 Found 971 hits with Last Name = 'johnson' and Initial = 'ar'
Found 971 hits with Last Name = 'johnson' and Initial = 'ar' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Collagenase 3
(Homo sapiens (Human)) | BDBM50305851
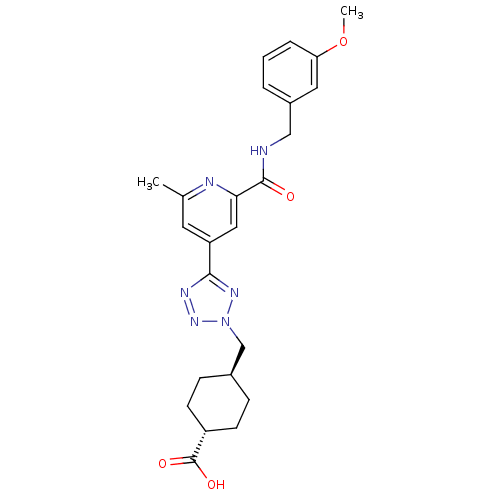
(CHEMBL596273 | trans-4-((5-(2-(3-methoxybenzylcarb...)Show SMILES COc1cccc(CNC(=O)c2cc(cc(C)n2)-c2nnn(C[C@H]3CC[C@@H](CC3)C(O)=O)n2)c1 |r,wU:26.30,wD:23.23,(7.62,-43.42,;8.41,-44.75,;9.95,-44.72,;10.71,-43.37,;12.25,-43.35,;13.03,-44.67,;12.28,-46.01,;13.07,-47.34,;14.61,-47.32,;15.4,-48.64,;14.64,-49.98,;16.94,-48.62,;17.69,-47.28,;19.23,-47.26,;20.02,-48.58,;19.27,-49.92,;20.07,-51.25,;17.72,-49.95,;19.99,-45.92,;19.34,-44.51,;20.47,-43.47,;21.82,-44.22,;23.22,-43.58,;24.56,-44.32,;24.59,-45.86,;25.93,-46.61,;27.25,-45.82,;27.22,-44.27,;25.88,-43.53,;28.6,-46.57,;28.63,-48.1,;29.92,-45.77,;21.52,-45.73,;10.74,-46.04,)| Show InChI InChI=1S/C24H28N6O4/c1-15-10-19(12-21(26-15)23(31)25-13-17-4-3-5-20(11-17)34-2)22-27-29-30(28-22)14-16-6-8-18(9-7-16)24(32)33/h3-5,10-12,16,18H,6-9,13-14H2,1-2H3,(H,25,31)(H,32,33)/t16-,18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs |
Bioorg Med Chem Lett 20: 576-80 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.081
BindingDB Entry DOI: 10.7270/Q2JS9QHB |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50305844
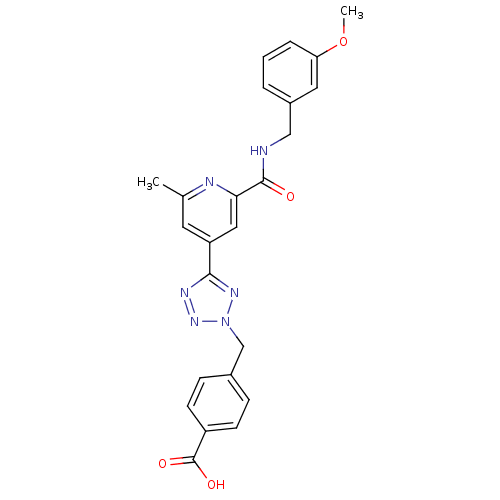
(4-((5-(2-(3-methoxybenzylcarbamoyl)-6-methylpyridi...)Show SMILES COc1cccc(CNC(=O)c2cc(cc(C)n2)-c2nnn(Cc3ccc(cc3)C(O)=O)n2)c1 Show InChI InChI=1S/C24H22N6O4/c1-15-10-19(12-21(26-15)23(31)25-13-17-4-3-5-20(11-17)34-2)22-27-29-30(28-22)14-16-6-8-18(9-7-16)24(32)33/h3-12H,13-14H2,1-2H3,(H,25,31)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs |
Bioorg Med Chem Lett 20: 576-80 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.081
BindingDB Entry DOI: 10.7270/Q2JS9QHB |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50305858
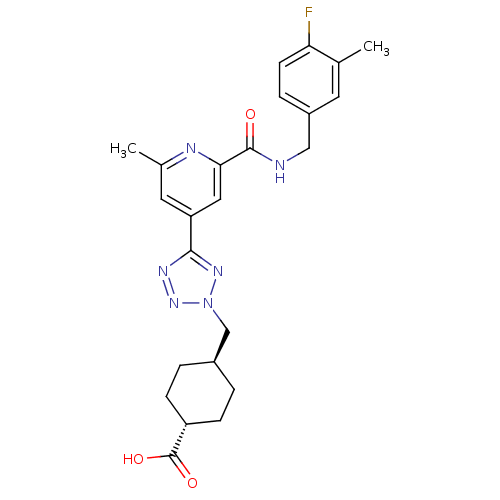
(CHEMBL605928 | trans-4-((5-(2-(4-fluoro-3-methylbe...)Show SMILES Cc1cc(cc(n1)C(=O)NCc1ccc(F)c(C)c1)-c1nnn(C[C@H]2CC[C@@H](CC2)C(O)=O)n1 |r,wU:27.32,wD:24.25,(4.82,-51.39,;4.03,-50.06,;4.78,-48.72,;3.99,-47.4,;2.44,-47.42,;1.69,-48.76,;2.48,-50.09,;.15,-48.78,;-.6,-50.12,;-.64,-47.46,;-2.18,-47.48,;-2.96,-46.15,;-2.21,-44.81,;-3,-43.49,;-4.54,-43.51,;-5.33,-42.19,;-5.29,-44.86,;-6.83,-44.89,;-4.5,-46.18,;4.74,-46.06,;4.1,-44.65,;5.23,-43.61,;6.57,-44.36,;7.97,-43.72,;9.32,-44.46,;9.34,-46,;10.69,-46.75,;12.01,-45.96,;11.98,-44.41,;10.63,-43.67,;13.36,-46.71,;13.39,-48.24,;14.68,-45.91,;6.27,-45.87,)| Show InChI InChI=1S/C24H27FN6O3/c1-14-9-17(5-8-20(14)25)12-26-23(32)21-11-19(10-15(2)27-21)22-28-30-31(29-22)13-16-3-6-18(7-4-16)24(33)34/h5,8-11,16,18H,3-4,6-7,12-13H2,1-2H3,(H,26,32)(H,33,34)/t16-,18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs |
Bioorg Med Chem Lett 20: 576-80 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.081
BindingDB Entry DOI: 10.7270/Q2JS9QHB |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50305856
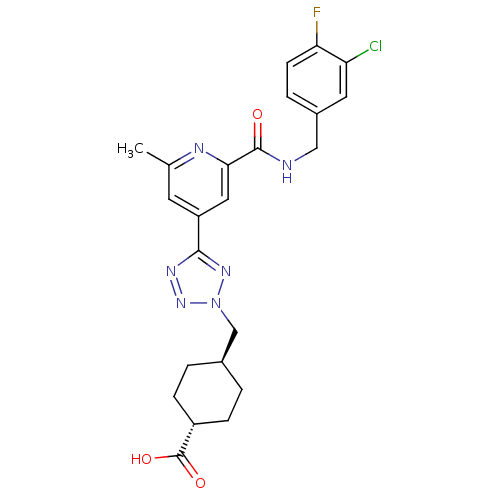
(CHEMBL603206 | trans-4-((5-(2-(3-chloro-4-fluorobe...)Show SMILES Cc1cc(cc(n1)C(=O)NCc1ccc(F)c(Cl)c1)-c1nnn(C[C@H]2CC[C@@H](CC2)C(O)=O)n1 |r,wU:27.32,wD:24.25,(3.2,-37.11,;2.41,-35.78,;3.16,-34.44,;2.37,-33.12,;.83,-33.14,;.07,-34.48,;.86,-35.81,;-1.47,-34.5,;-2.22,-35.84,;-2.25,-33.18,;-3.79,-33.2,;-4.58,-31.87,;-3.83,-30.53,;-4.62,-29.21,;-6.16,-29.23,;-6.95,-27.91,;-6.91,-30.58,;-8.45,-30.61,;-6.12,-31.9,;3.12,-31.78,;2.48,-30.37,;3.61,-29.33,;4.96,-30.08,;6.36,-29.44,;7.7,-30.18,;7.72,-31.72,;9.07,-32.47,;10.39,-31.68,;10.36,-30.13,;9.02,-29.39,;11.74,-32.43,;11.77,-33.96,;13.06,-31.63,;4.66,-31.59,)| Show InChI InChI=1S/C23H24ClFN6O3/c1-13-8-17(10-20(27-13)22(32)26-11-15-4-7-19(25)18(24)9-15)21-28-30-31(29-21)12-14-2-5-16(6-3-14)23(33)34/h4,7-10,14,16H,2-3,5-6,11-12H2,1H3,(H,26,32)(H,33,34)/t14-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs |
Bioorg Med Chem Lett 20: 576-80 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.081
BindingDB Entry DOI: 10.7270/Q2JS9QHB |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50305855
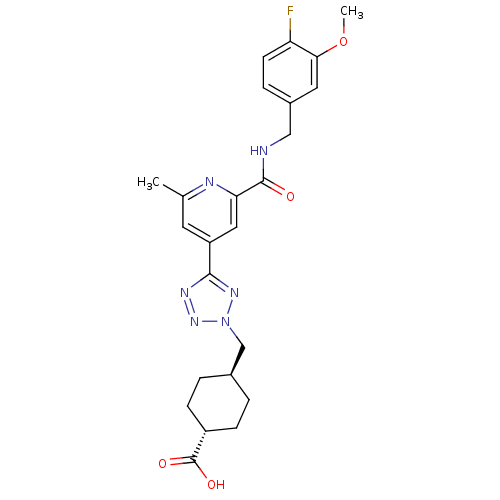
(CHEMBL595156 | trans-4-((5-(2-(4-fluoro-3-methoxyb...)Show SMILES COc1cc(CNC(=O)c2cc(cc(C)n2)-c2nnn(C[C@H]3CC[C@@H](CC3)C(O)=O)n2)ccc1F |r,wU:24.28,wD:21.21,(17.07,-17.7,;17.86,-19.02,;19.4,-18.99,;20.19,-20.31,;21.73,-20.28,;22.52,-21.61,;24.06,-21.59,;24.85,-22.91,;24.09,-24.26,;26.39,-22.89,;27.14,-21.55,;28.68,-21.53,;29.47,-22.85,;28.72,-24.2,;29.52,-25.52,;27.17,-24.22,;29.44,-20.19,;28.79,-18.78,;29.92,-17.74,;31.27,-18.49,;32.67,-17.85,;34.01,-18.6,;34.04,-20.13,;35.38,-20.88,;36.7,-20.09,;36.67,-18.54,;35.33,-17.8,;38.05,-20.84,;38.08,-22.38,;39.37,-20.04,;30.97,-20,;22.48,-18.94,;21.7,-17.62,;20.16,-17.64,;19.37,-16.32,)| Show InChI InChI=1S/C24H27FN6O4/c1-14-9-18(22-28-30-31(29-22)13-15-3-6-17(7-4-15)24(33)34)11-20(27-14)23(32)26-12-16-5-8-19(25)21(10-16)35-2/h5,8-11,15,17H,3-4,6-7,12-13H2,1-2H3,(H,26,32)(H,33,34)/t15-,17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs |
Bioorg Med Chem Lett 20: 576-80 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.081
BindingDB Entry DOI: 10.7270/Q2JS9QHB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244490
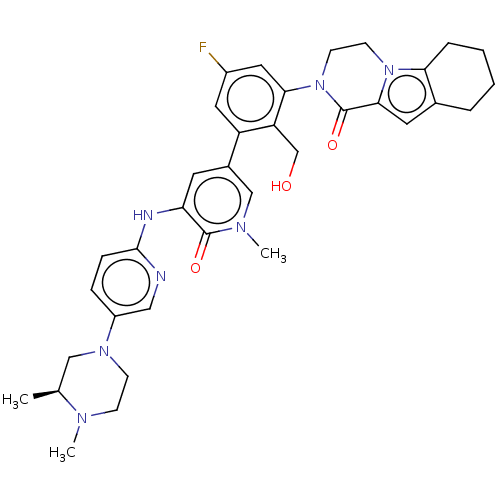
(CHEMBL4102992)Show SMILES C[C@H]1CN(CCN1C)c1ccc(Nc2cc(cn(C)c2=O)-c2cc(F)cc(N3CCn4c5CCCCc5cc4C3=O)c2CO)nc1 |r| Show InChI InChI=1S/C35H40FN7O3/c1-22-19-41(11-10-39(22)2)26-8-9-33(37-18-26)38-29-14-24(20-40(3)34(29)45)27-16-25(36)17-31(28(27)21-44)43-13-12-42-30-7-5-4-6-23(30)15-32(42)35(43)46/h8-9,14-18,20,22,44H,4-7,10-13,19,21H2,1-3H3,(H,37,38)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM111951
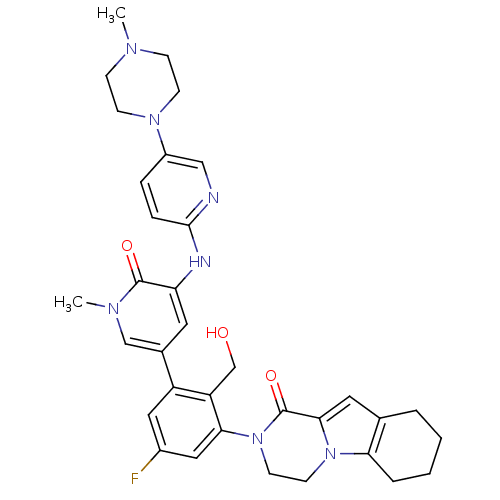
(US8618107, 197)Show SMILES CN1CCN(CC1)c1ccc(Nc2cc(cn(C)c2=O)-c2cc(F)cc(N3CCn4c5CCCCc5cc4C3=O)c2CO)nc1 Show InChI InChI=1S/C34H38FN7O3/c1-38-9-11-40(12-10-38)25-7-8-32(36-19-25)37-28-15-23(20-39(2)33(28)44)26-17-24(35)18-30(27(26)21-43)42-14-13-41-29-6-4-3-5-22(29)16-31(41)34(42)45/h7-8,15-20,43H,3-6,9-14,21H2,1-2H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244489

(CHEMBL4095379)Show SMILES C[C@@H]1CN(C)CCN1c1ccc(Nc2cc(cn(C)c2=O)-c2cc(F)cc(N3CCn4c5CCCCc5cc4C3=O)c2CO)nc1 |r| Show InChI InChI=1S/C35H40FN7O3/c1-22-19-39(2)10-11-41(22)26-8-9-33(37-18-26)38-29-14-24(20-40(3)34(29)45)27-16-25(36)17-31(28(27)21-44)43-13-12-42-30-7-5-4-6-23(30)15-32(42)35(43)46/h8-9,14-18,20,22,44H,4-7,10-13,19,21H2,1-3H3,(H,37,38)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244491
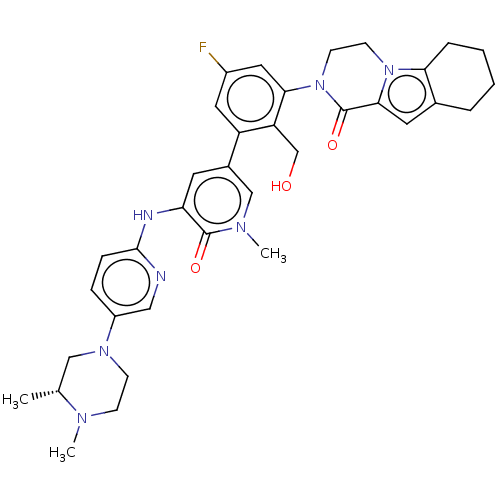
(CHEMBL4092794)Show SMILES C[C@@H]1CN(CCN1C)c1ccc(Nc2cc(cn(C)c2=O)-c2cc(F)cc(N3CCn4c5CCCCc5cc4C3=O)c2CO)nc1 |r| Show InChI InChI=1S/C35H40FN7O3/c1-22-19-41(11-10-39(22)2)26-8-9-33(37-18-26)38-29-14-24(20-40(3)34(29)45)27-16-25(36)17-31(28(27)21-44)43-13-12-42-30-7-5-4-6-23(30)15-32(42)35(43)46/h8-9,14-18,20,22,44H,4-7,10-13,19,21H2,1-3H3,(H,37,38)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244488
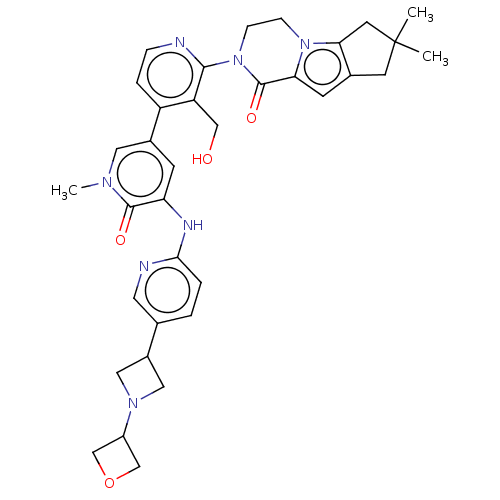
(CHEMBL4069790)Show SMILES Cn1cc(cc(Nc2ccc(cn2)C2CN(C2)C2COC2)c1=O)-c1ccnc(N2CCn3c4CC(C)(C)Cc4cc3C2=O)c1CO Show InChI InChI=1S/C35H39N7O4/c1-35(2)12-22-11-29-34(45)42(9-8-41(29)30(22)13-35)32-27(18-43)26(6-7-36-32)23-10-28(33(44)39(3)15-23)38-31-5-4-21(14-37-31)24-16-40(17-24)25-19-46-20-25/h4-7,10-11,14-15,24-25,43H,8-9,12-13,16-20H2,1-3H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244494
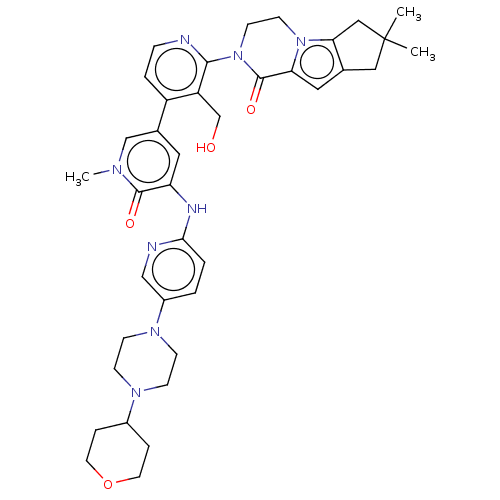
(CHEMBL4090117)Show SMILES Cn1cc(cc(Nc2ccc(cn2)N2CCN(CC2)C2CCOCC2)c1=O)-c1ccnc(N2CCn3c4CC(C)(C)Cc4cc3C2=O)c1CO Show InChI InChI=1S/C38H46N8O4/c1-38(2)20-25-19-32-37(49)46(15-14-45(32)33(25)21-38)35-30(24-47)29(6-9-39-35)26-18-31(36(48)42(3)23-26)41-34-5-4-28(22-40-34)44-12-10-43(11-13-44)27-7-16-50-17-8-27/h4-6,9,18-19,22-23,27,47H,7-8,10-17,20-21,24H2,1-3H3,(H,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244467
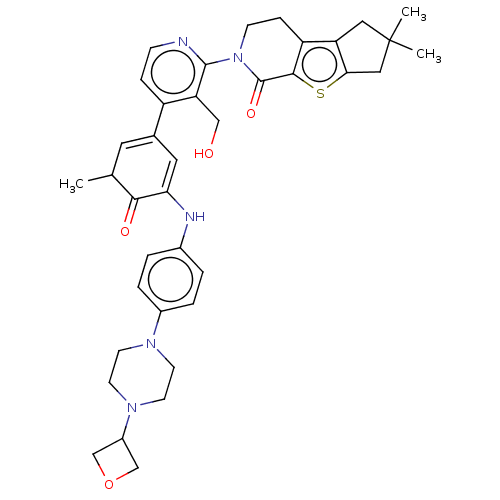
