 Found 1151 hits with Last Name = 'matthews' and Initial = 'd'
Found 1151 hits with Last Name = 'matthews' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50046660

(2-[4-(2-Amino-6-methyl-4-oxo-3,4-dihydro-quinazoli...)Show SMILES Cc1ccc2nc(N)[nH]c(=O)c2c1Sc1ccc(cc1)C(=O)N[C@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C21H20N4O6S/c1-10-2-7-13-16(19(29)25-21(22)24-13)17(10)32-12-5-3-11(4-6-12)18(28)23-14(20(30)31)8-9-15(26)27/h2-7,14H,8-9H2,1H3,(H,23,28)(H,26,27)(H,30,31)(H3,22,24,25,29)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for TS activity against human Thymidylate synthase by tight binding kinetics |
J Med Chem 36: 733-46 (1993)
BindingDB Entry DOI: 10.7270/Q2HM57JF |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50367916

(METHYLTRIENOLONE | Metribolone | R-1881)Show SMILES C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3C=C[C@]12C |r,c:16,19,t:9| Show InChI InChI=1S/C19H24O2/c1-18-9-7-15-14-6-4-13(20)11-12(14)3-5-16(15)17(18)8-10-19(18,2)21/h7,9,11,16-17,21H,3-6,8,10H2,1-2H3/t16-,17+,18+,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methyltrienolone from androgen receptor (unknown origin) expressed in HEK293 cell lysate incubated overnight by microbeta scinti... |
J Med Chem 59: 750-5 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01168
BindingDB Entry DOI: 10.7270/Q2H70HQ9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50367916

(METHYLTRIENOLONE | Metribolone | R-1881)Show SMILES C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3C=C[C@]12C |r,c:16,19,t:9| Show InChI InChI=1S/C19H24O2/c1-18-9-7-15-14-6-4-13(20)11-12(14)3-5-16(15)17(18)8-10-19(18,2)21/h7,9,11,16-17,21H,3-6,8,10H2,1-2H3/t16-,17+,18+,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methyltrienolone from progesterone receptor (unknown origin) expressed in HEK293 cell lysate incubated overnight by microbeta sc... |
J Med Chem 59: 750-5 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01168
BindingDB Entry DOI: 10.7270/Q2H70HQ9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50367916

(METHYLTRIENOLONE | Metribolone | R-1881)Show SMILES C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3C=C[C@]12C |r,c:16,19,t:9| Show InChI InChI=1S/C19H24O2/c1-18-9-7-15-14-6-4-13(20)11-12(14)3-5-16(15)17(18)8-10-19(18,2)21/h7,9,11,16-17,21H,3-6,8,10H2,1-2H3/t16-,17+,18+,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-aldosterone from mineralocorticoid receptor (unknown origin) expressed in HEK293 cell lysate incubated overnight by microbeta sc... |
J Med Chem 59: 750-5 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01168
BindingDB Entry DOI: 10.7270/Q2H70HQ9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50145862
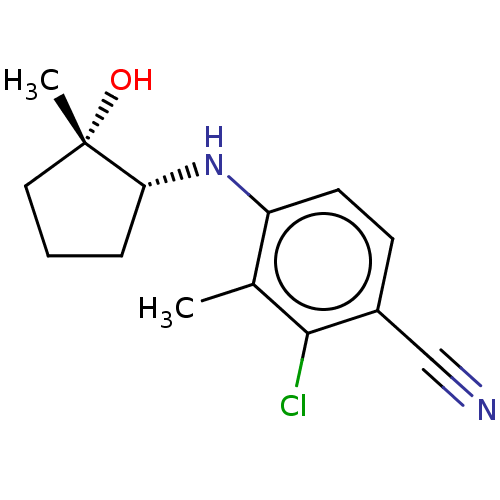
(CHEMBL3765171)Show InChI InChI=1S/C14H17ClN2O/c1-9-11(6-5-10(8-16)13(9)15)17-12-4-3-7-14(12,2)18/h5-6,12,17-18H,3-4,7H2,1-2H3/t12-,14+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methyltrienolone from androgen receptor (unknown origin) expressed in HEK293 cell lysate incubated overnight by microbeta scinti... |
J Med Chem 59: 750-5 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01168
BindingDB Entry DOI: 10.7270/Q2H70HQ9 |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(Dog) | BDBM50026636

