 Found 224 hits with Last Name = 'sankaran' and Initial = 'b'
Found 224 hits with Last Name = 'sankaran' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lethal factor
(Bacillus anthracis) | BDBM50379543
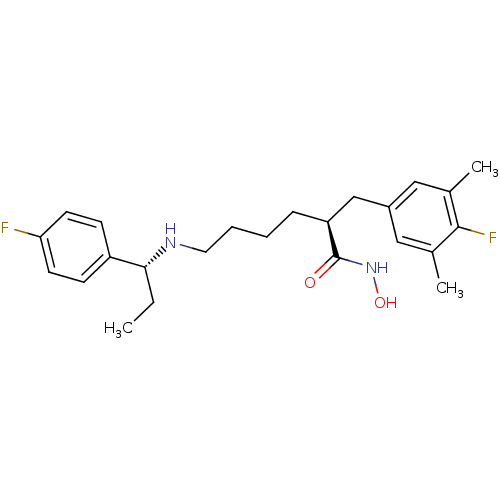
(CHEMBL2012752)Show SMILES CC[C@@H](NCCCC[C@@H](Cc1cc(C)c(F)c(C)c1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H32F2N2O2/c1-4-22(19-8-10-21(25)11-9-19)27-12-6-5-7-20(24(29)28-30)15-18-13-16(2)23(26)17(3)14-18/h8-11,13-14,20,22,27,30H,4-7,12,15H2,1-3H3,(H,28,29)/t20-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50379542
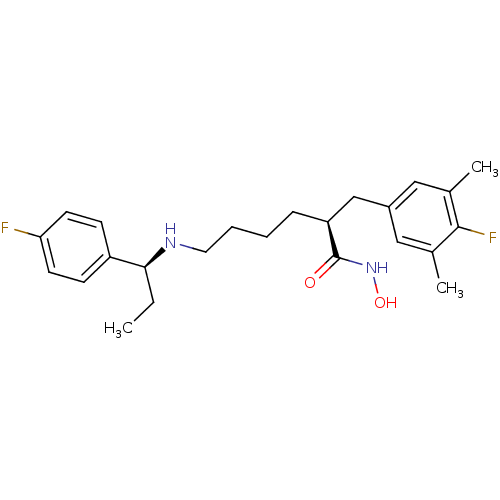
(CHEMBL2012753)Show SMILES CC[C@H](NCCCC[C@@H](Cc1cc(C)c(F)c(C)c1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H32F2N2O2/c1-4-22(19-8-10-21(25)11-9-19)27-12-6-5-7-20(24(29)28-30)15-18-13-16(2)23(26)17(3)14-18/h8-11,13-14,20,22,27,30H,4-7,12,15H2,1-3H3,(H,28,29)/t20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50340754
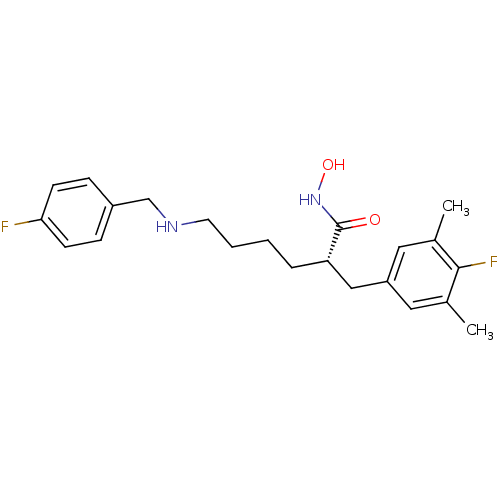
((S)-2-(4-fluoro-3,5-dimethylbenzyl)-6-(4-fluoroben...)Show SMILES Cc1cc(C[C@H](CCCCNCc2ccc(F)cc2)C(=O)NO)cc(C)c1F |r| Show InChI InChI=1S/C22H28F2N2O2/c1-15-11-18(12-16(2)21(15)24)13-19(22(27)26-28)5-3-4-10-25-14-17-6-8-20(23)9-7-17/h6-9,11-12,19,25,28H,3-5,10,13-14H2,1-2H3,(H,26,27)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50379536
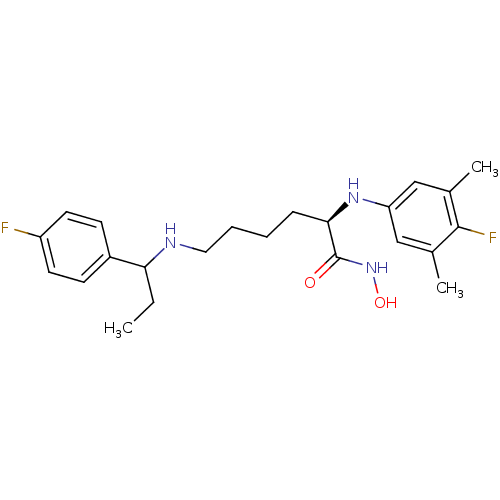
(CHEMBL2012836)Show SMILES CCC(NCCCC[C@@H](Nc1cc(C)c(F)c(C)c1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C23H31F2N3O2/c1-4-20(17-8-10-18(24)11-9-17)26-12-6-5-7-21(23(29)28-30)27-19-13-15(2)22(25)16(3)14-19/h8-11,13-14,20-21,26-27,30H,4-7,12H2,1-3H3,(H,28,29)/t20?,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50379541
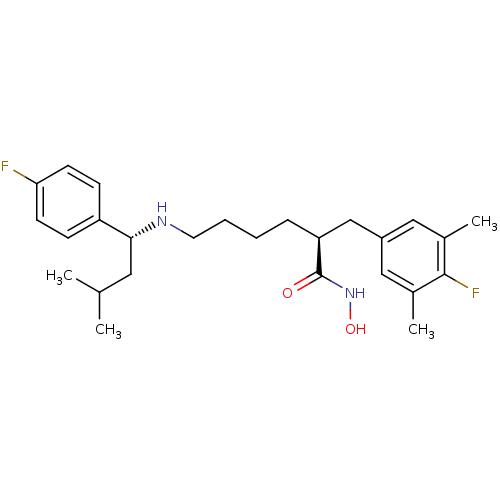
(CHEMBL2012832)Show SMILES CC(C)C[C@@H](NCCCC[C@@H](Cc1cc(C)c(F)c(C)c1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H36F2N2O2/c1-17(2)13-24(21-8-10-23(27)11-9-21)29-12-6-5-7-22(26(31)30-32)16-20-14-18(3)25(28)19(4)15-20/h8-11,14-15,17,22,24,29,32H,5-7,12-13,16H2,1-4H3,(H,30,31)/t22-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50329265
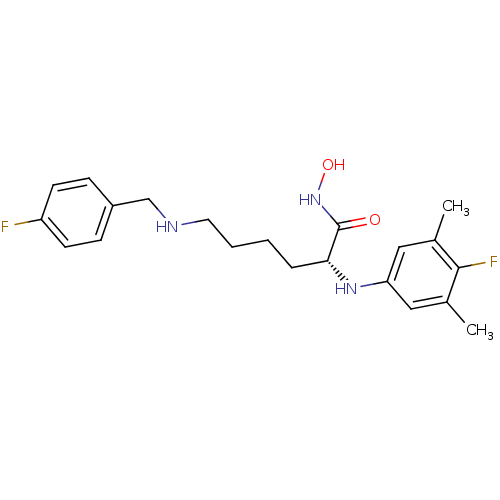
((R)-2-(4-fluoro-3,5-dimethylphenylamino)-6-(4-fluo...)Show SMILES Cc1cc(N[C@H](CCCCNCc2ccc(F)cc2)C(=O)NO)cc(C)c1F |r| Show InChI InChI=1S/C21H27F2N3O2/c1-14-11-18(12-15(2)20(14)23)25-19(21(27)26-28)5-3-4-10-24-13-16-6-8-17(22)9-7-16/h6-9,11-12,19,24-25,28H,3-5,10,13H2,1-2H3,(H,26,27)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50379533
(CHEMBL2012838)Show SMILES CCC(NCCCC[C@@H](C[C@@H](OC)c1ccc(F)cc1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H32F2N2O3/c1-3-22(17-7-11-20(25)12-8-17)27-15-5-4-6-19(24(29)28-30)16-23(31-2)18-9-13-21(26)14-10-18/h7-14,19,22-23,27,30H,3-6,15-16H2,1-2H3,(H,28,29)/t19-,22?,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50379540
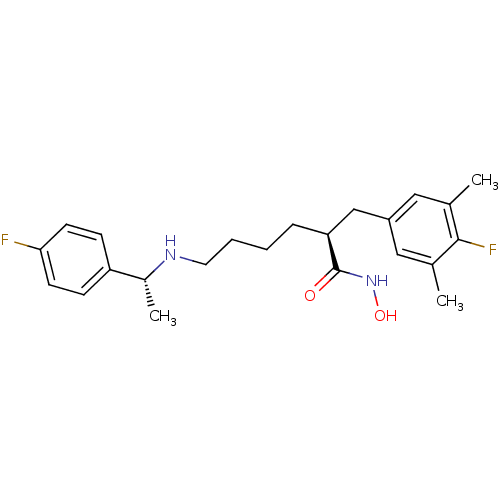
(CHEMBL2012750)Show SMILES C[C@@H](NCCCC[C@@H](Cc1cc(C)c(F)c(C)c1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C23H30F2N2O2/c1-15-12-18(13-16(2)22(15)25)14-20(23(28)27-29)6-4-5-11-26-17(3)19-7-9-21(24)10-8-19/h7-10,12-13,17,20,26,29H,4-6,11,14H2,1-3H3,(H,27,28)/t17-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50379535
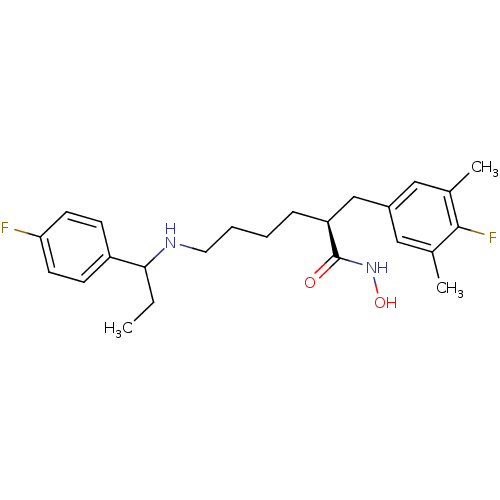
(CHEMBL2010824)Show SMILES CCC(NCCCC[C@@H](Cc1cc(C)c(F)c(C)c1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H32F2N2O2/c1-4-22(19-8-10-21(25)11-9-19)27-12-6-5-7-20(24(29)28-30)15-18-13-16(2)23(26)17(3)14-18/h8-11,13-14,20,22,27,30H,4-7,12,15H2,1-3H3,(H,28,29)/t20-,22?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50379544
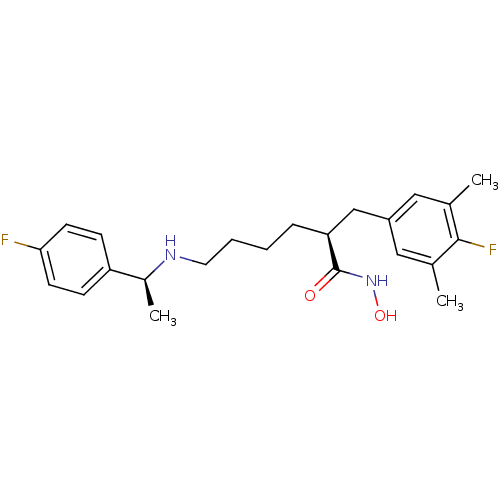
(CHEMBL2012751)Show SMILES C[C@H](NCCCC[C@@H](Cc1cc(C)c(F)c(C)c1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C23H30F2N2O2/c1-15-12-18(13-16(2)22(15)25)14-20(23(28)27-29)6-4-5-11-26-17(3)19-7-9-21(24)10-8-19/h7-10,12-13,17,20,26,29H,4-6,11,14H2,1-3H3,(H,27,28)/t17-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50340758
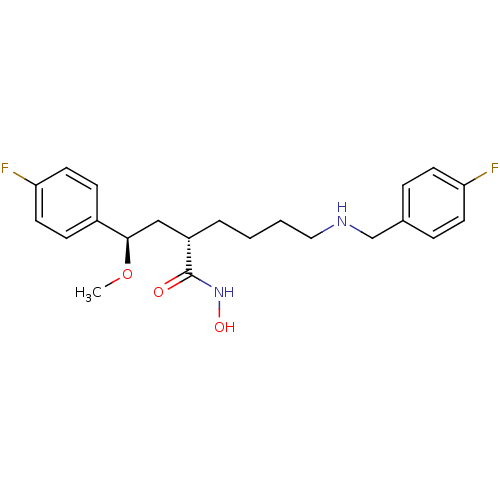