(CHEMBL4063638)Show SMILES CC1C=C(C=C(Nc2ccc(cc2)N2CCN(CC2)C2COC2)C1=O)c1ccnc(N2CCc3c4CC(C)(C)Cc4sc3C2=O)c1CO |c:2,t:4| Show InChI InChI=1S/C38H43N5O4S/c1-23-16-24(17-32(34(23)45)40-25-4-6-26(7-5-25)41-12-14-42(15-13-41)27-21-47-22-27)28-8-10-39-36(31(28)20-44)43-11-9-29-30-18-38(2,3)19-33(30)48-35(29)37(43)46/h4-8,10,16-17,23,27,40,44H,9,11-15,18-22H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50305857
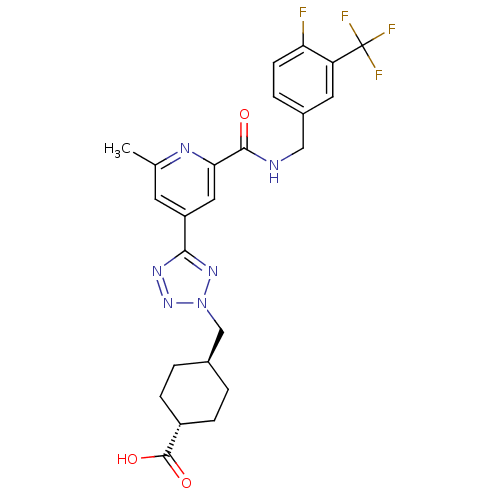
(CHEMBL594932 | trans-4-((5-(2-(4-fluoro-3-(trifluo...)Show SMILES Cc1cc(cc(n1)C(=O)NCc1ccc(F)c(c1)C(F)(F)F)-c1nnn(C[C@H]2CC[C@@H](CC2)C(O)=O)n1 |r,wU:30.35,wD:27.28,(30.42,-38.58,;29.63,-37.25,;30.37,-35.91,;29.59,-34.59,;28.04,-34.61,;27.29,-35.95,;28.08,-37.28,;25.75,-35.97,;24.99,-37.31,;24.96,-34.65,;23.42,-34.67,;22.63,-33.34,;23.38,-32,;22.6,-30.68,;21.06,-30.7,;20.27,-29.38,;20.3,-32.05,;21.09,-33.37,;18.76,-32.08,;17.97,-30.75,;18.02,-33.42,;17.22,-32.07,;30.34,-33.25,;29.7,-31.84,;30.83,-30.8,;32.17,-31.55,;33.57,-30.91,;34.92,-31.65,;34.94,-33.19,;36.28,-33.94,;37.6,-33.15,;37.57,-31.6,;36.23,-30.86,;38.96,-33.9,;38.98,-35.43,;40.28,-33.1,;31.87,-33.06,)| Show InChI InChI=1S/C24H24F4N6O3/c1-13-8-17(21-31-33-34(32-21)12-14-2-5-16(6-3-14)23(36)37)10-20(30-13)22(35)29-11-15-4-7-19(25)18(9-15)24(26,27)28/h4,7-10,14,16H,2-3,5-6,11-12H2,1H3,(H,29,35)(H,36,37)/t14-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs |
Bioorg Med Chem Lett 20: 576-80 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.081
BindingDB Entry DOI: 10.7270/Q2JS9QHB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244502

(CHEMBL4085043)Show SMILES C[C@H]1CN(C)CCN1c1ccc(Nc2cc(cn(C)c2=O)-c2cc(F)cc(N3CCn4c5CCCCc5cc4C3=O)c2CO)nc1 |r| Show InChI InChI=1S/C35H40FN7O3/c1-22-19-39(2)10-11-41(22)26-8-9-33(37-18-26)38-29-14-24(20-40(3)34(29)45)27-16-25(36)17-31(28(27)21-44)43-13-12-42-30-7-5-4-6-23(30)15-32(42)35(43)46/h8-9,14-18,20,22,44H,4-7,10-13,19,21H2,1-3H3,(H,37,38)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM111952
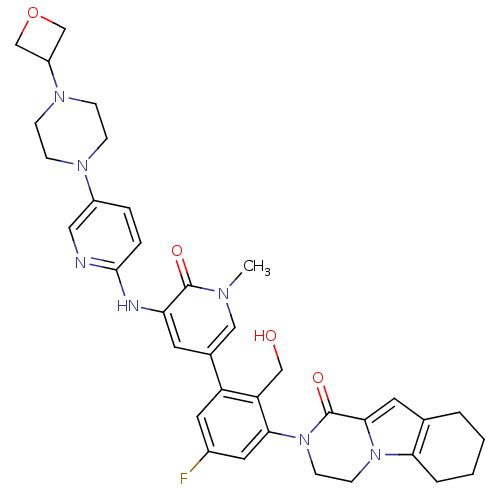
(US8618107, 210)Show SMILES Cn1cc(cc(Nc2ccc(cn2)N2CCN(CC2)C2COC2)c1=O)-c1cc(F)cc(N2CCn3c4CCCCc4cc3C2=O)c1CO Show InChI InChI=1S/C36H40FN7O4/c1-40-19-24(14-30(35(40)46)39-34-7-6-26(18-38-34)41-8-10-42(11-9-41)27-21-48-22-27)28-16-25(37)17-32(29(28)20-45)44-13-12-43-31-5-3-2-4-23(31)15-33(43)36(44)47/h6-7,14-19,27,45H,2-5,8-13,20-22H2,1H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244493
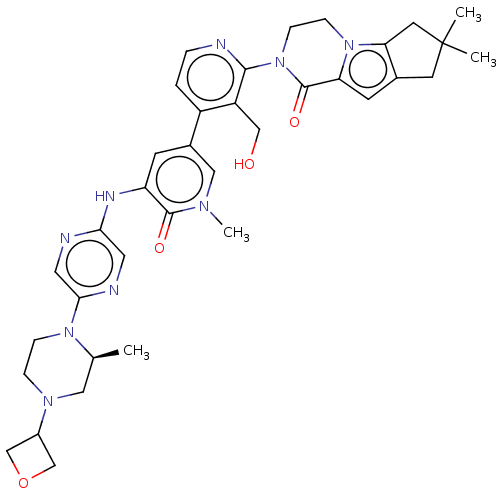
(CHEMBL4070991)Show SMILES C[C@H]1CN(CCN1c1cnc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)cn1)C1COC1 |r| Show InChI InChI=1S/C36H43N9O4/c1-22-17-42(25-20-49-21-25)7-8-43(22)32-16-38-31(15-39-32)40-28-11-24(18-41(4)34(28)47)26-5-6-37-33(27(26)19-46)45-10-9-44-29(35(45)48)12-23-13-36(2,3)14-30(23)44/h5-6,11-12,15-16,18,22,25,46H,7-10,13-14,17,19-21H2,1-4H3,(H,38,40)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244440
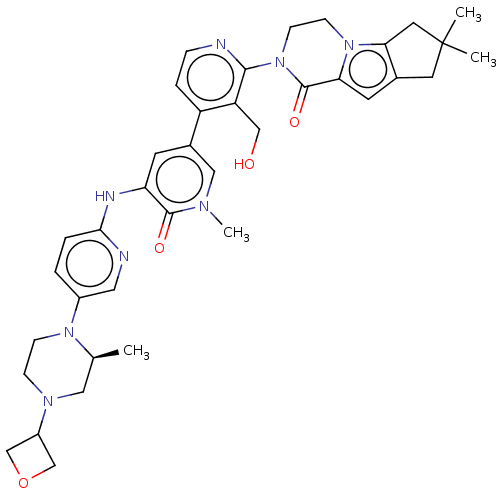
(CHEMBL4065122)Show SMILES C[C@H]1CN(CCN1c1ccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)nc1)C1COC1 |r| Show InChI InChI=1S/C37H44N8O4/c1-23-18-42(27-21-49-22-27)9-10-43(23)26-5-6-33(39-17-26)40-30-13-25(19-41(4)35(30)47)28-7-8-38-34(29(28)20-46)45-12-11-44-31(36(45)48)14-24-15-37(2,3)16-32(24)44/h5-8,13-14,17,19,23,27,46H,9-12,15-16,18,20-22H2,1-4H3,(H,39,40)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244492
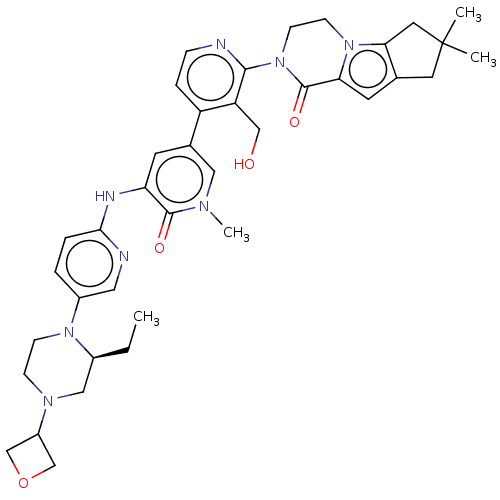
(CHEMBL4087543)Show SMILES CC[C@H]1CN(CCN1c1ccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)nc1)C1COC1 |r| Show InChI InChI=1S/C38H46N8O4/c1-5-26-20-43(28-22-50-23-28)10-11-44(26)27-6-7-34(40-18-27)41-31-14-25(19-42(4)36(31)48)29-8-9-39-35(30(29)21-47)46-13-12-45-32(37(46)49)15-24-16-38(2,3)17-33(24)45/h6-9,14-15,18-19,26,28,47H,5,10-13,16-17,20-23H2,1-4H3,(H,40,41)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244500
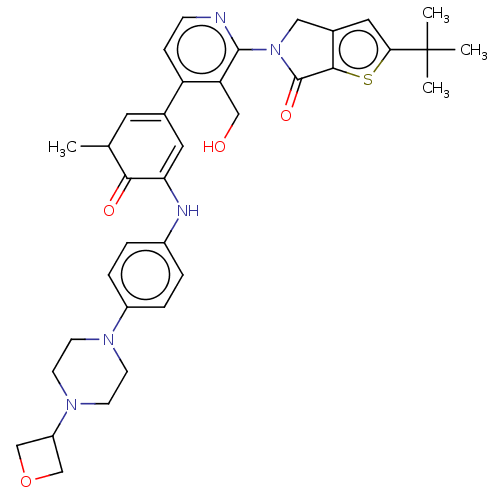
