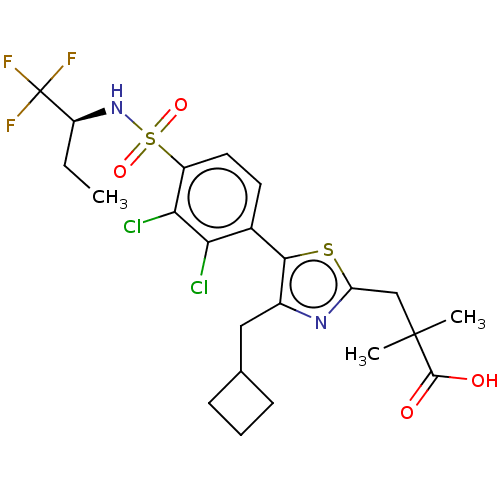 (CHEMBL4647899) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Binding affinity to recombinant human RORgammat transfected in human HEK293T cells incubated for 24 hrs by dual-glo luciferase reporter gene assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127174 BindingDB Entry DOI: 10.7270/Q2862M1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM392076 (US10301272, Example 7/9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Binding affinity to recombinant human RORgammat transfected in human HEK293T cells incubated for 24 hrs by dual-glo luciferase reporter gene assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127174 BindingDB Entry DOI: 10.7270/Q2862M1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM392006 (US10301272, Example 6/4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Binding affinity to recombinant human RORgammat transfected in human HEK293T cells incubated for 24 hrs by dual-glo luciferase reporter gene assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127174 BindingDB Entry DOI: 10.7270/Q2862M1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM285758 (US10080744, Example 3/25 | US10080744, Example 4/1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Cells used in this assay were transiently co-transfected with three different plasmids, one expressing the GAL4-DNA binding domain (DBD)-RORγt f... | US Patent US10080744 (2018) BindingDB Entry DOI: 10.7270/Q21J9CVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM285762 (US10080744, Example 3/29) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Cells used in this assay were transiently co-transfected with three different plasmids, one expressing the GAL4-DNA binding domain (DBD)-RORγt f... | US Patent US10080744 (2018) BindingDB Entry DOI: 10.7270/Q21J9CVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50425168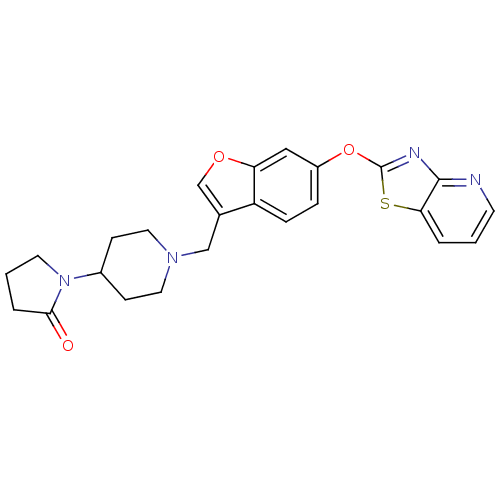 (CHEMBL2313571) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H expressed in Sf9 cells using LTA4 as substrate incubated for 10 mins prior to substrate addition measured after... | Bioorg Med Chem Lett 23: 811-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.074 BindingDB Entry DOI: 10.7270/Q27H1KW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM285753 (US10080744, Example 3/20) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Cells used in this assay were transiently co-transfected with three different plasmids, one expressing the GAL4-DNA binding domain (DBD)-RORγt f... | US Patent US10080744 (2018) BindingDB Entry DOI: 10.7270/Q21J9CVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402391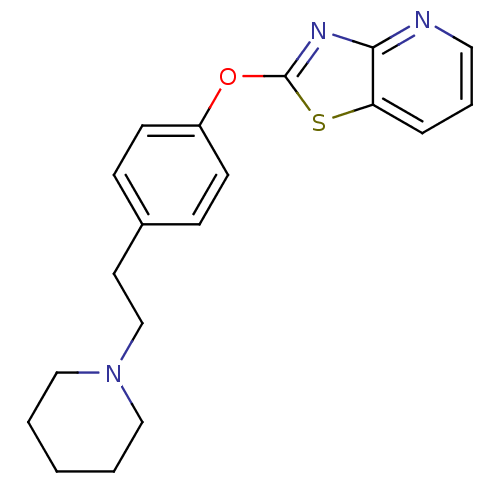 (CHEMBL2207742) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402403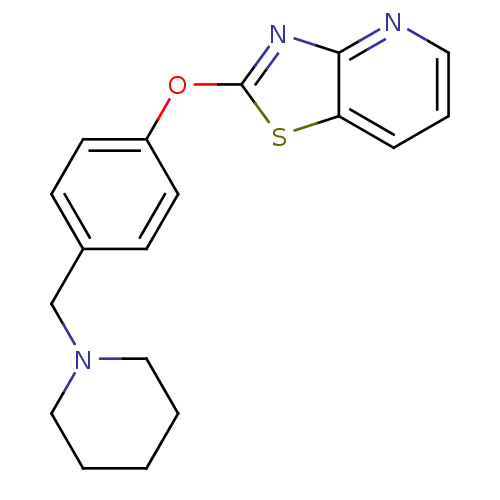 (CHEMBL2207730) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402384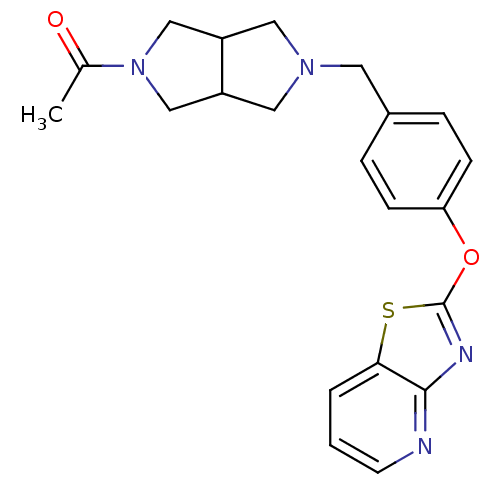 (CHEMBL2207749) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM285771 (US10080744, Example 3/38) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Binding affinity to recombinant human RORgammat transfected in human HEK293T cells incubated for 24 hrs by dual-glo luciferase reporter gene assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127174 BindingDB Entry DOI: 10.7270/Q2862M1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM285799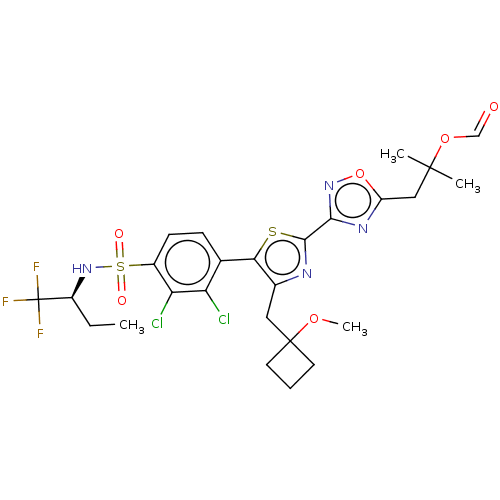 (US10080744, Example 11/10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Cells used in this assay were transiently co-transfected with three different plasmids, one expressing the GAL4-DNA binding domain (DBD)-RORγt f... | US Patent US10080744 (2018) BindingDB Entry DOI: 10.7270/Q21J9CVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM285771 (US10080744, Example 3/38) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Cells