((S)-6-(4-fluorobenzylamino)-2-((R)-2-(4-fluorophen...)Show SMILES CO[C@H](C[C@H](CCCCNCc1ccc(F)cc1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C22H28F2N2O3/c1-29-21(17-7-11-20(24)12-8-17)14-18(22(27)26-28)4-2-3-13-25-15-16-5-9-19(23)10-6-16/h5-12,18,21,25,28H,2-4,13-15H2,1H3,(H,26,27)/t18-,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lethal factor
(Bacillus anthracis) | BDBM50379539
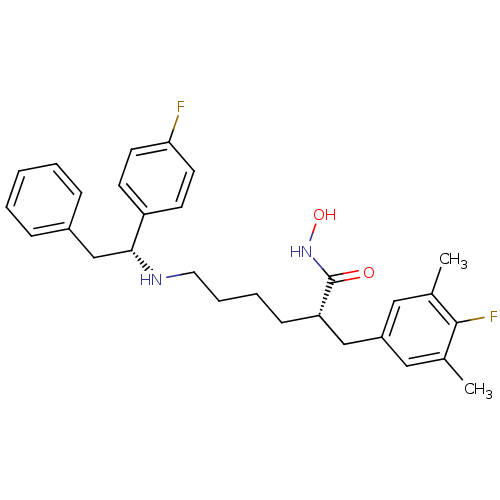
(CHEMBL2012834)Show SMILES Cc1cc(C[C@H](CCCCN[C@H](Cc2ccccc2)c2ccc(F)cc2)C(=O)NO)cc(C)c1F |r| Show InChI InChI=1S/C29H34F2N2O2/c1-20-16-23(17-21(2)28(20)31)18-25(29(34)33-35)10-6-7-15-32-27(19-22-8-4-3-5-9-22)24-11-13-26(30)14-12-24/h3-5,8-9,11-14,16-17,25,27,32,35H,6-7,10,15,18-19H2,1-2H3,(H,33,34)/t25-,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50379534
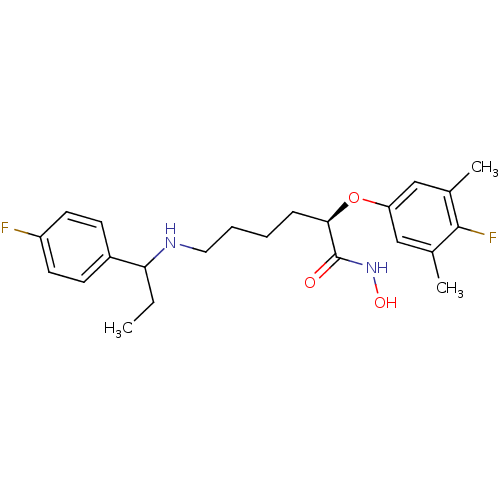
(CHEMBL2012837)Show SMILES CCC(NCCCC[C@@H](Oc1cc(C)c(F)c(C)c1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C23H30F2N2O3/c1-4-20(17-8-10-18(24)11-9-17)26-12-6-5-7-21(23(28)27-29)30-19-13-15(2)22(25)16(3)14-19/h8-11,13-14,20-21,26,29H,4-7,12H2,1-3H3,(H,27,28)/t20?,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50340768
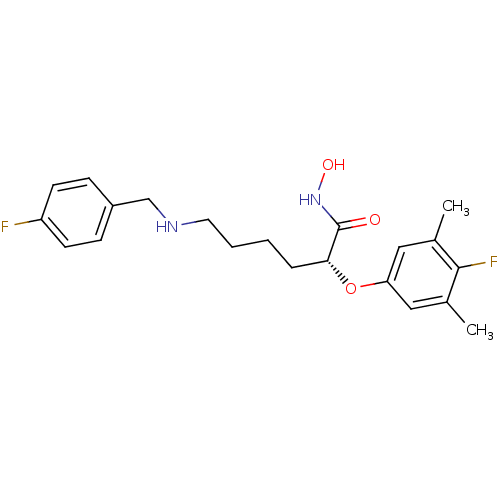
((R)-2-(4-fluoro-3,5-dimethylphenoxy)-6-(4-fluorobe...)Show SMILES Cc1cc(O[C@H](CCCCNCc2ccc(F)cc2)C(=O)NO)cc(C)c1F |r| Show InChI InChI=1S/C21H26F2N2O3/c1-14-11-18(12-15(2)20(14)23)28-19(21(26)25-27)5-3-4-10-24-13-16-6-8-17(22)9-7-16/h6-9,11-12,19,24,27H,3-5,10,13H2,1-2H3,(H,25,26)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50379538
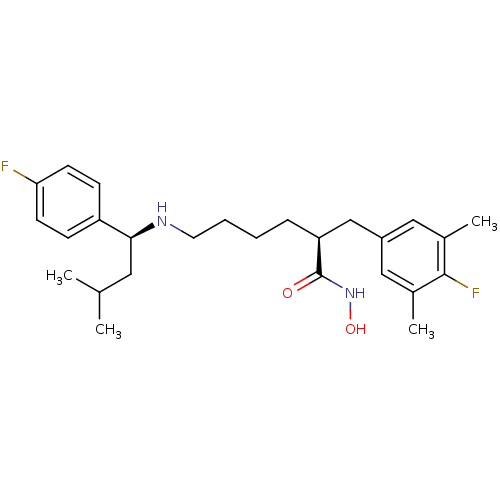
(CHEMBL2012833)Show SMILES CC(C)C[C@H](NCCCC[C@@H](Cc1cc(C)c(F)c(C)c1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H36F2N2O2/c1-17(2)13-24(21-8-10-23(27)11-9-21)29-12-6-5-7-22(26(31)30-32)16-20-14-18(3)25(28)19(4)15-20/h8-11,14-15,17,22,24,29,32H,5-7,12-13,16H2,1-4H3,(H,30,31)/t22-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50379537
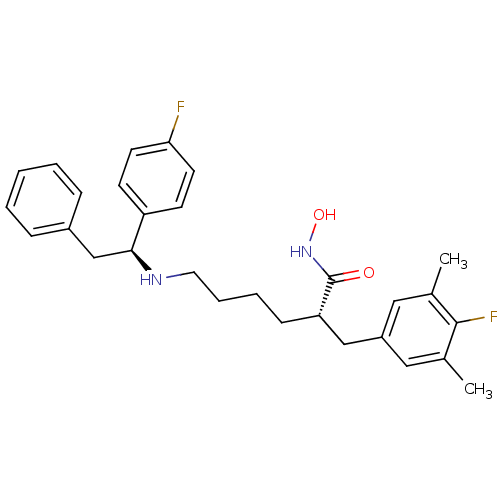
(CHEMBL2012835)Show SMILES Cc1cc(C[C@H](CCCCN[C@@H](Cc2ccccc2)c2ccc(F)cc2)C(=O)NO)cc(C)c1F |r| Show InChI InChI=1S/C29H34F2N2O2/c1-20-16-23(17-21(2)28(20)31)18-25(29(34)33-35)10-6-7-15-32-27(19-22-8-4-3-5-9-22)24-11-13-26(30)14-12-24/h3-5,8-9,11-14,16-17,25,27,32,35H,6-7,10,15,18-19H2,1-2H3,(H,33,34)/t25-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM423466
(WO2006061714, P39.1 | WO2006061714-ID-11 | cmdc.20...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C=O Show InChI InChI=1S/C26H38N4O6/c1-16(2)12-21(24(33)28-20(14-31)13-19-10-11-27-23(19)32)29-25(34)22(17(3)4)30-26(35)36-15-18-8-6-5-7-9-18/h5-9,14,16-17,19-22H,10-13,15H2,1-4H3,(H,27,32)(H,28,33)(H,29,34)(H,30,35)/t19-,20-,21-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.30 | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University
| Assay Description
The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... |
ChemMedChem (2020)
Article DOI: 10.1002/cmdc.202000924
BindingDB Entry DOI: 10.7270/Q2G163V1 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM442754
(MPI4)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C=O Show InChI InChI=1S/C29H36N4O6/c1-19(2)25(33-29(38)39-18-21-11-7-4-8-12-21)28(37)32-24(15-20-9-5-3-6-10-20)27(36)31-23(17-34)16-22-13-14-30-26(22)35/h3-12,17,19,22-25H,13-16,18H2,1-2H3,(H,30,35)(H,31,36)(H,32,37)(H,33,38)/t22-,23-,24-,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University
| Assay Description
The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... |
ChemMedChem (2020)
Article DOI: 10.1002/cmdc.202000924
BindingDB Entry DOI: 10.7270/Q2G163V1 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM484193
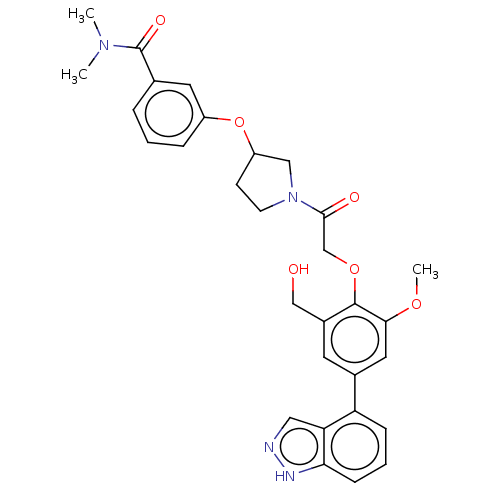
(CDD-1714)Show SMILES COc1cc(cc(CO)c1OCC(=O)N1CCC(C1)Oc1cccc(c1)C(=O)N(C)C)-c1cccc2[nH]ncc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
| Assay Description
To determine the Ki values of active compounds, 25 nM Mpro was mixed with increasing concentrations of compounds (from 4 nM to 4,000 nM with twofold ... |