(CHEMBL4093188)Show SMILES CC1C=C(C=C(Nc2ccc(cc2)N2CCN(CC2)C2COC2)C1=O)c1ccnc(N2Cc3cc(sc3C2=O)C(C)(C)C)c1CO |c:2,t:4| Show InChI InChI=1S/C36H41N5O4S/c1-22-15-23(28-9-10-37-34(29(28)19-42)41-18-24-17-31(36(2,3)4)46-33(24)35(41)44)16-30(32(22)43)38-25-5-7-26(8-6-25)39-11-13-40(14-12-39)27-20-45-21-27/h5-10,15-17,22,27,38,42H,11-14,18-21H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244486

(CHEMBL4097832)Show SMILES COCCCN1CCN(CC1)c1ccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)nc1 Show InChI InChI=1S/C37H46N8O4/c1-37(2)20-25-19-31-36(48)45(16-15-44(31)32(25)21-37)34-29(24-46)28(8-9-38-34)26-18-30(35(47)41(3)23-26)40-33-7-6-27(22-39-33)43-13-11-42(12-14-43)10-5-17-49-4/h6-9,18-19,22-23,46H,5,10-17,20-21,24H2,1-4H3,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244501
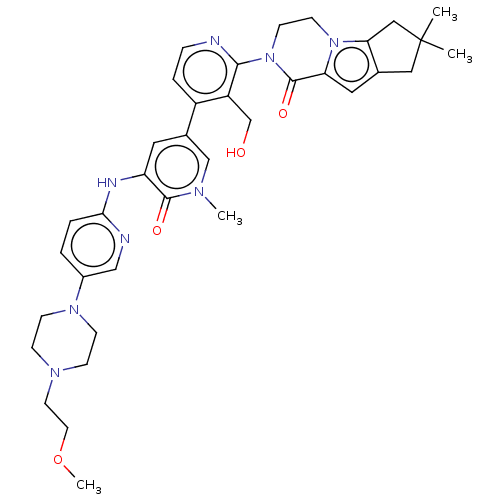
(CHEMBL4062634)Show SMILES COCCN1CCN(CC1)c1ccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)nc1 Show InChI InChI=1S/C36H44N8O4/c1-36(2)19-24-18-30-35(47)44(14-13-43(30)31(24)20-36)33-28(23-45)27(7-8-37-33)25-17-29(34(46)40(3)22-25)39-32-6-5-26(21-38-32)42-11-9-41(10-12-42)15-16-48-4/h5-8,17-18,21-22,45H,9-16,19-20,23H2,1-4H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244497

(CHEMBL4074792)Show SMILES C[C@@H]1CN([C@@H](C)CN1C1COC1)c1ccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)nc1 |r| Show InChI InChI=1S/C38H46N8O4/c1-23-18-46(28-21-50-22-28)24(2)17-45(23)27-6-7-34(40-16-27)41-31-12-26(19-42(5)36(31)48)29-8-9-39-35(30(29)20-47)44-11-10-43-32(37(44)49)13-25-14-38(3,4)15-33(25)43/h6-9,12-13,16,19,23-24,28,47H,10-11,14-15,17-18,20-22H2,1-5H3,(H,40,41)/t23-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM111939

(US8618107, 105)Show SMILES Cn1cc(cc(Nc2ccncn2)c1=O)-c1cccc(N2CCc3c4CC(C)(C)Cc4sc3C2=O)c1CO Show InChI InChI=1S/C29H29N5O3S/c1-29(2)12-20-19-8-10-34(28(37)26(19)38-24(20)13-29)23-6-4-5-18(21(23)15-35)17-11-22(27(36)33(3)14-17)32-25-7-9-30-16-31-25/h4-7,9,11,14,16,35H,8,10,12-13,15H2,1-3H3,(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length His-tagged BTK expressed in baculovirus expression system by Z-LYTE assay |
ACS Med Chem Lett 8: 608-613 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00103
BindingDB Entry DOI: 10.7270/Q24M96ZH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Collagenase 3
(Homo sapiens (Human)) | BDBM50305854
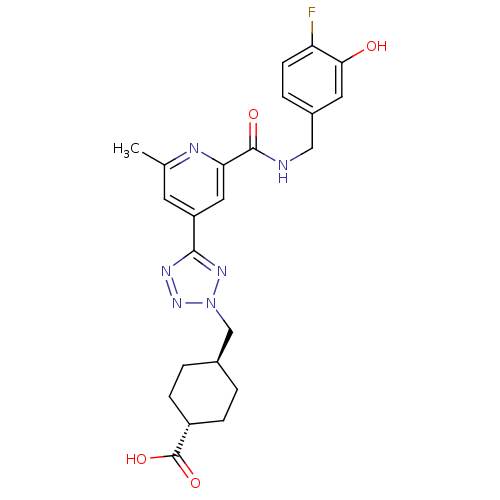
(CHEMBL604139 | trans-4-((5-(2-(4-fluoro-3-hydroxyb...)Show SMILES Cc1cc(cc(n1)C(=O)NCc1ccc(F)c(O)c1)-c1nnn(C[C@H]2CC[C@@H](CC2)C(O)=O)n1 |r,wU:27.32,wD:24.25,(2.92,-23.18,;2.12,-21.86,;2.87,-20.52,;2.08,-19.2,;.54,-19.22,;-.21,-20.56,;.57,-21.89,;-1.75,-20.58,;-2.51,-21.92,;-2.54,-19.26,;-4.08,-19.27,;-4.87,-17.95,;-4.12,-16.61,;-4.9,-15.29,;-6.44,-15.31,;-7.23,-13.98,;-7.2,-16.66,;-8.74,-16.68,;-6.41,-17.98,;2.84,-17.86,;2.19,-16.45,;3.32,-15.41,;4.67,-16.16,;6.07,-15.51,;7.41,-16.26,;7.44,-17.8,;8.78,-18.54,;10.1,-17.75,;10.07,-16.21,;8.73,-15.46,;11.45,-18.5,;11.48,-20.04,;12.77,-17.71,;4.37,-17.67,)| Show InChI InChI=1S/C23H25FN6O4/c1-13-8-17(10-19(26-13)22(32)25-11-15-4-7-18(24)20(31)9-15)21-27-29-30(28-21)12-14-2-5-16(6-3-14)23(33)34/h4,7-10,14,16,31H,2-3,5-6,11-12H2,1H3,(H,25,32)(H,33,34)/t14-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs |
Bioorg Med Chem Lett 20: 576-80 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.081
BindingDB Entry DOI: 10.7270/Q2JS9QHB |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50305850

(CHEMBL593258 | trans-4-((5-(2-(3-hydroxybenzylcarb...)Show SMILES Cc1cc(cc(n1)C(=O)NCc1cccc(O)c1)-c1nnn(C[C@H]2CC[C@@H](CC2)C(O)=O)n1 |r,wU:26.31,wD:23.24,(29.74,-40.23,;28.95,-38.91,;29.7,-37.57,;28.91,-36.25,;27.36,-36.27,;26.61,-37.61,;27.4,-38.94,;25.07,-37.63,;24.32,-38.97,;24.28,-36.3,;22.74,-36.32,;21.96,-35,;22.71,-33.66,;21.92,-32.34,;20.38,-32.35,;19.63,-33.71,;18.09,-33.73,;20.42,-35.03,;29.66,-34.9,;29.02,-33.5,;30.15,-32.45,;31.49,-33.21,;32.89,-32.56,;34.24,-33.31,;34.26,-34.85,;35.61,-35.59,;36.93,-34.8,;36.9,-33.25,;35.55,-32.51,;38.28,-35.55,;38.31,-37.09,;39.6,-34.76,;31.19,-34.72,)| Show InChI InChI=1S/C23H26N6O4/c1-14-9-18(11-20(25-14)22(31)24-12-16-3-2-4-19(30)10-16)21-26-28-29(27-21)13-15-5-7-17(8-6-15)23(32)33/h2-4,9-11,15,17,30H,5-8,12-13H2,1H3,(H,24,31)(H,32,33)/t15-,17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs |
Bioorg Med Chem Lett 20: 576-80 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.081
BindingDB Entry DOI: 10.7270/Q2JS9QHB |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50305852
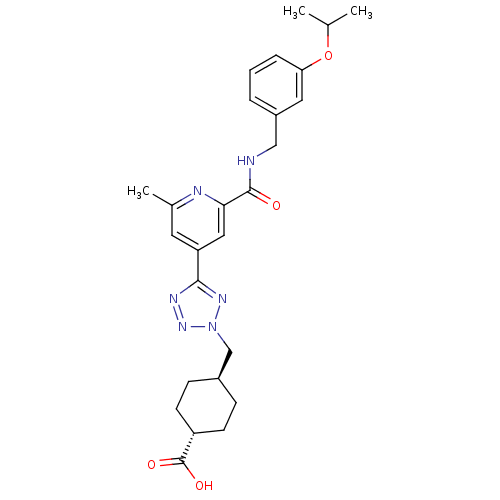
(CHEMBL603857 | trans-4-((5-(2-(3-isopropoxybenzylc...)Show SMILES CC(C)Oc1cccc(CNC(=O)c2cc(cc(C)n2)-c2nnn(C[C@H]3CC[C@@H](CC3)C(O)=O)n2)c1 |r,wU:28.32,wD:25.25,(-8.9,-3.29,;-7.36,-3.27,;-6.57,-4.59,;-6.61,-1.92,;-5.07,-1.9,;-4.32,-.54,;-2.78,-.53,;-1.99,-1.85,;-2.74,-3.19,;-1.96,-4.51,;-.42,-4.49,;.37,-5.82,;-.38,-7.16,;1.91,-5.8,;2.66,-4.45,;4.21,-4.44,;4.99,-5.76,;4.25,-7.1,;5.04,-8.42,;2.7,-7.13,;4.96,-3.09,;4.32,-1.69,;5.45,-.64,;6.79,-1.4,;8.19,-.75,;9.54,-1.5,;9.56,-3.03,;10.9,-3.78,;12.23,-2.99,;12.2,-1.44,;10.85,-.7,;13.58,-3.74,;13.61,-5.28,;14.9,-2.95,;6.49,-2.91,;-4.28,-3.21,)| Show InChI InChI=1S/C26H32N6O4/c1-16(2)36-22-6-4-5-19(12-22)14-27-25(33)23-13-21(11-17(3)28-23)24-29-31-32(30-24)15-18-7-9-20(10-8-18)26(34)35/h4-6,11-13,16,18,20H,7-10,14-15H2,1-3H3,(H,27,33)(H,34,35)/t18-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs |
Bioorg Med Chem Lett 20: 576-80 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.081
BindingDB Entry DOI: 10.7270/Q2JS9QHB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244483
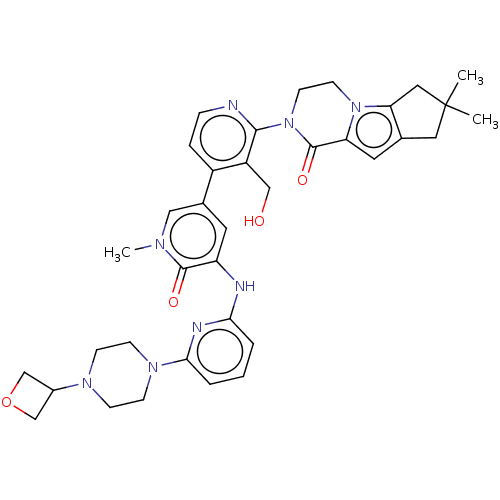
(CHEMBL4082268)Show SMILES Cn1cc(cc(Nc2cccc(n2)N2CCN(CC2)C2COC2)c1=O)-c1ccnc(N2CCn3c4CC(C)(C)Cc4cc3C2=O)c1CO Show InChI InChI=1S/C36H42N8O4/c1-36(2)17-23-16-29-35(47)44(14-13-43(29)30(23)18-36)33-27(20-45)26(7-8-37-33)24-15-28(34(46)40(3)19-24)38-31-5-4-6-32(39-31)42-11-9-41(10-12-42)25-21-48-22-25/h4-8,15-16,19,25,45H,9-14,17-18,20-22H2,1-3H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244484
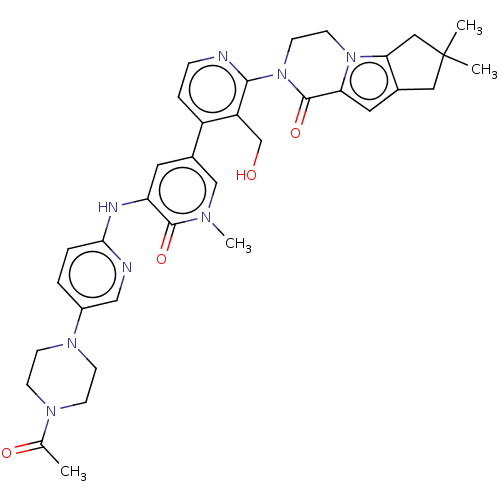
(CHEMBL4060356)Show SMILES CC(=O)N1CCN(CC1)c1ccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)nc1 Show InChI InChI=1S/C35H40N8O4/c1-22(45)40-9-11-41(12-10-40)25-5-6-31(37-19-25)38-28-15-24(20-39(4)33(28)46)26-7-8-36-32(27(26)21-44)43-14-13-42-29(34(43)47)16-23-17-35(2,3)18-30(23)42/h5-8,15-16,19-20,44H,9-14,17-18,21H2,1-4H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244495
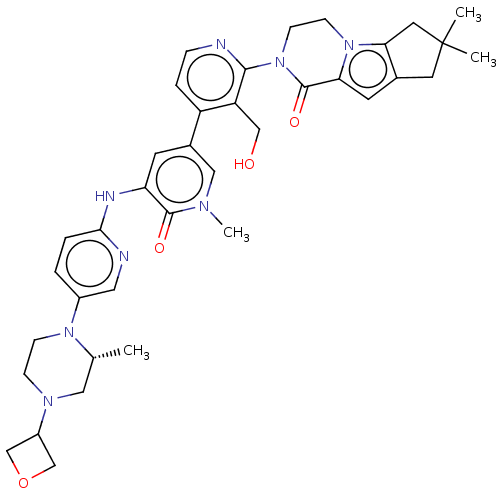
(CHEMBL4066176)Show SMILES C[C@@H]1CN(CCN1c1ccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)nc1)C1COC1 |r| Show InChI InChI=1S/C37H44N8O4/c1-23-18-42(27-21-49-22-27)9-10-43(23)26-5-6-33(39-17-26)40-30-13-25(19-41(4)35(30)47)28-7-8-38-34(29(28)20-46)45-12-11-44-31(36(45)48)14-24-15-37(2,3)16-32(24)44/h5-8,13-14,17,19,23,27,46H,9-12,15-16,18,20-22H2,1-4H3,(H,39,40)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244499
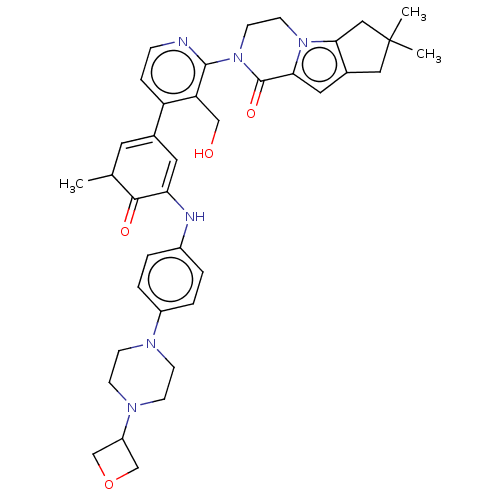
(CHEMBL4086408)Show SMILES CC1C=C(C=C(Nc2ccc(cc2)N2CCN(CC2)C2COC2)C1=O)c1ccnc(N2CCn3c4CC(C)(C)Cc4cc3C2=O)c1CO |c:2,t:4| Show InChI InChI=1S/C38H44N6O4/c1-24-16-25(17-32(35(24)46)40-27-4-6-28(7-5-27)41-10-12-42(13-11-41)29-22-48-23-29)30-8-9-39-36(31(30)21-45)44-15-14-43-33(37(44)47)18-26-19-38(2,3)20-34(26)43/h4-9,16-18,24,29,40,45H,10-15,19-23H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244464
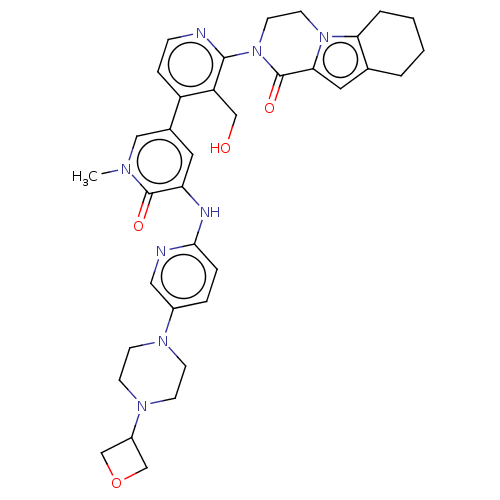
(CHEMBL4085477)Show SMILES Cn1cc(cc(Nc2ccc(cn2)N2CCN(CC2)C2COC2)c1=O)-c1ccnc(N2CCn3c4CCCCc4cc3C2=O)c1CO Show InChI InChI=1S/C35H40N8O4/c1-39-19-24(16-29(34(39)45)38-32-7-6-25(18-37-32)40-10-12-41(13-11-40)26-21-47-22-26)27-8-9-36-33(28(27)20-44)43-15-14-42-30-5-3-2-4-23(30)17-31(42)35(43)46/h6-9,16-19,26,44H,2-5,10-15,20-22H2,1H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50305833

(4-((5-(2-(3-methoxybenzylcarbamoyl)pyridin-4-yl)-2...)Show SMILES COc1cccc(CNC(=O)c2cc(ccn2)-c2nnn(Cc3ccc(cc3)C(O)=O)n2)c1 Show InChI InChI=1S/C23H20N6O4/c1-33-19-4-2-3-16(11-19)13-25-22(30)20-12-18(9-10-24-20)21-26-28-29(27-21)14-15-5-7-17(8-6-15)23(31)32/h2-12H,13-14H2,1H3,(H,25,30)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs |
Bioorg Med Chem Lett 20: 576-80 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.081
BindingDB Entry DOI: 10.7270/Q2JS9QHB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244469
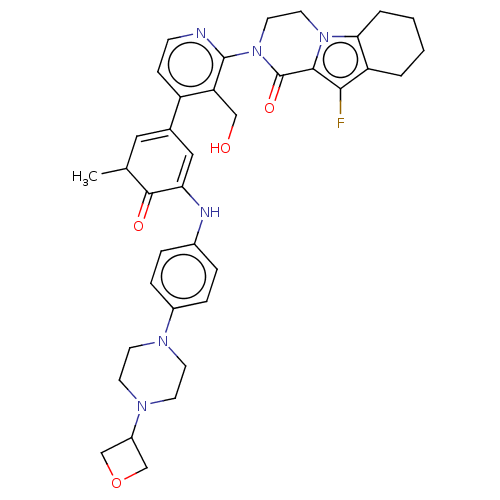
(CHEMBL4090946)Show SMILES CC1C=C(C=C(Nc2ccc(cc2)N2CCN(CC2)C2COC2)C1=O)c1ccnc(N2CCn3c4CCCCc4c(F)c3C2=O)c1CO |c:2,t:4| Show InChI InChI=1S/C37H41FN6O4/c1-23-18-24(19-31(35(23)46)40-25-6-8-26(9-7-25)41-12-14-42(15-13-41)27-21-48-22-27)28-10-11-39-36(30(28)20-45)44-17-16-43-32-5-3-2-4-29(32)33(38)34(43)37(44)47/h6-11,18-19,23,27,40,45H,2-5,12-17,20-22H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50305845
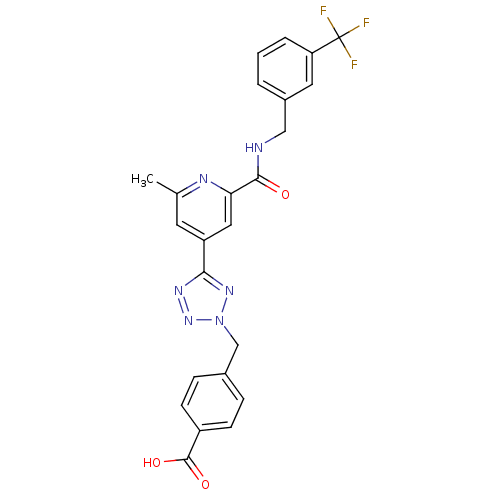
(4-((5-(2-methyl-6-(3-(trifluoromethyl)benzylcarbam...)Show SMILES Cc1cc(cc(n1)C(=O)NCc1cccc(c1)C(F)(F)F)-c1nnn(Cc2ccc(cc2)C(O)=O)n1 Show InChI InChI=1S/C24H19F3N6O3/c1-14-9-18(21-30-32-33(31-21)13-15-5-7-17(8-6-15)23(35)36)11-20(29-14)22(34)28-12-16-3-2-4-19(10-16)24(25,26)27/h2-11H,12-13H2,1H3,(H,28,34)(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs |
Bioorg Med Chem Lett 20: 576-80 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.081
BindingDB Entry DOI: 10.7270/Q2JS9QHB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244485

(CHEMBL4071754)Show SMILES CC(=O)N1CC2(C1)CN(C2)c1ccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)nc1 Show InChI InChI=1S/C36H40N8O4/c1-22(46)41-18-36(19-41)20-42(21-36)25-5-6-31(38-15-25)39-28-11-24(16-40(4)33(28)47)26-7-8-37-32(27(26)17-45)44-10-9-43-29(34(44)48)12-23-13-35(2,3)14-30(23)43/h5-8,11-12,15-16,45H,9-10,13-14,17-21H2,1-4H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50305840
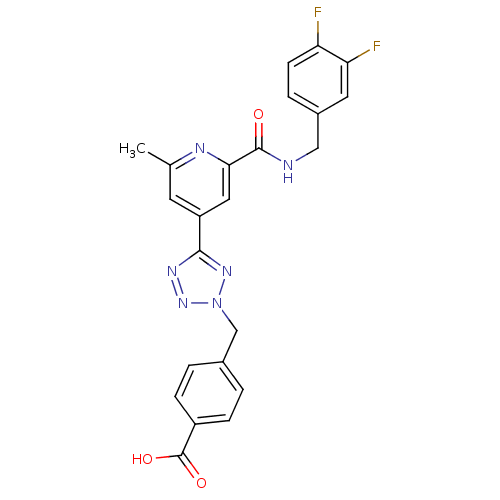
(4-((5-(2-(3,4-difluorobenzylcarbamoyl)-6-methylpyr...)Show SMILES Cc1cc(cc(n1)C(=O)NCc1ccc(F)c(F)c1)-c1nnn(Cc2ccc(cc2)C(O)=O)n1 Show InChI InChI=1S/C23H18F2N6O3/c1-13-8-17(10-20(27-13)22(32)26-11-15-4-7-18(24)19(25)9-15)21-28-30-31(29-21)12-14-2-5-16(6-3-14)23(33)34/h2-10H,11-12H2,1H3,(H,26,32)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs |
Bioorg Med Chem Lett 20: 576-80 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.081
BindingDB Entry DOI: 10.7270/Q2JS9QHB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244487
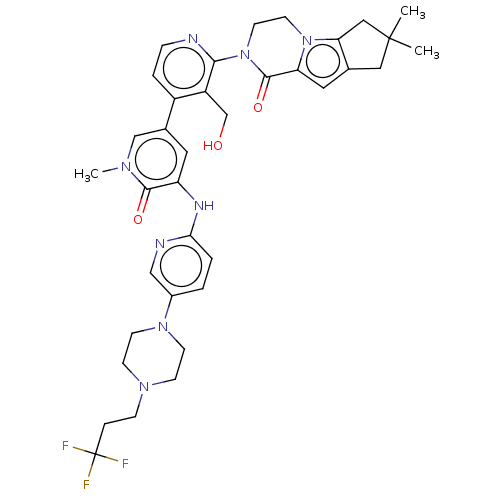
(CHEMBL4079803)Show SMILES Cn1cc(cc(Nc2ccc(cn2)N2CCN(CCC(F)(F)F)CC2)c1=O)-c1ccnc(N2CCn3c4CC(C)(C)Cc4cc3C2=O)c1CO Show InChI InChI=1S/C36H41F3N8O3/c1-35(2)18-23-17-29-34(50)47(15-14-46(29)30(23)19-35)32-27(22-48)26(6-8-40-32)24-16-28(33(49)43(3)21-24)42-31-5-4-25(20-41-31)45-12-10-44(11-13-45)9-7-36(37,38)39/h4-6,8,16-17,20-21,48H,7,9-15,18-19,22H2,1-3H3,(H,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50305847
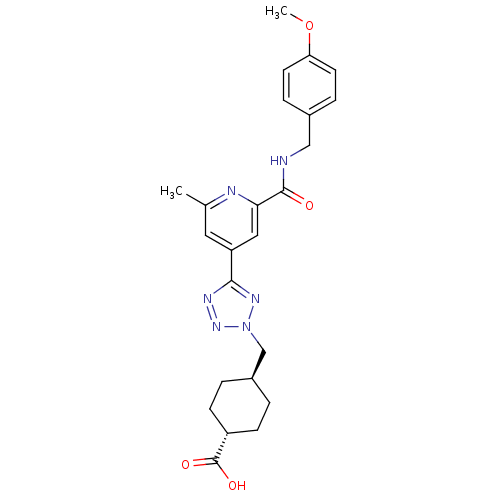
(CHEMBL596304 | trans-4-((5-(2-(4-methoxybenzylcarb...)Show SMILES COc1ccc(CNC(=O)c2cc(cc(C)n2)-c2nnn(C[C@H]3CC[C@@H](CC3)C(O)=O)n2)cc1 |r,wU:25.29,wD:22.22,(-9.22,-14.95,;-7.68,-14.93,;-6.89,-16.25,;-5.35,-16.23,;-4.56,-17.55,;-5.31,-18.89,;-4.52,-20.22,;-2.98,-20.2,;-2.2,-21.52,;-2.95,-22.86,;-.66,-21.5,;.1,-20.16,;1.64,-20.14,;2.43,-21.46,;1.68,-22.8,;2.47,-24.12,;.13,-22.83,;2.39,-18.8,;1.75,-17.39,;2.88,-16.35,;4.23,-17.1,;5.62,-16.45,;6.97,-17.2,;6.99,-18.74,;8.34,-19.49,;9.66,-18.69,;9.63,-17.15,;8.28,-16.41,;11.01,-19.44,;11.04,-20.98,;12.33,-18.65,;3.92,-18.61,;-6.85,-18.92,;-7.64,-17.6,)| Show InChI InChI=1S/C24H28N6O4/c1-15-11-19(12-21(26-15)23(31)25-13-16-5-9-20(34-2)10-6-16)22-27-29-30(28-22)14-17-3-7-18(8-4-17)24(32)33/h5-6,9-12,17-18H,3-4,7-8,13-14H2,1-2H3,(H,25,31)(H,32,33)/t17-,18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs |
Bioorg Med Chem Lett 20: 576-80 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.081
BindingDB Entry DOI: 10.7270/Q2JS9QHB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244468
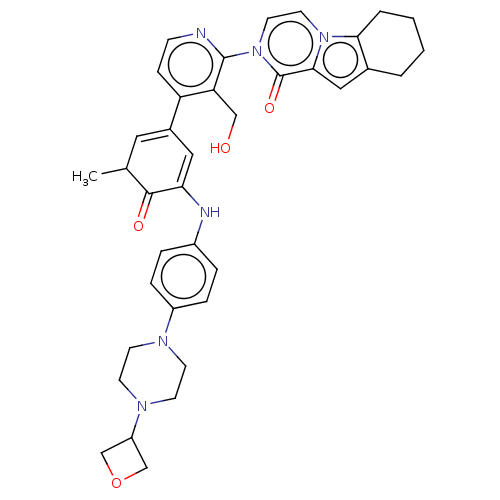