used in this assay were transiently co-transfected with three different plasmids, one expressing the GAL4-DNA binding domain (DBD)-RORγt f... | US Patent US10080744 (2018) BindingDB Entry DOI: 10.7270/Q21J9CVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM220233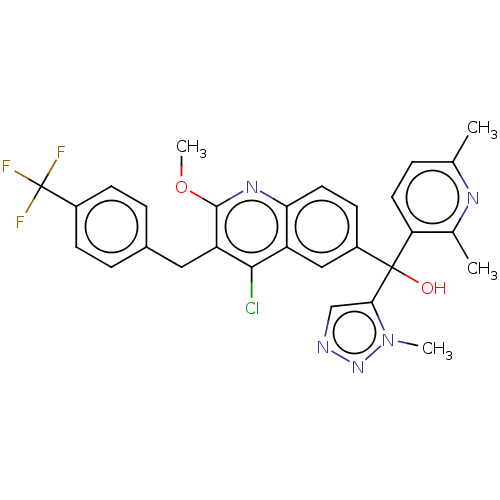 (US9290476, 77A | US9290476, 77B | US9290476, 77C) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inverse agonist activity at RORgammat in human PBMC derived CD4-positive T cells assessed as suppression of T cell differentiation to Th17 cells by m... | Bioorg Med Chem Lett 29: 1463-1470 (2019) Article DOI: 10.1016/j.bmcl.2019.04.021 BindingDB Entry DOI: 10.7270/Q2862KRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM285802 (US10080744, Example 12/1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Cells used in this assay were transiently co-transfected with three different plasmids, one expressing the GAL4-DNA binding domain (DBD)-RORγt f... | US Patent US10080744 (2018) BindingDB Entry DOI: 10.7270/Q21J9CVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM285733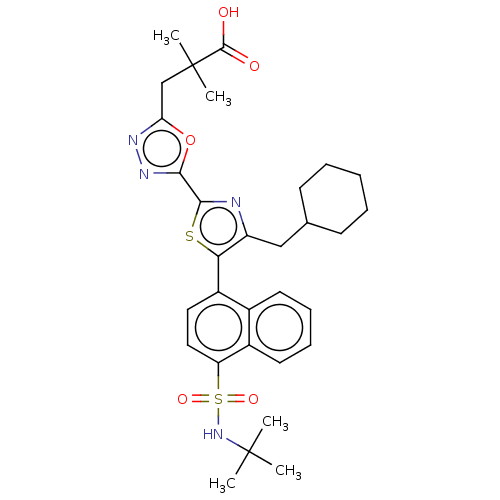 (US10080744, Example 3/1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Cells used in this assay were transiently co-transfected with three different plasmids, one expressing the GAL4-DNA binding domain (DBD)-RORγt f... | US Patent US10080744 (2018) BindingDB Entry DOI: 10.7270/Q21J9CVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50543668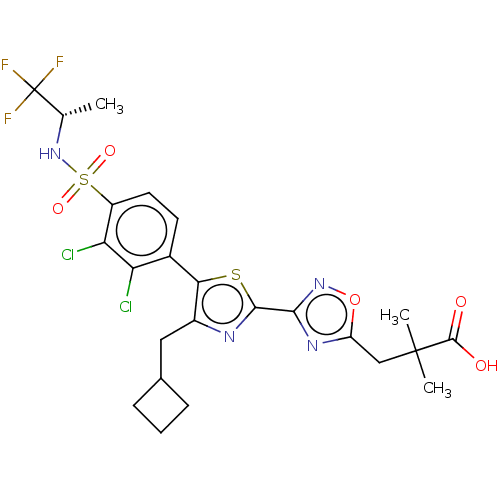 (CHEMBL4632527) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Binding affinity to recombinant human RORgammat transfected in human HEK293T cells incubated for 24 hrs by dual-glo luciferase reporter gene assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127174 BindingDB Entry DOI: 10.7270/Q2862M1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM285759 (US10080744, Example 3/26) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Binding affinity to recombinant human RORgammat transfected in human HEK293T cells incubated for 24 hrs by dual-glo luciferase reporter gene assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127174 BindingDB Entry DOI: 10.7270/Q2862M1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM285789 (US10080744, Example 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Cells used in this assay were transiently co-transfected with three different plasmids, one expressing the GAL4-DNA binding domain (DBD)-RORγt f... | US Patent US10080744 (2018) BindingDB Entry DOI: 10.7270/Q21J9CVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM285763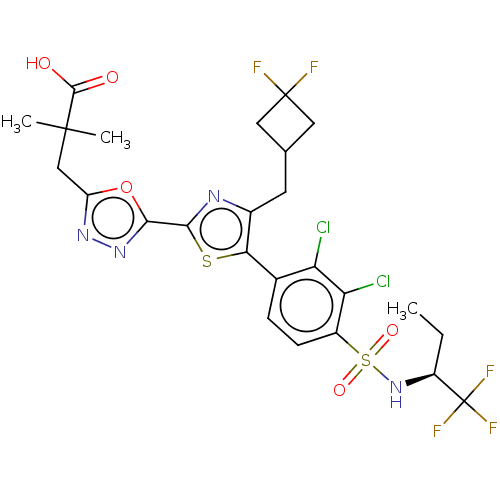 (US10080744, Example 3/30) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Cells used in this assay were transiently co-transfected with three different plasmids, one expressing the GAL4-DNA binding domain (DBD)-RORγt f... | US Patent US10080744 (2018) BindingDB Entry DOI: 10.7270/Q21J9CVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM285759 (US10080744, Example 3/26) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Cells used in this assay were transiently co-transfected with three different plasmids, one expressing the GAL4-DNA binding domain (DBD)-RORγt f... | US Patent US10080744 (2018) BindingDB Entry DOI: 10.7270/Q21J9CVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50425170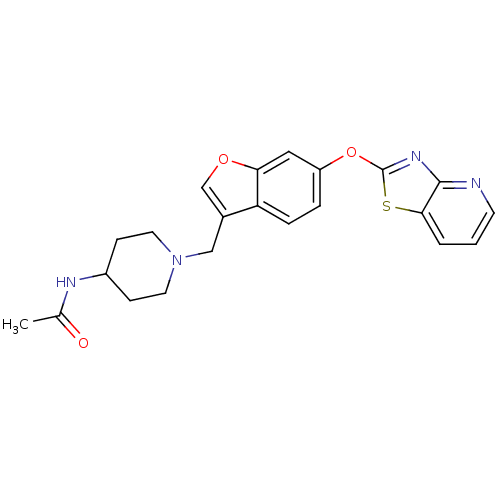 (CHEMBL2313569) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H expressed in Sf9 cells using LTA4 as substrate incubated for 10 mins prior to substrate addition measured after... | Bioorg Med Chem Lett 23: 811-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.074 BindingDB Entry DOI: 10.7270/Q27H1KW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM220233 (US9290476, 77A | US9290476, 77B | US9290476, 77C) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inverse agonist activity at GAL4-DNA binding domain-fused human RORgammat LBD expressed in pGL4.31 transfected HEK293T cells assessed as inhibition o... | Bioorg Med Chem Lett 29: 1463-1470 (2019) Article DOI: 10.1016/j.bmcl.2019.04.021 BindingDB Entry DOI: 10.7270/Q2862KRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24206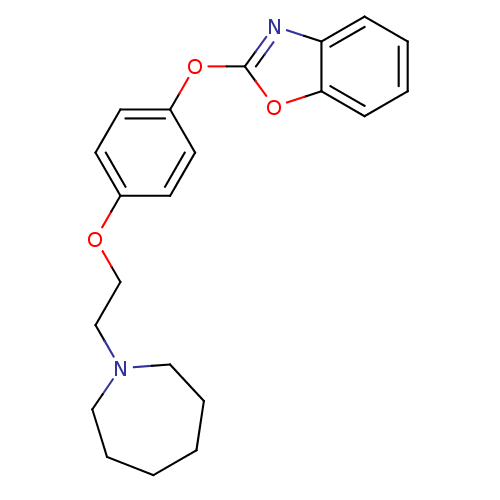 (2-{4-[2-(azepan-1-yl)ethoxy]phenoxy}-1,3-benzoxazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402392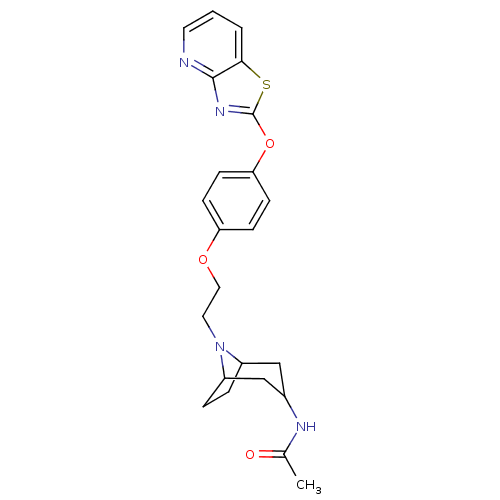 (CHEMBL2207741) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM285811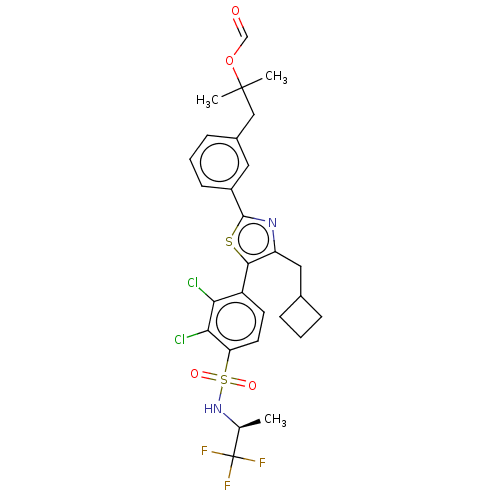 (US10080744, Example 15) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Cells used in this assay were transiently co-transfected with three different plasmids, one expressing the GAL4-DNA binding domain (DBD)-RORγt f... | US Patent US10080744 (2018) BindingDB Entry DOI: 10.7270/Q21J9CVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM285798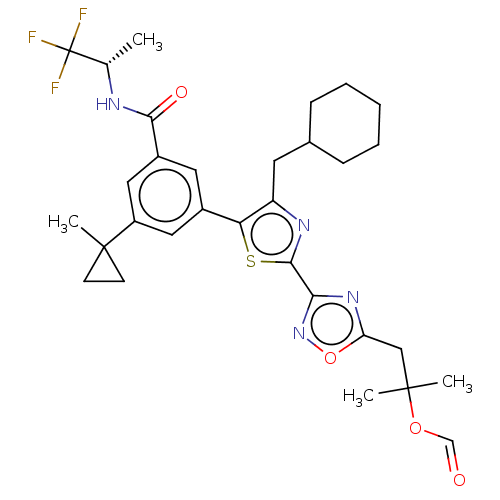 (US10080744, Example 11/9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Cells used in this assay were transiently co-transfected with three different plasmids, one expressing the GAL4-DNA binding domain (DBD)-RORγt f... | US Patent US10080744 (2018) BindingDB Entry DOI: 10.7270/Q21J9CVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM285742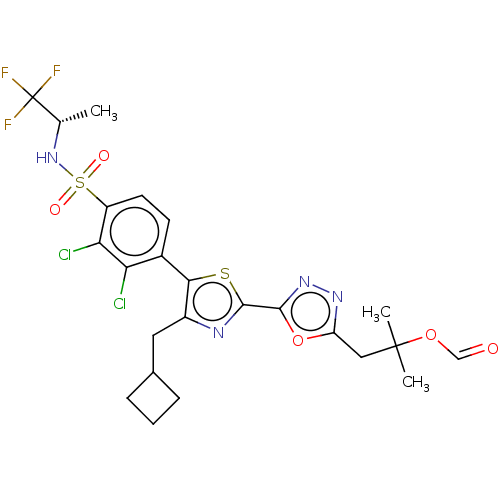 (US10080744, Example 3/9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Cells used in this assay were transiently co-transfected with three different plasmids, one expressing the GAL4-DNA binding domain (DBD)-RORγt f... | US Patent US10080744 (2018) BindingDB Entry DOI: 10.7270/Q21J9CVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50543663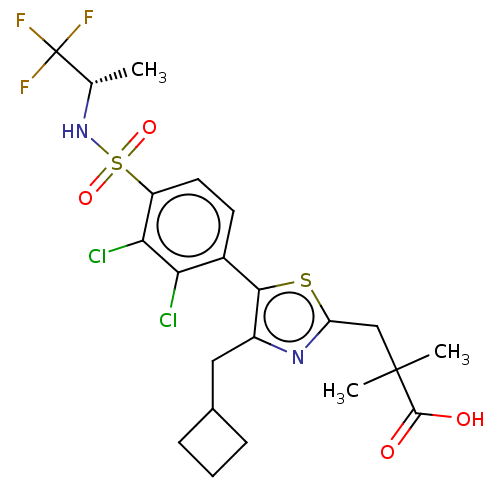 (CHEMBL4647734) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Binding affinity to recombinant human RORgammat transfected in human HEK293T cells incubated for 24 hrs by dual-glo luciferase reporter gene assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127174 BindingDB Entry DOI: 10.7270/Q2862M1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM285800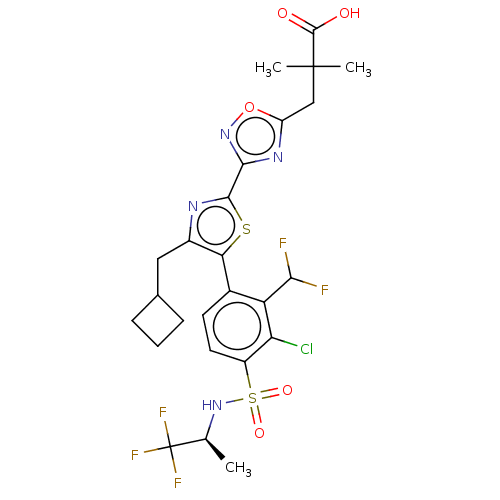 (US10080744, Example 11/11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Cells used in this assay were transiently co-transfected with three different plasmids, one expressing the GAL4-DNA binding domain (DBD)-RORγt f... | US Patent US10080744 (2018) BindingDB Entry DOI: 10.7270/Q21J9CVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50542475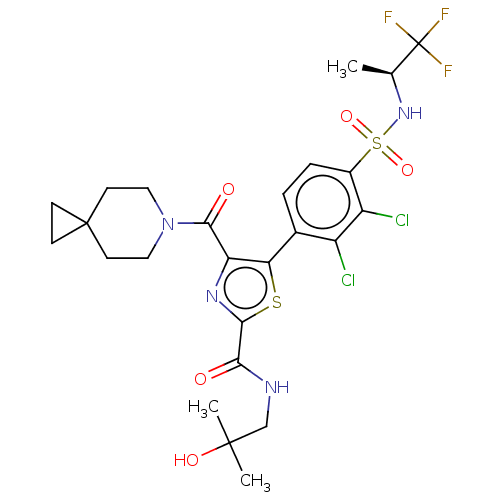 (CHEMBL4639401) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inverse agonist activity at human RORgammat LBD by M1H cell based assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127205 BindingDB Entry DOI: 10.7270/Q2RF5ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM285795 (US10080744, Example 11/6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Cells used in this assay were transiently co-transfected with three different plasmids, one expressing the GAL4-DNA binding domain (DBD)-RORγt f... | US Patent US10080744 (2018) BindingDB Entry DOI: 10.7270/Q21J9CVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM285774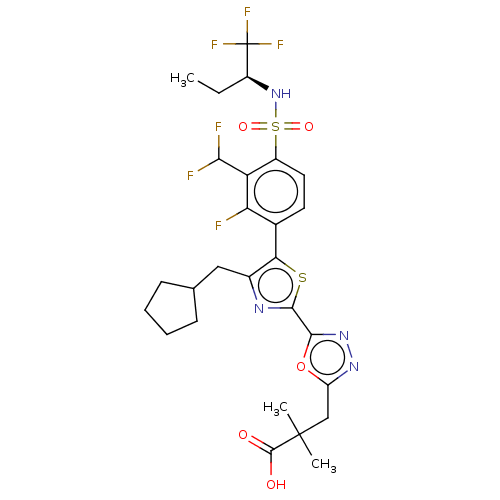 (US10080744, Example 3/41) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Cells used in this assay were transiently co-transfected with three different plasmids, one expressing the GAL4-DNA binding domain (DBD)-RORγt f... | US Patent US10080744 (2018) BindingDB Entry DOI: 10.7270/Q21J9CVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50425151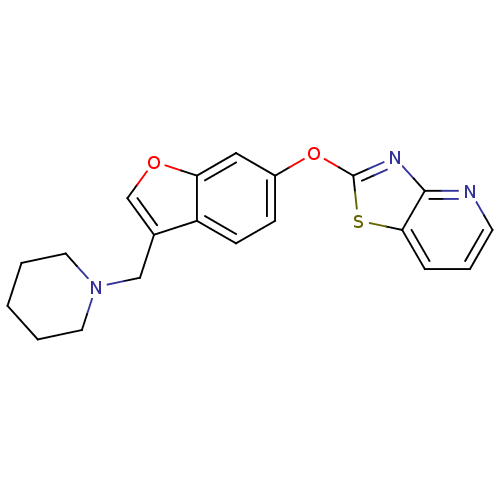 (CHEMBL2313563) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H