(17alpha-hydroxy-20alpha-yohimban-16beta-carboxylic...)Show SMILES COC(=O)[C@@H]1[C@@H](O)CC[C@@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15+,17+,18+,19+/m1/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 236: 90-6 (1986)
BindingDB Entry DOI: 10.7270/Q2T72FXQ |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50046665
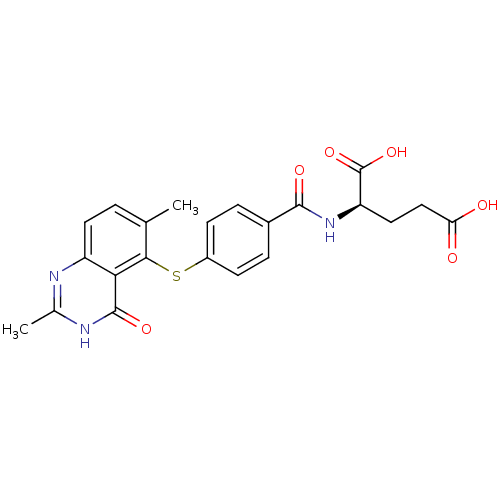
(2-[4-(2,6-Dimethyl-4-oxo-3,4-dihydro-quinazolin-5-...)Show SMILES Cc1nc2ccc(C)c(Sc3ccc(cc3)C(=O)N[C@H](CCC(O)=O)C(O)=O)c2c(=O)[nH]1 Show InChI InChI=1S/C22H21N3O6S/c1-11-3-8-15-18(21(29)24-12(2)23-15)19(11)32-14-6-4-13(5-7-14)20(28)25-16(22(30)31)9-10-17(26)27/h3-8,16H,9-10H2,1-2H3,(H,25,28)(H,26,27)(H,30,31)(H,23,24,29)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for TS activity against human Thymidylate synthase by tight binding kinetics |
J Med Chem 36: 733-46 (1993)
BindingDB Entry DOI: 10.7270/Q2HM57JF |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50145863
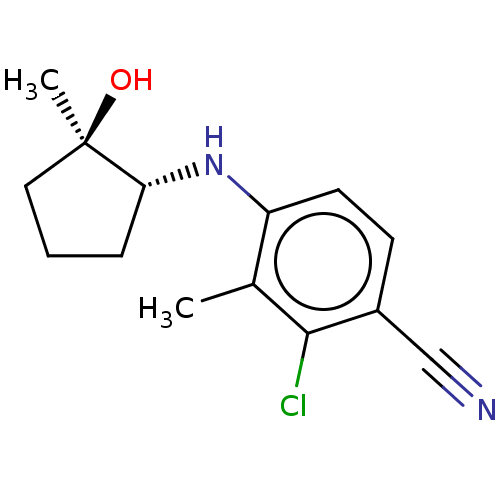
(CHEMBL3764185)Show SMILES Cc1c(N[C@@H]2CCC[C@@]2(C)O)ccc(C#N)c1Cl |r| Show InChI InChI=1S/C14H17ClN2O/c1-9-11(6-5-10(8-16)13(9)15)17-12-4-3-7-14(12,2)18/h5-6,12,17-18H,3-4,7H2,1-2H3/t12-,14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methyltrienolone from androgen receptor (unknown origin) expressed in HEK293 cell lysate incubated overnight by microbeta scinti... |
J Med Chem 59: 750-5 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01168
BindingDB Entry DOI: 10.7270/Q2H70HQ9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-2B adrenergic receptor
(Dog) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 236: 90-6 (1986)
BindingDB Entry DOI: 10.7270/Q2T72FXQ |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(RAT) | BDBM50019848
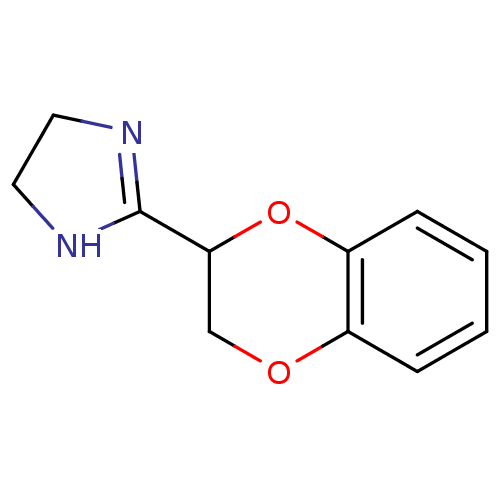
(2-(2,3-dihydro-1,4-benzodioxin-2-yl)-4,5-dihydro-1...)Show InChI InChI=1S/C11H12N2O2/c1-2-4-9-8(3-1)14-7-10(15-9)11-12-5-6-13-11/h1-4,10H,5-7H2,(H,12,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 236: 90-6 (1986)
BindingDB Entry DOI: 10.7270/Q2T72FXQ |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50055234
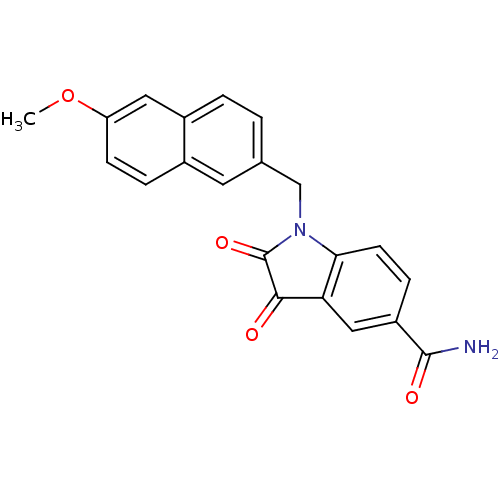
(1-(6-Methoxy-naphthalen-2-ylmethyl)-2,3-dioxo-2,3-...)Show SMILES COc1ccc2cc(CN3C(=O)C(=O)c4cc(ccc34)C(N)=O)ccc2c1 Show InChI InChI=1S/C21H16N2O4/c1-27-16-6-4-13-8-12(2-3-14(13)9-16)11-23-18-7-5-15(20(22)25)10-17(18)19(24)21(23)26/h2-10H,11H2,1H3,(H2,22,25) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human rhinovirus 3C protease |
J Med Chem 39: 5072-82 (1997)
Article DOI: 10.1021/jm960603e
BindingDB Entry DOI: 10.7270/Q2K936MP |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(GUINEA PIG) | BDBM50019848