Proc Natl Acad Sci U S A 118: (2021)
Article DOI: 10.1073/pnas.2111172118
BindingDB Entry DOI: 10.7270/Q2W380FK |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
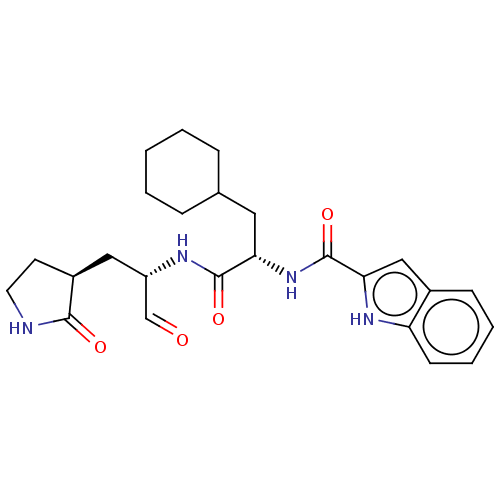
(2019-nCoV) | BDBM420296
(Advanced SARS-CoV-2 Inhibitor 11a | MPI10 | acs.jm...)Show SMILES O=C[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@H](CC1CCCCC1)NC(=O)c1cc2ccccc2[nH]1 Show InChI InChI=1S/C25H32N4O4/c30-15-19(13-18-10-11-26-23(18)31)27-24(32)21(12-16-6-2-1-3-7-16)29-25(33)22-14-17-8-4-5-9-20(17)28-22/h4-5,8-9,14-16,18-19,21,28H,1-3,6-7,10-13H2,(H,26,31)(H,27,32)(H,29,33)/t18-,19-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| Article
PubMed
| 30 | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University
| Assay Description
The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... |
ChemMedChem (2020)
Article DOI: 10.1002/cmdc.202000924
BindingDB Entry DOI: 10.7270/Q2G163V1 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM419133
(BDBM429386 | GC376)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(O)S([O-])(=O)=O Show InChI InChI=1S/C21H31N3O8S/c1-13(2)10-16(24-21(28)32-12-14-6-4-3-5-7-14)19(26)23-17(20(27)33(29,30)31)11-15-8-9-22-18(15)25/h3-7,13,15-17,20,27H,8-12H2,1-2H3,(H,22,25)(H,23,26)(H,24,28)(H,29,30,31)/p-1/t15?,16-,17-,20?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University
| Assay Description
The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... |
ChemMedChem (2020)
Article DOI: 10.1002/cmdc.202000924
BindingDB Entry DOI: 10.7270/Q2G163V1 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM442755
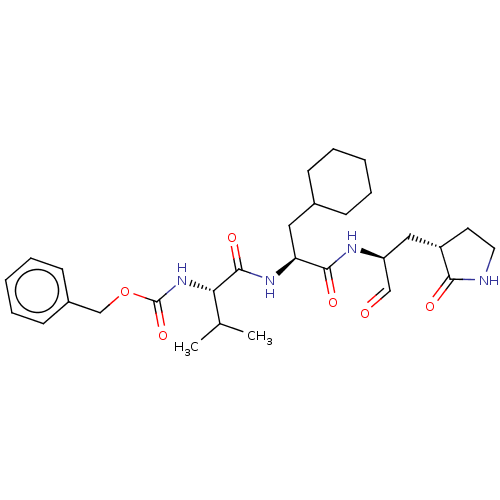
(MPI5)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C=O Show InChI InChI=1S/C29H42N4O6/c1-19(2)25(33-29(38)39-18-21-11-7-4-8-12-21)28(37)32-24(15-20-9-5-3-6-10-20)27(36)31-23(17-34)16-22-13-14-30-26(22)35/h4,7-8,11-12,17,19-20,22-25H,3,5-6,9-10,13-16,18H2,1-2H3,(H,30,35)(H,31,36)(H,32,37)(H,33,38)/t22-,23-,24-,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University
| Assay Description
The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... |
ChemMedChem (2020)
Article DOI: 10.1002/cmdc.202000924
BindingDB Entry DOI: 10.7270/Q2G163V1 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM484197
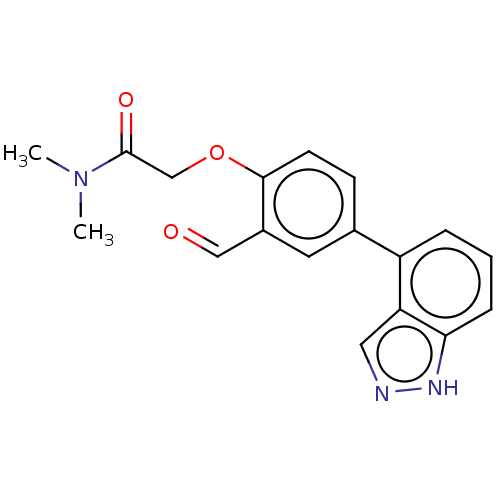
(CDD-1976 | WO2023004291, Compound CDD-1976)Show InChI InChI=1S/C18H17N3O3/c1-21(2)18(23)11-24-17-7-6-12(8-13(17)10-22)14-4-3-5-16-15(14)9-19-20-16/h3-10H,11H2,1-2H3,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
| Assay Description
To determine the Ki values of active compounds, 25 nM Mpro was mixed with increasing concentrations of compounds (from 4 nM to 4,000 nM with twofold ... |
Proc Natl Acad Sci U S A 118: (2021)
Article DOI: 10.1073/pnas.2111172118
BindingDB Entry DOI: 10.7270/Q2W380FK |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM442792
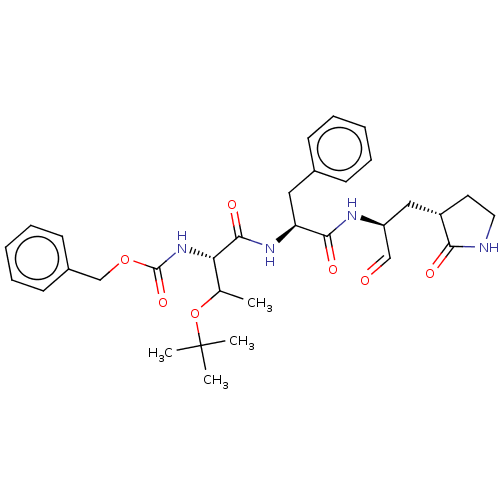
(MPI7)Show SMILES CC(OC(C)(C)C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C=O Show InChI InChI=1S/C32H42N4O7/c1-21(43-32(2,3)4)27(36-31(41)42-20-23-13-9-6-10-14-23)30(40)35-26(17-22-11-7-5-8-12-22)29(39)34-25(19-37)18-24-15-16-33-28(24)38/h5-14,19,21,24-27H,15-18,20H2,1-4H3,(H,33,38)(H,34,39)(H,35,40)(H,36,41)/t21?,24-,25-,26-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 46 | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University
| Assay Description
The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... |
ChemMedChem (2020)
Article DOI: 10.1002/cmdc.202000924
BindingDB Entry DOI: 10.7270/Q2G163V1 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM442794
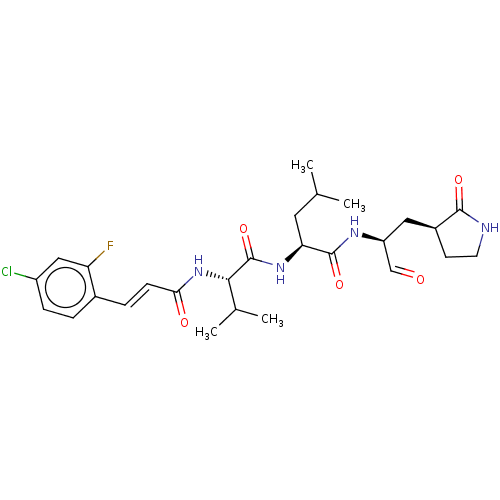
(MPI9)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)\C=C\c1ccc(Cl)cc1F)C(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C=O Show InChI InChI=1S/C27H36ClFN4O5/c1-15(2)11-22(26(37)31-20(14-34)12-18-9-10-30-25(18)36)32-27(38)24(16(3)4)33-23(35)8-6-17-5-7-19(28)13-21(17)29/h5-8,13-16,18,20,22,24H,9-12H2,1-4H3,(H,30,36)(H,31,37)(H,32,38)(H,33,35)/b8-6+/t18-,20-,22-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 55 | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University