expressed in Sf9 cells using LTA4 as substrate incubated for 10 mins prior to substrate addition measured after... | Bioorg Med Chem Lett 23: 811-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.074 BindingDB Entry DOI: 10.7270/Q27H1KW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM285769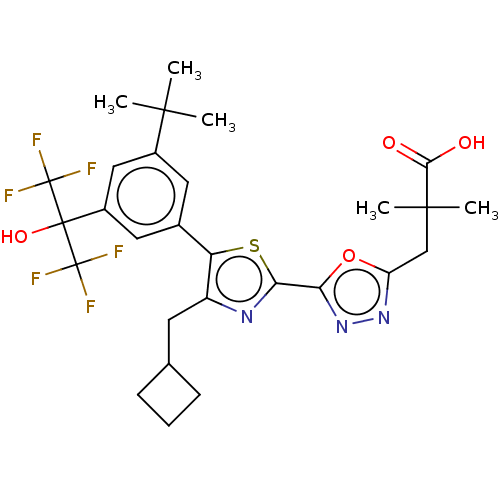 (US10080744, Example 3/36) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Cells used in this assay were transiently co-transfected with three different plasmids, one expressing the GAL4-DNA binding domain (DBD)-RORγt f... | US Patent US10080744 (2018) BindingDB Entry DOI: 10.7270/Q21J9CVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM285803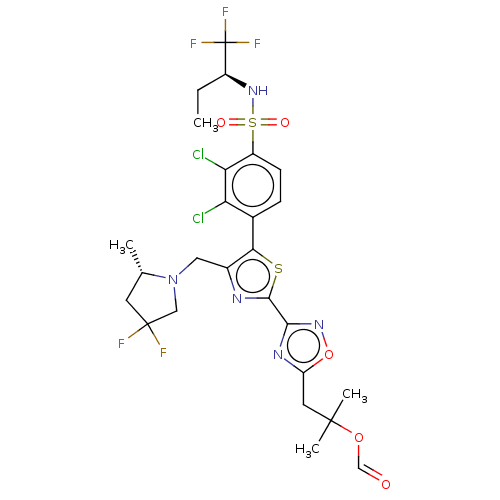 (US10080744, Example 12/2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Cells used in this assay were transiently co-transfected with three different plasmids, one expressing the GAL4-DNA binding domain (DBD)-RORγt f... | US Patent US10080744 (2018) BindingDB Entry DOI: 10.7270/Q21J9CVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM285812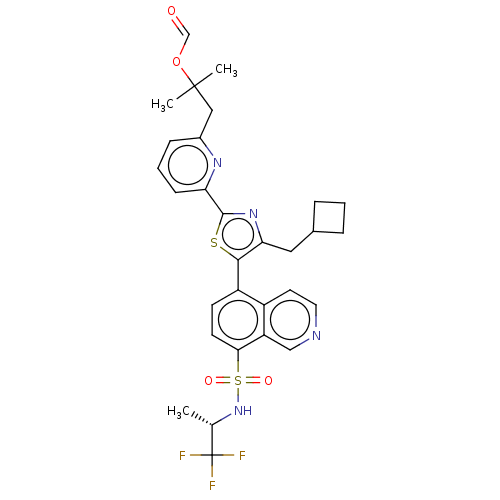 (US10080744, Example 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Cells used in this assay were transiently co-transfected with three different plasmids, one expressing the GAL4-DNA binding domain (DBD)-RORγt f... | US Patent US10080744 (2018) BindingDB Entry DOI: 10.7270/Q21J9CVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM285779 (US10080744, Example 4/2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Cells used in this assay were transiently co-transfected with three different plasmids, one expressing the GAL4-DNA binding domain (DBD)-RORγt f... | US Patent US10080744 (2018) BindingDB Entry DOI: 10.7270/Q21J9CVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402383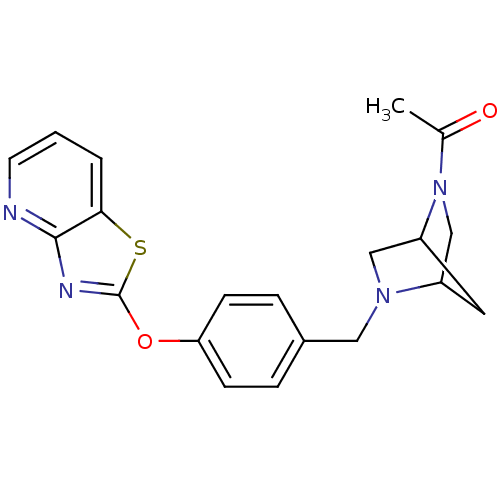 (CHEMBL2207750) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50510354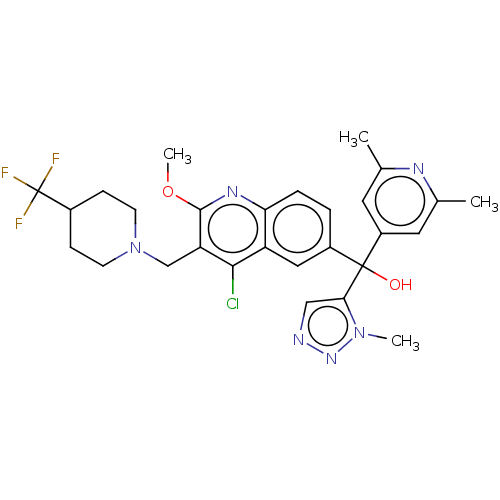 (CHEMBL4441287) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inverse agonist activity at GAL4-DNA binding domain-fused human full length RORgammat LBD expressed in pGL4.31 transfected HEK293T cells assessed as ... | Bioorg Med Chem Lett 29: 1463-1470 (2019) Article DOI: 10.1016/j.bmcl.2019.04.021 BindingDB Entry DOI: 10.7270/Q2862KRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50542493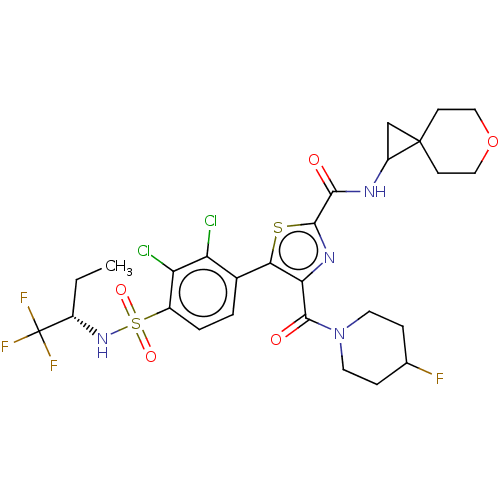 (CHEMBL4635066) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inverse agonist activity at human RORgammat LBD by M1H cell based assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127205 BindingDB Entry DOI: 10.7270/Q2RF5ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM285735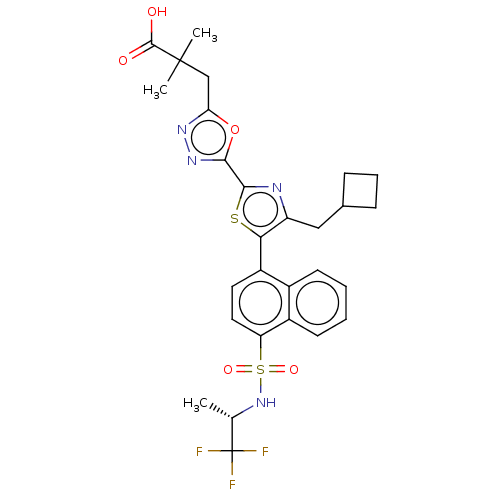 (US10080744, Example 3/3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description Cells used in this assay were transiently co-transfected with three different plasmids, one expressing the GAL4-DNA binding domain (DBD)-RORγt f... | US Patent US10080744 (2018) BindingDB Entry DOI: 10.7270/Q21J9CVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50451743 (CHEMBL4205065) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, LLC Curated by ChEMBL | Assay Description Inverse agonist activity at GAL4 DBD-fused wild type human RORgammat LBD (850 to 1635 residues) expressed in HEK293T cells assessed as inhibition of ... | Bioorg Med Chem Lett 27: 5277-5283 (2017) Article DOI: 10.1016/j.bmcl.2017.10.027 BindingDB Entry DOI: 10.7270/Q2V98BN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402395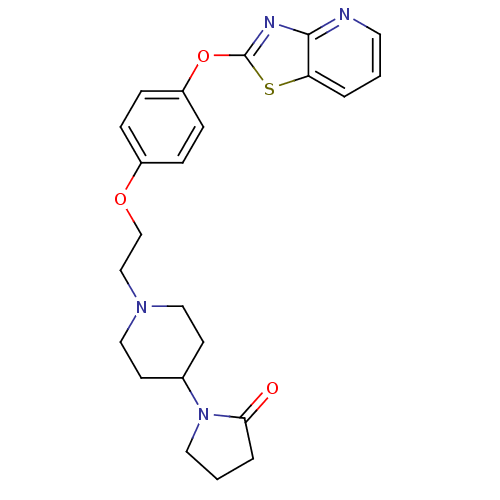 (CHEMBL2207738) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402405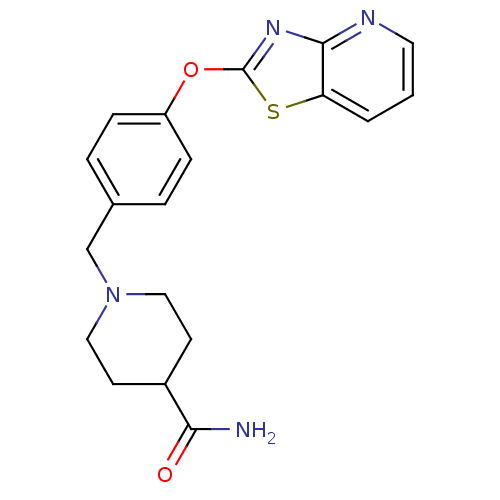 (CHEMBL2207752) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1388 total ) | Next | Last >> |