(2-(2,3-dihydro-1,4-benzodioxin-2-yl)-4,5-dihydro-1...)Show InChI InChI=1S/C11H12N2O2/c1-2-4-9-8(3-1)14-7-10(15-9)11-12-5-6-13-11/h1-4,10H,5-7H2,(H,12,13) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 236: 90-6 (1986)
BindingDB Entry DOI: 10.7270/Q2T72FXQ |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50055213

(1-(3,5-Dihydroxy-benzyl)-2,3-dioxo-2,3-dihydro-1H-...)Show SMILES NC(=O)c1ccc2N(Cc3cc(O)cc(O)c3)C(=O)C(=O)c2c1 Show InChI InChI=1S/C16H12N2O5/c17-15(22)9-1-2-13-12(5-9)14(21)16(23)18(13)7-8-3-10(19)6-11(20)4-8/h1-6,19-20H,7H2,(H2,17,22) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human rhinovirus 3C protease |
J Med Chem 39: 5072-82 (1997)
Article DOI: 10.1021/jm960603e
BindingDB Entry DOI: 10.7270/Q2K936MP |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50055232

(1-(6-Hydroxy-naphthalen-2-ylmethyl)-2,3-dioxo-2,3-...)Show SMILES NC(=O)c1ccc2N(Cc3ccc4cc(O)ccc4c3)C(=O)C(=O)c2c1 Show InChI InChI=1S/C20H14N2O4/c21-19(25)14-4-6-17-16(9-14)18(24)20(26)22(17)10-11-1-2-13-8-15(23)5-3-12(13)7-11/h1-9,23H,10H2,(H2,21,25) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human rhinovirus 3C protease |
J Med Chem 39: 5072-82 (1997)
Article DOI: 10.1021/jm960603e
BindingDB Entry DOI: 10.7270/Q2K936MP |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50055218
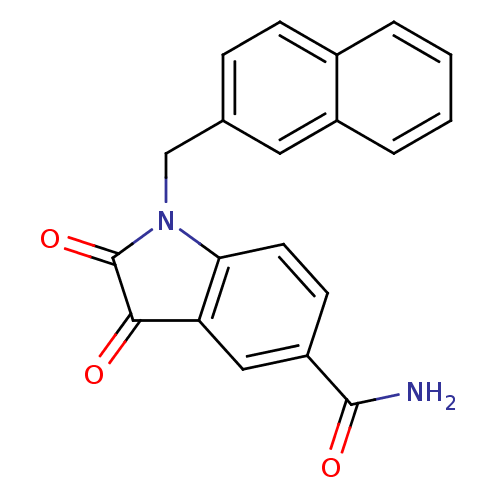
(1-(2-naphthlmethyl) isatin-5-carboxamide | 1-Napht...)Show SMILES NC(=O)c1ccc2N(Cc3ccc4ccccc4c3)C(=O)C(=O)c2c1 Show InChI InChI=1S/C20H14N2O3/c21-19(24)15-7-8-17-16(10-15)18(23)20(25)22(17)11-12-5-6-13-3-1-2-4-14(13)9-12/h1-10H,11H2,(H2,21,24) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human rhinovirus 3C protease |
J Med Chem 39: 5072-82 (1997)
Article DOI: 10.1021/jm960603e
BindingDB Entry DOI: 10.7270/Q2K936MP |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50065598