| Assay Description
The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... |
ChemMedChem (2020)
Article DOI: 10.1002/cmdc.202000924
BindingDB Entry DOI: 10.7270/Q2G163V1 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM442762
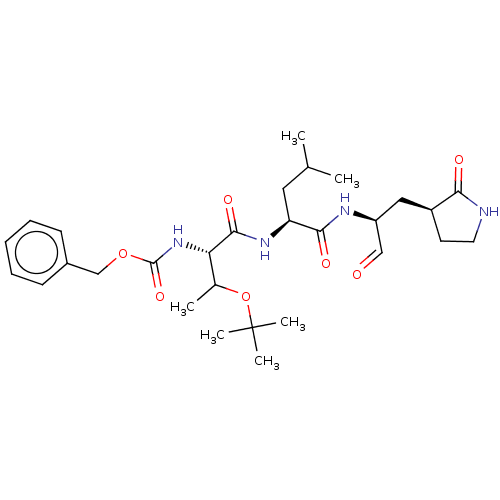
(MPI6)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)OC(C)(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C=O Show InChI InChI=1S/C29H44N4O7/c1-18(2)14-23(26(36)31-22(16-34)15-21-12-13-30-25(21)35)32-27(37)24(19(3)40-29(4,5)6)33-28(38)39-17-20-10-8-7-9-11-20/h7-11,16,18-19,21-24H,12-15,17H2,1-6H3,(H,30,35)(H,31,36)(H,32,37)(H,33,38)/t19?,21-,22-,23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 59 | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University
| Assay Description
The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... |
ChemMedChem (2020)
Article DOI: 10.1002/cmdc.202000924
BindingDB Entry DOI: 10.7270/Q2G163V1 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM484196
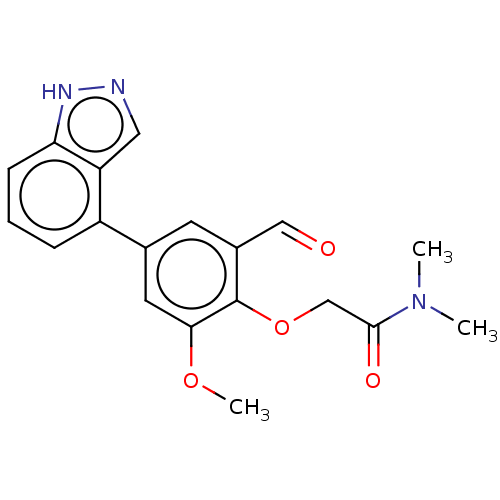
(CDD-1713 | WO2023004291, Compound CDD-1713)Show SMILES COc1cc(cc(C=O)c1OCC(=O)N(C)C)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C19H19N3O4/c1-22(2)18(24)11-26-19-13(10-23)7-12(8-17(19)25-3)14-5-4-6-16-15(14)9-20-21-16/h4-10H,11H2,1-3H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
| Assay Description
To determine the Ki values of active compounds, 25 nM Mpro was mixed with increasing concentrations of compounds (from 4 nM to 4,000 nM with twofold ... |
Proc Natl Acad Sci U S A 118: (2021)
Article DOI: 10.1073/pnas.2111172118
BindingDB Entry DOI: 10.7270/Q2W380FK |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM442746
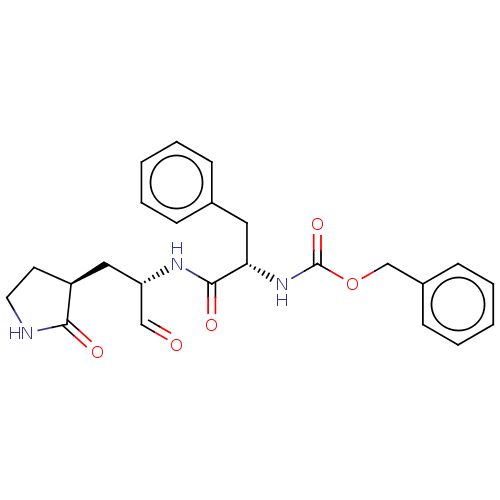
(MPI1)Show SMILES O=C[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C24H27N3O5/c28-15-20(14-19-11-12-25-22(19)29)26-23(30)21(13-17-7-3-1-4-8-17)27-24(31)32-16-18-9-5-2-6-10-18/h1-10,15,19-21H,11-14,16H2,(H,25,29)(H,26,30)(H,27,31)/t19-,20-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 98 | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University
| Assay Description
The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... |
ChemMedChem (2020)
Article DOI: 10.1002/cmdc.202000924
BindingDB Entry DOI: 10.7270/Q2G163V1 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM429372
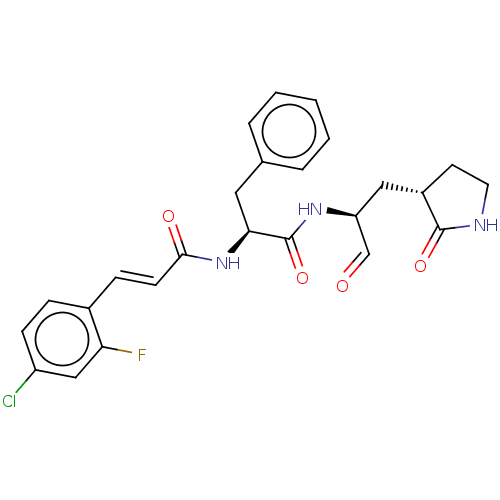
(MPI2 | med.21724, Compound 43)Show SMILES Fc1cc(Cl)ccc1\C=C\C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C=O Show InChI InChI=1S/C25H25ClFN3O4/c26-19-8-6-17(21(27)14-19)7-9-23(32)30-22(12-16-4-2-1-3-5-16)25(34)29-20(15-31)13-18-10-11-28-24(18)33/h1-9,14-15,18,20,22H,10-13H2,(H,28,33)(H,29,34)(H,30,32)/b9-7+/t18-,20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 101 | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University
| Assay Description
The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... |
ChemMedChem (2020)
Article DOI: 10.1002/cmdc.202000924
BindingDB Entry DOI: 10.7270/Q2G163V1 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM429100
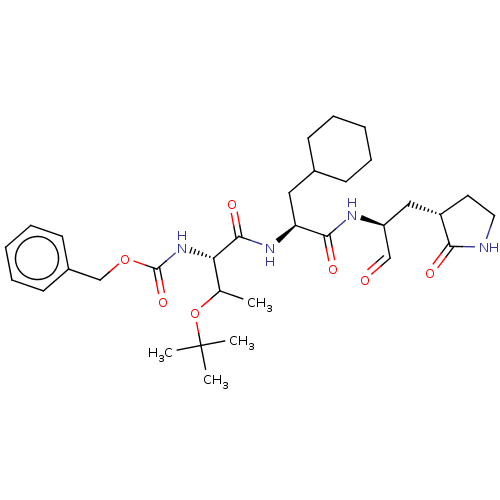
(MPI8 | jm5b01461, Compound 45)Show SMILES CC(OC(C)(C)C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C=O Show InChI InChI=1S/C32H48N4O7/c1-21(43-32(2,3)4)27(36-31(41)42-20-23-13-9-6-10-14-23)30(40)35-26(17-22-11-7-5-8-12-22)29(39)34-25(19-37)18-24-15-16-33-28(24)38/h6,9-10,13-14,19,21-22,24-27H,5,7-8,11-12,15-18,20H2,1-4H3,(H,33,38)(H,34,39)(H,35,40)(H,36,41)/t21?,24-,25-,26-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 103 | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University
| Assay Description
The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... |
ChemMedChem (2020)
Article DOI: 10.1002/cmdc.202000924
BindingDB Entry DOI: 10.7270/Q2G163V1 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50340758