(CHEMBL4104307)Show SMILES CC1C=C(C=C(Nc2ccc(cc2)N2CCN(CC2)C2COC2)C1=O)c1ccnc(c1CO)-n1ccn2c3CCCCc3cc2c1=O |c:2,t:4| Show InChI InChI=1S/C37H40N6O4/c1-24-18-26(19-32(35(24)45)39-27-6-8-28(9-7-27)40-12-14-41(15-13-40)29-22-47-23-29)30-10-11-38-36(31(30)21-44)43-17-16-42-33-5-3-2-4-25(33)20-34(42)37(43)46/h6-11,16-20,24,29,39,44H,2-5,12-15,21-23H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50305842
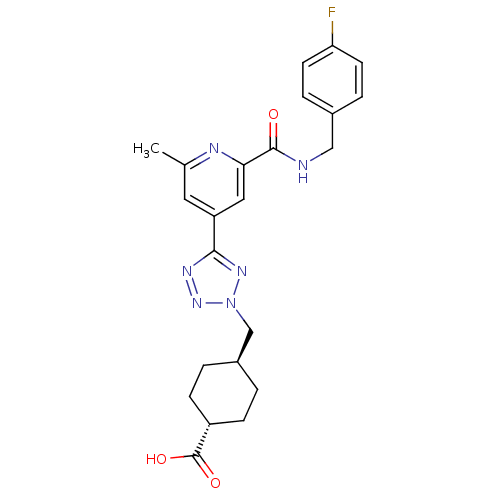
(CHEMBL603656 | trans-4-((5-(2-(4-fluorobenzylcarba...)Show SMILES Cc1cc(cc(n1)C(=O)NCc1ccc(F)cc1)-c1nnn(C[C@H]2CC[C@@H](CC2)C(O)=O)n1 |r,wU:26.31,wD:23.24,(30.11,-12.24,;29.32,-10.92,;30.07,-9.57,;29.28,-8.26,;27.74,-8.27,;26.98,-9.62,;27.77,-10.94,;25.44,-9.64,;24.69,-10.98,;24.66,-8.31,;23.12,-8.33,;22.33,-7.01,;23.08,-5.67,;22.3,-4.34,;20.75,-4.36,;19.97,-3.04,;20,-5.72,;20.79,-7.03,;30.03,-6.91,;29.39,-5.51,;30.52,-4.46,;31.87,-5.21,;33.27,-4.57,;34.61,-5.32,;34.63,-6.85,;35.98,-7.6,;37.3,-6.81,;37.27,-5.26,;35.93,-4.52,;38.65,-7.56,;38.68,-9.1,;39.97,-6.77,;31.57,-6.73,)| Show InChI InChI=1S/C23H25FN6O3/c1-14-10-18(11-20(26-14)22(31)25-12-15-4-8-19(24)9-5-15)21-27-29-30(28-21)13-16-2-6-17(7-3-16)23(32)33/h4-5,8-11,16-17H,2-3,6-7,12-13H2,1H3,(H,25,31)(H,32,33)/t16-,17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs |
Bioorg Med Chem Lett 20: 576-80 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.081
BindingDB Entry DOI: 10.7270/Q2JS9QHB |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50305843
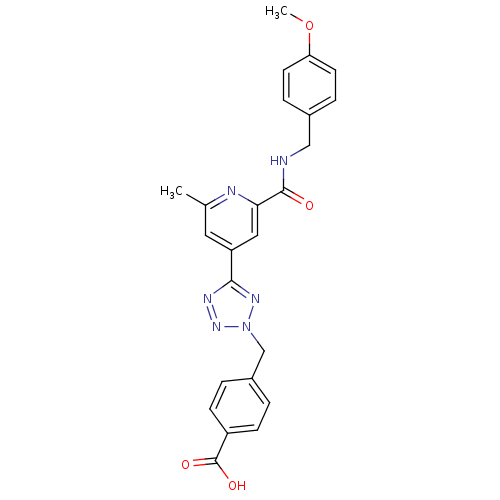
(4-((5-(2-(4-methoxybenzylcarbamoyl)-6-methylpyridi...)Show SMILES COc1ccc(CNC(=O)c2cc(cc(C)n2)-c2nnn(Cc3ccc(cc3)C(O)=O)n2)cc1 Show InChI InChI=1S/C24H22N6O4/c1-15-11-19(12-21(26-15)23(31)25-13-16-5-9-20(34-2)10-6-16)22-27-29-30(28-22)14-17-3-7-18(8-4-17)24(32)33/h3-12H,13-14H2,1-2H3,(H,25,31)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs |
Bioorg Med Chem Lett 20: 576-80 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.081
BindingDB Entry DOI: 10.7270/Q2JS9QHB |
More data for this
Ligand-Target Pair | |
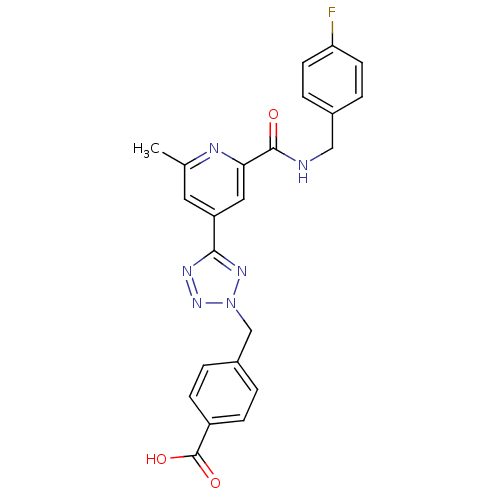
Collagenase 3
(Homo sapiens (Human)) | BDBM50305838
(4-((5-(2-(4-fluorobenzylcarbamoyl)-6-methylpyridin...)Show SMILES Cc1cc(cc(n1)C(=O)NCc1ccc(F)cc1)-c1nnn(Cc2ccc(cc2)C(O)=O)n1 Show InChI InChI=1S/C23H19FN6O3/c1-14-10-18(11-20(26-14)22(31)25-12-15-4-8-19(24)9-5-15)21-27-29-30(28-21)13-16-2-6-17(7-3-16)23(32)33/h2-11H,12-13H2,1H3,(H,25,31)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs |
Bioorg Med Chem Lett 20: 576-80 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.081
BindingDB Entry DOI: 10.7270/Q2JS9QHB |
More data for this
Ligand-Target Pair | |
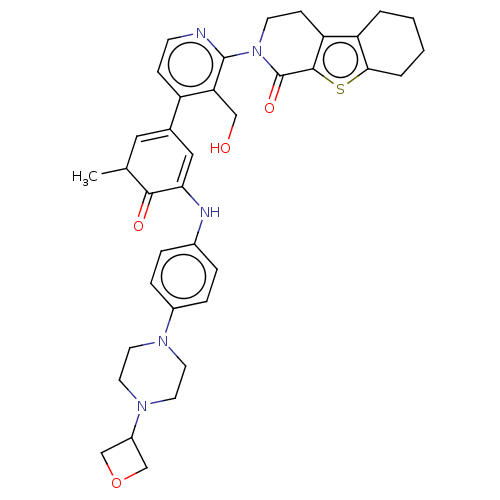
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244466
(CHEMBL4075845)Show SMILES CC1C=C(C=C(Nc2ccc(cc2)N2CCN(CC2)C2COC2)C1=O)c1ccnc(N2CCc3c4CCCCc4sc3C2=O)c1CO |c:2,t:4| Show InChI InChI=1S/C37H41N5O4S/c1-23-18-24(19-32(34(23)44)39-25-6-8-26(9-7-25)40-14-16-41(17-15-40)27-21-46-22-27)28-10-12-38-36(31(28)20-43)42-13-11-30-29-4-2-3-5-33(29)47-35(30)37(42)45/h6-10,12,18-19,23,27,39,43H,2-5,11,13-17,20-22H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
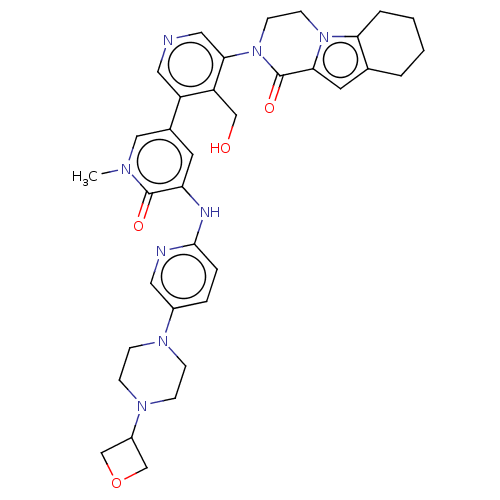
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244498
(CHEMBL4101904)Show SMILES Cn1cc(cc(Nc2ccc(cn2)N2CCN(CC2)C2COC2)c1=O)-c1cncc(N2CCn3c4CCCCc4cc3C2=O)c1CO Show InChI InChI=1S/C35H40N8O4/c1-39-19-24(14-29(34(39)45)38-33-7-6-25(16-37-33)40-8-10-41(11-9-40)26-21-47-22-26)27-17-36-18-32(28(27)20-44)43-13-12-42-30-5-3-2-4-23(30)15-31(42)35(43)46/h6-7,14-19,26,44H,2-5,8-13,20-22H2,1H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50305849
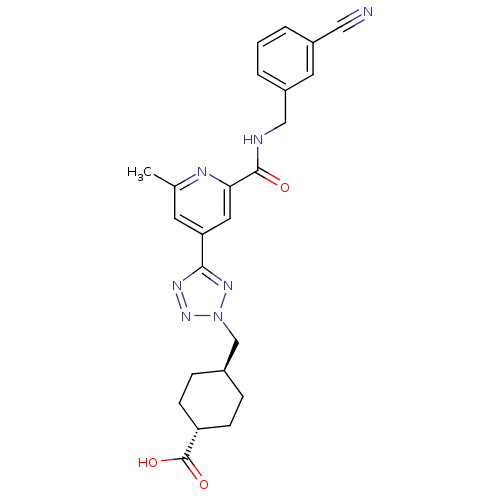