(CHEMBL419332 | {(S)-1-[(S)-1-((S)-3-Dimethylcarbam...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(=O)N(C)C)C=O Show InChI InChI=1S/C30H40N4O6/c1-21(2)17-25(33-30(39)40-20-23-13-9-6-10-14-23)29(38)32-26(18-22-11-7-5-8-12-22)28(37)31-24(19-35)15-16-27(36)34(3)4/h5-14,19,21,24-26H,15-18,20H2,1-4H3,(H,31,37)(H,32,38)(H,33,39)/t24-,25-,26-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against HRV-14 3C protease. |
J Med Chem 41: 2786-805 (1998)
Article DOI: 10.1021/jm980071x
BindingDB Entry DOI: 10.7270/Q2F76BPF |
More data for this
Ligand-Target Pair | |
Alpha-2A/Alpha-2B/Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM31046
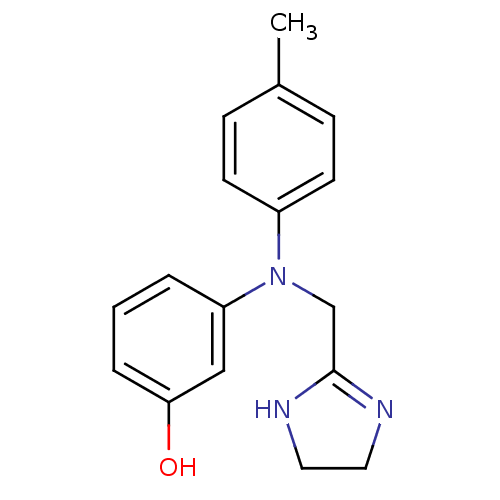
(3-[4,5-dihydro-1H-imidazol-2-ylmethyl-(4-methylphe...)Show InChI InChI=1S/C17H19N3O/c1-13-5-7-14(8-6-13)20(12-17-18-9-10-19-17)15-3-2-4-16(21)11-15/h2-8,11,21H,9-10,12H2,1H3,(H,18,19) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against alpha-2-adrenergic receptor using 10 nM [3H]yohimbine in human platelet membranes from three separate experiments using 10 i... |
J Med Chem 27: 918-21 (1984)
BindingDB Entry DOI: 10.7270/Q2125VWH |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(GUINEA PIG) | BDBM50026636