((S)-6-(4-fluorobenzylamino)-2-((R)-2-(4-fluorophen...)Show SMILES CO[C@H](C[C@H](CCCCNCc1ccc(F)cc1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C22H28F2N2O3/c1-29-21(17-7-11-20(24)12-8-17)14-18(22(27)26-28)4-2-3-13-25-15-16-5-9-19(23)10-6-16/h5-12,18,21,25,28H,2-4,13-15H2,1H3,(H,26,27)/t18-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-12 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM484198
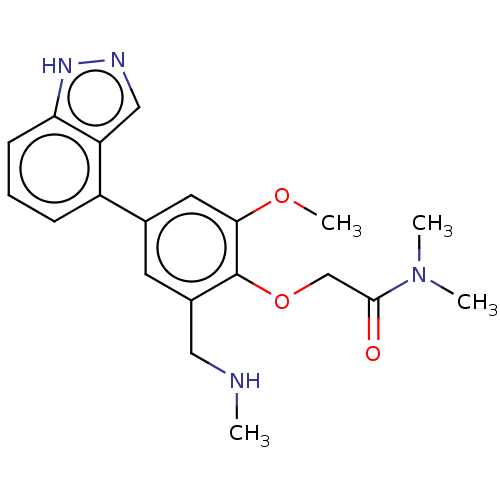
(CDD-1777)Show SMILES CNCc1cc(cc(OC)c1OCC(=O)N(C)C)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C20H24N4O3/c1-21-10-14-8-13(15-6-5-7-17-16(15)11-22-23-17)9-18(26-4)20(14)27-12-19(25)24(2)3/h5-9,11,21H,10,12H2,1-4H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
| Assay Description
To determine the Ki values of active compounds, 25 nM Mpro was mixed with increasing concentrations of compounds (from 4 nM to 4,000 nM with twofold ... |
Proc Natl Acad Sci U S A 118: (2021)
Article DOI: 10.1073/pnas.2111172118
BindingDB Entry DOI: 10.7270/Q2W380FK |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50379533