(CHEMBL593257 | trans-4-((5-(2-(3-cyanobenzylcarbam...)Show SMILES Cc1cc(cc(n1)C(=O)NCc1cccc(c1)C#N)-c1nnn(C[C@H]2CC[C@@H](CC2)C(O)=O)n1 |r,wU:27.32,wD:24.25,(4.38,-39.29,;3.59,-37.97,;4.33,-36.63,;3.55,-35.31,;2,-35.32,;1.25,-36.67,;2.04,-38,;-.29,-36.69,;-1.05,-38.03,;-1.08,-35.36,;-2.62,-35.38,;-3.41,-34.06,;-2.66,-32.72,;-3.44,-31.4,;-4.98,-31.41,;-5.74,-32.77,;-4.95,-34.08,;-7.28,-32.8,;-8.82,-32.82,;4.3,-33.96,;3.66,-32.56,;4.79,-31.51,;6.13,-32.27,;7.53,-31.62,;8.88,-32.37,;8.9,-33.9,;10.24,-34.65,;11.56,-33.86,;11.53,-32.31,;10.19,-31.57,;12.92,-34.61,;12.94,-36.15,;14.24,-33.82,;5.83,-33.78,)| Show InChI InChI=1S/C24H25N7O3/c1-15-9-20(11-21(27-15)23(32)26-13-18-4-2-3-17(10-18)12-25)22-28-30-31(29-22)14-16-5-7-19(8-6-16)24(33)34/h2-4,9-11,16,19H,5-8,13-14H2,1H3,(H,26,32)(H,33,34)/t16-,19- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs |
Bioorg Med Chem Lett 20: 576-80 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.081
BindingDB Entry DOI: 10.7270/Q2JS9QHB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244465
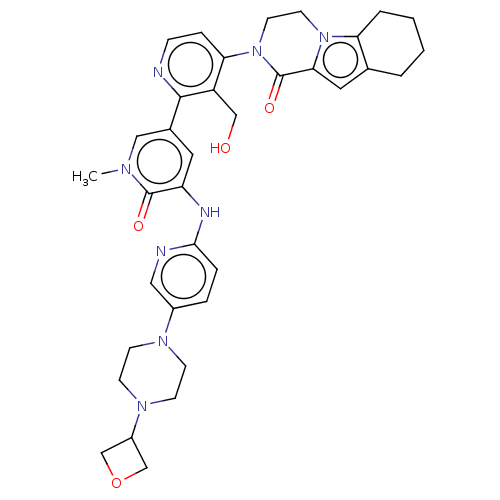
(CHEMBL4075253)Show SMILES Cn1cc(cc(Nc2ccc(cn2)N2CCN(CC2)C2COC2)c1=O)-c1nccc(N2CCn3c4CCCCc4cc3C2=O)c1CO Show InChI InChI=1S/C35H40N8O4/c1-39-19-24(16-28(34(39)45)38-32-7-6-25(18-37-32)40-10-12-41(13-11-40)26-21-47-22-26)33-27(20-44)30(8-9-36-33)43-15-14-42-29-5-3-2-4-23(29)17-31(42)35(43)46/h6-9,16-19,26,44H,2-5,10-15,20-22H2,1H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50305853
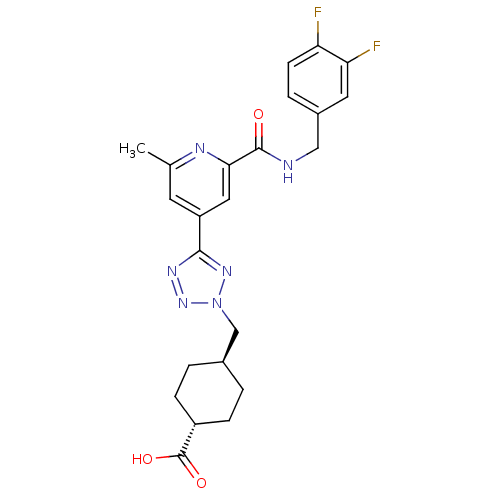
(CHEMBL595842 | trans-4-((5-(2-(3,4-difluorobenzylc...)Show SMILES Cc1cc(cc(n1)C(=O)NCc1ccc(F)c(F)c1)-c1nnn(C[C@H]2CC[C@@H](CC2)C(O)=O)n1 |r,wU:27.32,wD:24.25,(31.3,-8.85,;30.5,-7.53,;31.25,-6.18,;30.46,-4.86,;28.92,-4.88,;28.17,-6.23,;28.95,-7.55,;26.63,-6.24,;25.87,-7.59,;25.84,-4.92,;24.3,-4.94,;23.51,-3.62,;24.26,-2.28,;23.48,-.95,;21.94,-.97,;21.15,.35,;21.18,-2.32,;19.64,-2.35,;21.97,-3.64,;31.22,-3.52,;30.58,-2.12,;31.71,-1.07,;33.05,-1.82,;34.45,-1.18,;35.79,-1.93,;35.82,-3.46,;37.16,-4.21,;38.48,-3.42,;38.45,-1.87,;37.11,-1.13,;39.84,-4.17,;39.86,-5.71,;41.16,-3.38,;32.75,-3.33,)| Show InChI InChI=1S/C23H24F2N6O3/c1-13-8-17(10-20(27-13)22(32)26-11-15-4-7-18(24)19(25)9-15)21-28-30-31(29-21)12-14-2-5-16(6-3-14)23(33)34/h4,7-10,14,16H,2-3,5-6,11-12H2,1H3,(H,26,32)(H,33,34)/t14-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs |
Bioorg Med Chem Lett 20: 576-80 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.081
BindingDB Entry DOI: 10.7270/Q2JS9QHB |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50305836
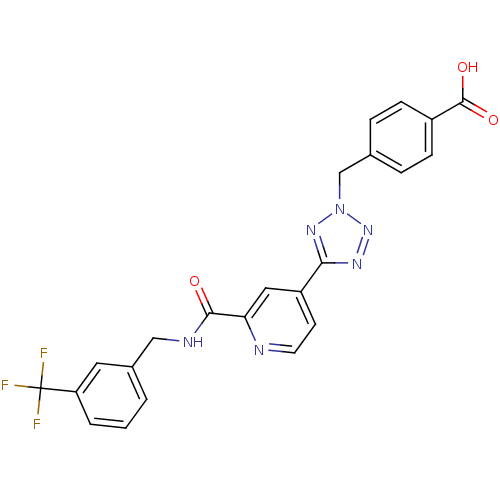
(4-((5-(2-(3-(trifluoromethyl)benzylcarbamoyl)pyrid...)Show SMILES OC(=O)c1ccc(Cn2nnc(n2)-c2ccnc(c2)C(=O)NCc2cccc(c2)C(F)(F)F)cc1 Show InChI InChI=1S/C23H17F3N6O3/c24-23(25,26)18-3-1-2-15(10-18)12-28-21(33)19-11-17(8-9-27-19)20-29-31-32(30-20)13-14-4-6-16(7-5-14)22(34)35/h1-11H,12-13H2,(H,28,33)(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs |
Bioorg Med Chem Lett 20: 576-80 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.081
BindingDB Entry DOI: 10.7270/Q2JS9QHB |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50305835
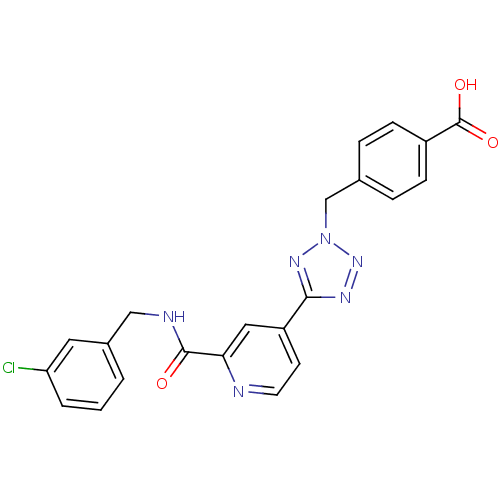
(4-((5-(2-(3-chlorobenzylcarbamoyl)pyridin-4-yl)-2H...)Show SMILES OC(=O)c1ccc(Cn2nnc(n2)-c2ccnc(c2)C(=O)NCc2cccc(Cl)c2)cc1 Show InChI InChI=1S/C22H17ClN6O3/c23-18-3-1-2-15(10-18)12-25-21(30)19-11-17(8-9-24-19)20-26-28-29(27-20)13-14-4-6-16(7-5-14)22(31)32/h1-11H,12-13H2,(H,25,30)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs |
Bioorg Med Chem Lett 20: 576-80 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.081
BindingDB Entry DOI: 10.7270/Q2JS9QHB |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50305837
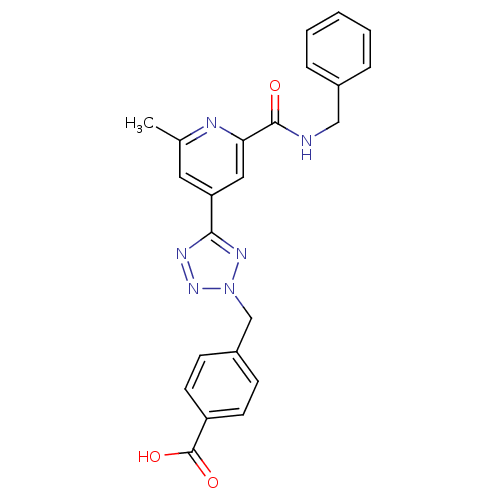
(4-((5-(2-(benzylcarbamoyl)-6-methylpyridin-4-yl)-2...)Show SMILES Cc1cc(cc(n1)C(=O)NCc1ccccc1)-c1nnn(Cc2ccc(cc2)C(O)=O)n1 Show InChI InChI=1S/C23H20N6O3/c1-15-11-19(12-20(25-15)22(30)24-13-16-5-3-2-4-6-16)21-26-28-29(27-21)14-17-7-9-18(10-8-17)23(31)32/h2-12H,13-14H2,1H3,(H,24,30)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs |
Bioorg Med Chem Lett 20: 576-80 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.081
BindingDB Entry DOI: 10.7270/Q2JS9QHB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data