(17alpha-hydroxy-20alpha-yohimban-16beta-carboxylic...)Show SMILES COC(=O)[C@@H]1[C@@H](O)CC[C@@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15+,17+,18+,19+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 236: 90-6 (1986)
BindingDB Entry DOI: 10.7270/Q2T72FXQ |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM50005335
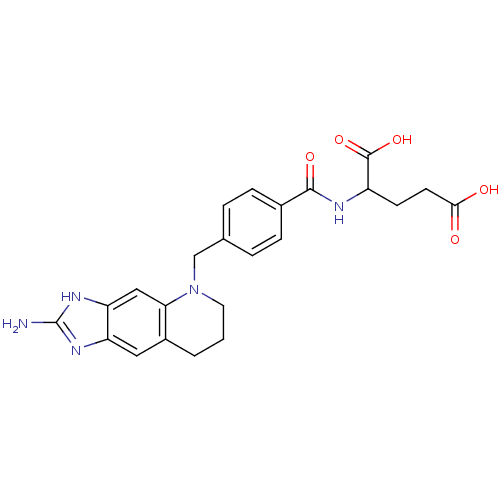
(2-[4-(2-Amino-1,6,7,8-tetrahydro-imidazo[4,5-g]qui...)Show SMILES Nc1nc2cc3CCCN(Cc4ccc(cc4)C(=O)NC(CCC(O)=O)C(O)=O)c3cc2[nH]1 Show InChI InChI=1S/C23H25N5O5/c24-23-26-17-10-15-2-1-9-28(19(15)11-18(17)27-23)12-13-3-5-14(6-4-13)21(31)25-16(22(32)33)7-8-20(29)30/h3-6,10-11,16H,1-2,7-9,12H2,(H,25,31)(H,29,30)(H,32,33)(H3,24,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli Thymidylate synthase (TS) |
J Med Chem 35: 847-58 (1992)
BindingDB Entry DOI: 10.7270/Q2X0660R |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50065588
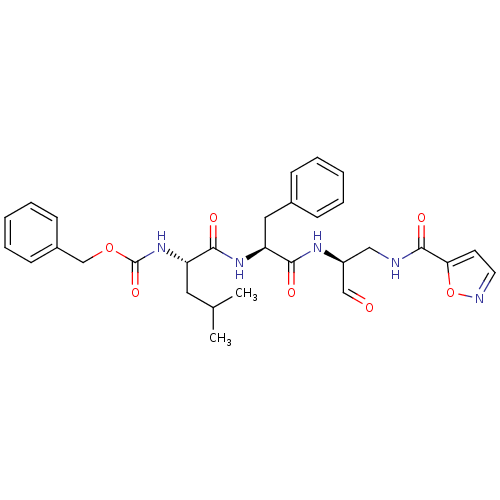
(CHEMBL96803 | [(S)-1-((S)-1-{(S)-1-Formyl-2-[(isox...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CNC(=O)c1ccno1)C=O Show InChI InChI=1S/C30H35N5O7/c1-20(2)15-24(35-30(40)41-19-22-11-7-4-8-12-22)28(38)34-25(16-21-9-5-3-6-10-21)27(37)33-23(18-36)17-31-29(39)26-13-14-32-42-26/h3-14,18,20,23-25H,15-17,19H2,1-2H3,(H,31,39)(H,33,37)(H,34,38)(H,35,40)/t23-,24-,25-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against HRV-14 3C protease. |
J Med Chem 41: 2786-805 (1998)
Article DOI: 10.1021/jm980071x
BindingDB Entry DOI: 10.7270/Q2F76BPF |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50065603
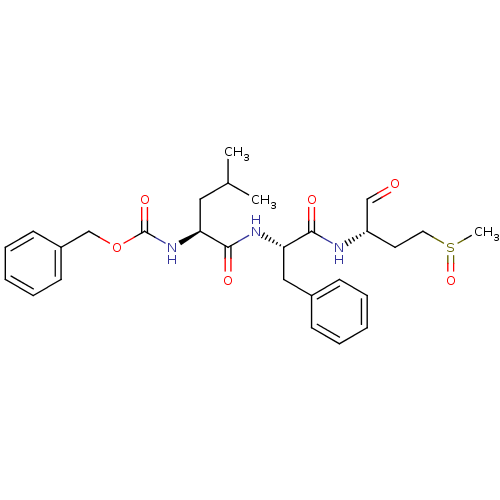
(CHEMBL96185 | {(S)-1-[(S)-1-((S)-1-Formyl-3-methan...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCS(C)=O)C=O Show InChI InChI=1S/C28H37N3O6S/c1-20(2)16-24(31-28(35)37-19-22-12-8-5-9-13-22)27(34)30-25(17-21-10-6-4-7-11-21)26(33)29-23(18-32)14-15-38(3)36/h4-13,18,20,23-25H,14-17,19H2,1-3H3,(H,29,33)(H,30,34)(H,31,35)/t23-,24-,25-,38?/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against HRV-14 3C protease. |
J Med Chem 41: 2786-805 (1998)
Article DOI: 10.1021/jm980071x
BindingDB Entry DOI: 10.7270/Q2F76BPF |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50145860

(CHEMBL3764446)Show SMILES Cc1c(N[C@@H]2COC[C@@]2(C)O)ccc(C#N)c1Cl |r| Show InChI InChI=1S/C13H15ClN2O2/c1-8-10(4-3-9(5-15)12(8)14)16-11-6-18-7-13(11,2)17/h3-4,11,16-17H,6-7H2,1-2H3/t11-,13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-methyltrienolone from androgen receptor (unknown origin) expressed in HEK293 cell lysate incubated overnight by microbeta scinti... |
J Med Chem 59: 750-5 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01168
BindingDB Entry DOI: 10.7270/Q2H70HQ9 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50065590