(CHEMBL2012838)Show SMILES CCC(NCCCC[C@@H](C[C@@H](OC)c1ccc(F)cc1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H32F2N2O3/c1-3-22(17-7-11-20(25)12-8-17)27-15-5-4-6-19(24(29)28-30)16-23(31-2)18-9-13-21(26)14-10-18/h7-14,19,22-23,27,30H,3-6,15-16H2,1-2H3,(H,28,29)/t19-,22?,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-9 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50379533

(CHEMBL2012838)Show SMILES CCC(NCCCC[C@@H](C[C@@H](OC)c1ccc(F)cc1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H32F2N2O3/c1-3-22(17-7-11-20(25)12-8-17)27-15-5-4-6-19(24(29)28-30)16-23(31-2)18-9-13-21(26)14-10-18/h7-14,19,22-23,27,30H,3-6,15-16H2,1-2H3,(H,28,29)/t19-,22?,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-12 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50340758

((S)-6-(4-fluorobenzylamino)-2-((R)-2-(4-fluorophen...)Show SMILES CO[C@H](C[C@H](CCCCNCc1ccc(F)cc1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C22H28F2N2O3/c1-29-21(17-7-11-20(24)12-8-17)14-18(22(27)26-28)4-2-3-13-25-15-16-5-9-19(23)10-6-16/h5-12,18,21,25,28H,2-4,13-15H2,1H3,(H,26,27)/t18-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-9 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50379533