(((S)-1-{(S)-1-[(S)-1-(Acetylamino-methyl)-2-oxo-et...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CNC(C)=O)C=O Show InChI InChI=1S/C28H36N4O6/c1-19(2)14-24(32-28(37)38-18-22-12-8-5-9-13-22)27(36)31-25(15-21-10-6-4-7-11-21)26(35)30-23(17-33)16-29-20(3)34/h4-13,17,19,23-25H,14-16,18H2,1-3H3,(H,29,34)(H,30,35)(H,31,36)(H,32,37)/t23-,24-,25-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against HRV-14 3C protease. |
J Med Chem 41: 2786-805 (1998)
Article DOI: 10.1021/jm980071x
BindingDB Entry DOI: 10.7270/Q2F76BPF |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(GUINEA PIG) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 236: 90-6 (1986)
BindingDB Entry DOI: 10.7270/Q2T72FXQ |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(RAT) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 236: 90-6 (1986)
BindingDB Entry DOI: 10.7270/Q2T72FXQ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM34012
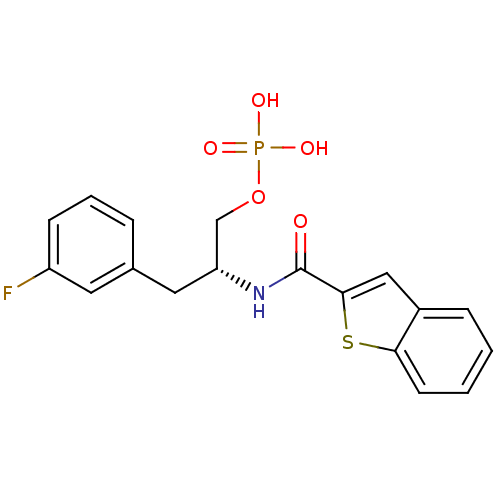
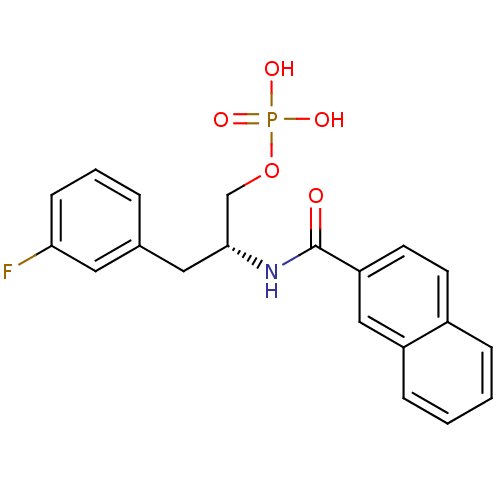
(3-fluorophenylalanine derivative, 21b)Show SMILES OP(O)(=O)OC[C@@H](Cc1cccc(F)c1)NC(=O)c1cc2ccccc2s1 |r| Show InChI InChI=1S/C18H17FNO5PS/c19-14-6-3-4-12(8-14)9-15(11-25-26(22,23)24)20-18(21)17-10-13-5-1-2-7-16(13)27-17/h1-8,10,15H,9,11H2,(H,20,21)(H2,22,23,24)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | -45.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 15 |
Pfizer
| Assay Description
The cis/trans conversion of cis-Suc-AEPFpNA peptide by the PPIase led to the cleavage of para nitroaniline by subtilisin which was monitored at 390 n... |
Bioorg Med Chem Lett 19: 5613-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.034
BindingDB Entry DOI: 10.7270/Q2XD100H |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50055217
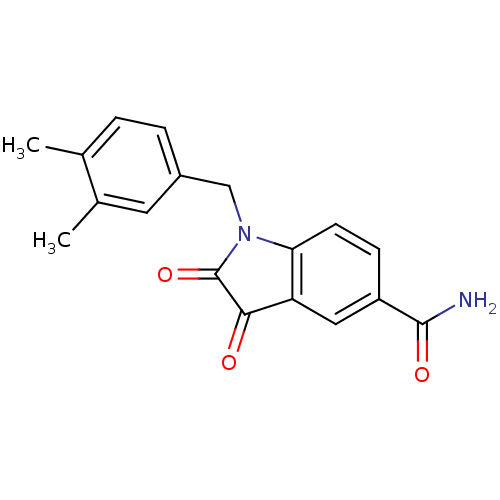
(1-(3,4-Dimethyl-benzyl)-2,3-dioxo-2,3-dihydro-1H-i...)Show InChI InChI=1S/C18H16N2O3/c1-10-3-4-12(7-11(10)2)9-20-15-6-5-13(17(19)22)8-14(15)16(21)18(20)23/h3-8H,9H2,1-2H3,(H2,19,22) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human rhinovirus 3C protease |
J Med Chem 39: 5072-82 (1997)
Article DOI: 10.1021/jm960603e
BindingDB Entry DOI: 10.7270/Q2K936MP |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50005329
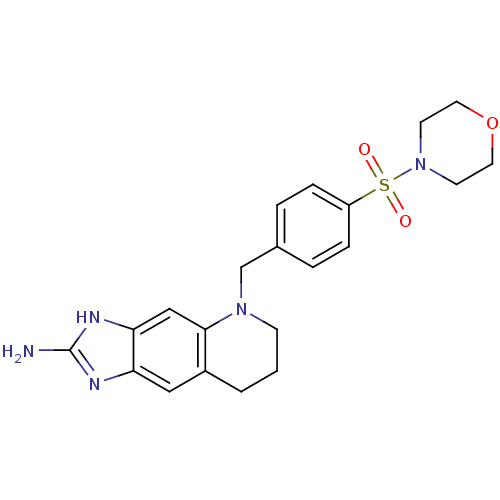
(5-[4-(Morpholine-4-sulfonyl)-benzyl]-5,6,7,8-tetra...)Show SMILES Nc1nc2cc3CCCN(Cc4ccc(cc4)S(=O)(=O)N4CCOCC4)c3cc2[nH]1 Show InChI InChI=1S/C21H25N5O3S/c22-21-23-18-12-16-2-1-7-25(20(16)13-19(18)24-21)14-15-3-5-17(6-4-15)30(27,28)26-8-10-29-11-9-26/h3-6,12-13H,1-2,7-11,14H2,(H3,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase (TS) |
J Med Chem 35: 847-58 (1992)
BindingDB Entry DOI: 10.7270/Q2X0660R |
More data for this
Ligand-Target Pair | |
Alpha-2A/Alpha-2B/Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50225305

(CHEMBL433730)Show InChI InChI=1S/C13H16ClN/c1-2-8-15-9-6-11-4-3-5-13(14)12(11)7-10-15/h2-5H,1,6-10H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against alpha-2-adrenergic receptor using 10 nM [3H]yohimbine in human platelet membranes from three separate experiments using 10 i... |
J Med Chem 27: 918-21 (1984)
BindingDB Entry DOI: 10.7270/Q2125VWH |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50065586
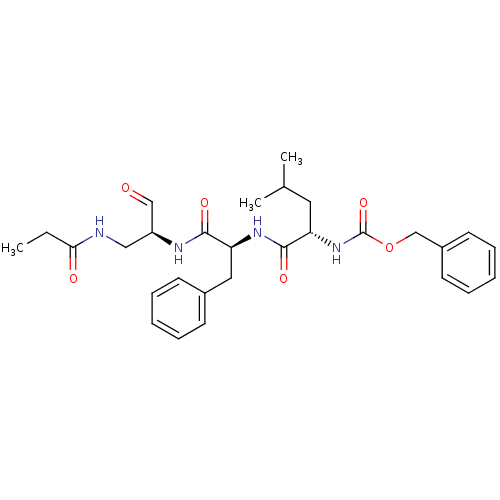
(((S)-3-Methyl-1-{(S)-1-[(S)-2-oxo-1-(propionylamin...)Show SMILES CCC(=O)NC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O Show InChI InChI=1S/C29H38N4O6/c1-4-26(35)30-17-23(18-34)31-27(36)25(16-21-11-7-5-8-12-21)32-28(37)24(15-20(2)3)33-29(38)39-19-22-13-9-6-10-14-22/h5-14,18,20,23-25H,4,15-17,19H2,1-3H3,(H,30,35)(H,31,36)(H,32,37)(H,33,38)/t23-,24-,25-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against HRV-14 3C protease. |
J Med Chem 41: 2786-805 (1998)
Article DOI: 10.1021/jm980071x
BindingDB Entry DOI: 10.7270/Q2F76BPF |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM34011
(3-fluorophenylalanine derivative, 21a)Show SMILES OP(O)(=O)OC[C@@H](Cc1cccc(F)c1)NC(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C20H19FNO5P/c21-18-7-3-4-14(10-18)11-19(13-27-28(24,25)26)22-20(23)17-9-8-15-5-1-2-6-16(15)12-17/h1-10,12,19H,11,13H2,(H,22,23)(H2,24,25,26)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | -44.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 15 |
Pfizer
| Assay Description
The cis/trans conversion of cis-Suc-AEPFpNA peptide by the PPIase led to the cleavage of para nitroaniline by subtilisin which was monitored at 390 n... |
Bioorg Med Chem Lett 19: 5613-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.034
BindingDB Entry DOI: 10.7270/Q2XD100H |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(GUINEA PIG) | BDBM50026636