(CHEMBL2012838)Show SMILES CCC(NCCCC[C@@H](C[C@@H](OC)c1ccc(F)cc1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H32F2N2O3/c1-3-22(17-7-11-20(25)12-8-17)27-15-5-4-6-19(24(29)28-30)16-23(31-2)18-9-13-21(26)14-10-18/h7-14,19,22-23,27,30H,3-6,15-16H2,1-2H3,(H,28,29)/t19-,22?,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-3 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50340758

((S)-6-(4-fluorobenzylamino)-2-((R)-2-(4-fluorophen...)Show SMILES CO[C@H](C[C@H](CCCCNCc1ccc(F)cc1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C22H28F2N2O3/c1-29-21(17-7-11-20(24)12-8-17)14-18(22(27)26-28)4-2-3-13-25-15-16-5-9-19(23)10-6-16/h5-12,18,21,25,28H,2-4,13-15H2,1H3,(H,26,27)/t18-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-1 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50379533

(CHEMBL2012838)Show SMILES CCC(NCCCC[C@@H](C[C@@H](OC)c1ccc(F)cc1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H32F2N2O3/c1-3-22(17-7-11-20(25)12-8-17)27-15-5-4-6-19(24(29)28-30)16-23(31-2)18-9-13-21(26)14-10-18/h7-14,19,22-23,27,30H,3-6,15-16H2,1-2H3,(H,28,29)/t19-,22?,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-1 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50340768

((R)-2-(4-fluoro-3,5-dimethylphenoxy)-6-(4-fluorobe...)Show SMILES Cc1cc(O[C@H](CCCCNCc2ccc(F)cc2)C(=O)NO)cc(C)c1F |r| Show InChI InChI=1S/C21H26F2N2O3/c1-14-11-18(12-15(2)20(14)23)28-19(21(26)25-27)5-3-4-10-24-13-16-6-8-17(22)9-7-16/h6-9,11-12,19,24,27H,3-5,10,13H2,1-2H3,(H,25,26)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-12 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50379535

(CHEMBL2010824)Show SMILES CCC(NCCCC[C@@H](Cc1cc(C)c(F)c(C)c1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H32F2N2O2/c1-4-22(19-8-10-21(25)11-9-19)27-12-6-5-7-20(24(29)28-30)15-18-13-16(2)23(26)17(3)14-18/h8-11,13-14,20,22,27,30H,4-7,12,15H2,1-3H3,(H,28,29)/t20-,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-12 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50340754

((S)-2-(4-fluoro-3,5-dimethylbenzyl)-6-(4-fluoroben...)Show SMILES Cc1cc(C[C@H](CCCCNCc2ccc(F)cc2)C(=O)NO)cc(C)c1F |r| Show InChI InChI=1S/C22H28F2N2O2/c1-15-11-18(12-16(2)21(15)24)13-19(22(27)26-28)5-3-4-10-25-14-17-6-8-20(23)9-7-17/h6-9,11-12,19,25,28H,3-5,10,13-14H2,1-2H3,(H,26,27)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-12 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50379536

(CHEMBL2012836)Show SMILES CCC(NCCCC[C@@H](Nc1cc(C)c(F)c(C)c1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C23H31F2N3O2/c1-4-20(17-8-10-18(24)11-9-17)26-12-6-5-7-21(23(29)28-30)27-19-13-15(2)22(25)16(3)14-19/h8-11,13-14,20-21,26-27,30H,4-7,12H2,1-3H3,(H,28,29)/t20?,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-12 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50329265

((R)-2-(4-fluoro-3,5-dimethylphenylamino)-6-(4-fluo...)Show SMILES Cc1cc(N[C@H](CCCCNCc2ccc(F)cc2)C(=O)NO)cc(C)c1F |r| Show InChI InChI=1S/C21H27F2N3O2/c1-14-11-18(12-15(2)20(14)23)25-19(21(27)26-28)5-3-4-10-24-13-16-6-8-17(22)9-7-16/h6-9,11-12,19,24-25,28H,3-5,10,13H2,1-2H3,(H,26,27)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-12 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50379534

(CHEMBL2012837)Show SMILES CCC(NCCCC[C@@H](Oc1cc(C)c(F)c(C)c1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C23H30F2N2O3/c1-4-20(17-8-10-18(24)11-9-17)26-12-6-5-7-21(23(28)27-29)30-19-13-15(2)22(25)16(3)14-19/h8-11,13-14,20-21,26,29H,4-7,12H2,1-3H3,(H,27,28)/t20?,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-9 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50340768

((R)-2-(4-fluoro-3,5-dimethylphenoxy)-6-(4-fluorobe...)Show SMILES Cc1cc(O[C@H](CCCCNCc2ccc(F)cc2)C(=O)NO)cc(C)c1F |r| Show InChI InChI=1S/C21H26F2N2O3/c1-14-11-18(12-15(2)20(14)23)28-19(21(26)25-27)5-3-4-10-24-13-16-6-8-17(22)9-7-16/h6-9,11-12,19,24,27H,3-5,10,13H2,1-2H3,(H,25,26)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-9 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50340754

((S)-2-(4-fluoro-3,5-dimethylbenzyl)-6-(4-fluoroben...)Show SMILES Cc1cc(C[C@H](CCCCNCc2ccc(F)cc2)C(=O)NO)cc(C)c1F |r| Show InChI InChI=1S/C22H28F2N2O2/c1-15-11-18(12-16(2)21(15)24)13-19(22(27)26-28)5-3-4-10-25-14-17-6-8-20(23)9-7-17/h6-9,11-12,19,25,28H,3-5,10,13-14H2,1-2H3,(H,26,27)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-9 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50379536

(CHEMBL2012836)Show SMILES CCC(NCCCC[C@@H](Nc1cc(C)c(F)c(C)c1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C23H31F2N3O2/c1-4-20(17-8-10-18(24)11-9-17)26-12-6-5-7-21(23(29)28-30)27-19-13-15(2)22(25)16(3)14-19/h8-11,13-14,20-21,26-27,30H,4-7,12H2,1-3H3,(H,28,29)/t20?,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-9 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50329265

((R)-2-(4-fluoro-3,5-dimethylphenylamino)-6-(4-fluo...)Show SMILES Cc1cc(N[C@H](CCCCNCc2ccc(F)cc2)C(=O)NO)cc(C)c1F |r| Show InChI InChI=1S/C21H27F2N3O2/c1-14-11-18(12-15(2)20(14)23)25-19(21(27)26-28)5-3-4-10-24-13-16-6-8-17(22)9-7-16/h6-9,11-12,19,24-25,28H,3-5,10,13H2,1-2H3,(H,26,27)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-9 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50340758

((S)-6-(4-fluorobenzylamino)-2-((R)-2-(4-fluorophen...)Show SMILES CO[C@H](C[C@H](CCCCNCc1ccc(F)cc1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C22H28F2N2O3/c1-29-21(17-7-11-20(24)12-8-17)14-18(22(27)26-28)4-2-3-13-25-15-16-5-9-19(23)10-6-16/h5-12,18,21,25,28H,2-4,13-15H2,1H3,(H,26,27)/t18-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-3 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50379534

(CHEMBL2012837)Show SMILES CCC(NCCCC[C@@H](Oc1cc(C)c(F)c(C)c1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C23H30F2N2O3/c1-4-20(17-8-10-18(24)11-9-17)26-12-6-5-7-21(23(28)27-29)30-19-13-15(2)22(25)16(3)14-19/h8-11,13-14,20-21,26,29H,4-7,12H2,1-3H3,(H,27,28)/t20?,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-3 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data