(17alpha-hydroxy-20alpha-yohimban-16beta-carboxylic...)Show SMILES COC(=O)[C@@H]1[C@@H](O)CC[C@@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15+,17+,18+,19+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 236: 90-6 (1986)
BindingDB Entry DOI: 10.7270/Q2T72FXQ |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM50005324

(4-[4-(2-Amino-1,6,7,8-tetrahydro-imidazo[4,5-g]qui...)Show SMILES Nc1nc2cc3CCCN(Cc4ccc(cc4)S(=O)(=O)c4ccc(O)cc4)c3cc2[nH]1 Show InChI InChI=1S/C23H22N4O3S/c24-23-25-20-12-16-2-1-11-27(22(16)13-21(20)26-23)14-15-3-7-18(8-4-15)31(29,30)19-9-5-17(28)6-10-19/h3-10,12-13,28H,1-2,11,14H2,(H3,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli Thymidylate synthase (TS) |
J Med Chem 35: 847-58 (1992)
BindingDB Entry DOI: 10.7270/Q2X0660R |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50367916

(METHYLTRIENOLONE | Metribolone | R-1881)Show SMILES C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3C=C[C@]12C |r,c:16,19,t:9| Show InChI InChI=1S/C19H24O2/c1-18-9-7-15-14-6-4-13(20)11-12(14)3-5-16(15)17(18)8-10-19(18,2)21/h7,9,11,16-17,21H,3-6,8,10H2,1-2H3/t16-,17+,18+,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dexamethasone from glucocorticoid receptor (unknown origin) expressed in HEK293 cell lysate incubated overnight by microbeta sci... |
J Med Chem 59: 750-5 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01168
BindingDB Entry DOI: 10.7270/Q2H70HQ9 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50055225

(1-(4-Methyl-benzyl)-2,3-dioxo-2,3-dihydro-1H-indol...)Show InChI InChI=1S/C17H14N2O3/c1-10-2-4-11(5-3-10)9-19-14-7-6-12(16(18)21)8-13(14)15(20)17(19)22/h2-8H,9H2,1H3,(H2,18,21) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human rhinovirus 3C protease |
J Med Chem 39: 5072-82 (1997)
Article DOI: 10.1021/jm960603e
BindingDB Entry DOI: 10.7270/Q2K936MP |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50065595
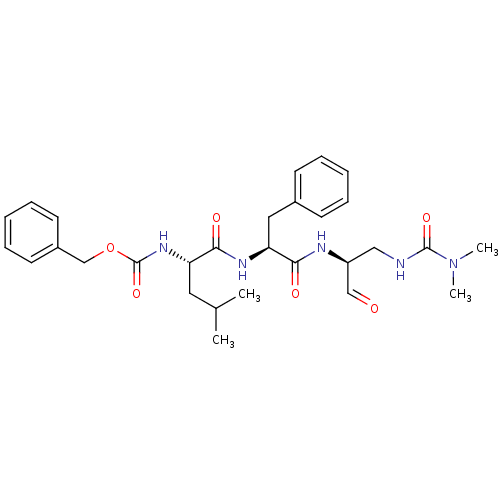
(((S)-1-{(S)-1-[(S)-2-(3,3-Dimethyl-ureido)-1-formy...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CNC(=O)N(C)C)C=O Show InChI InChI=1S/C29H39N5O6/c1-20(2)15-24(33-29(39)40-19-22-13-9-6-10-14-22)27(37)32-25(16-21-11-7-5-8-12-21)26(36)31-23(18-35)17-30-28(38)34(3)4/h5-14,18,20,23-25H,15-17,19H2,1-4H3,(H,30,38)(H,31,36)(H,32,37)(H,33,39)/t23-,24-,25-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against HRV-14 3C protease. |
J Med Chem 41: 2786-805 (1998)
Article DOI: 10.1021/jm980071x
BindingDB Entry DOI: 10.7270/Q2F76BPF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50005335

(2-[4-(2-Amino-1,6,7,8-tetrahydro-imidazo[4,5-g]qui...)Show SMILES Nc1nc2cc3CCCN(Cc4ccc(cc4)C(=O)NC(CCC(O)=O)C(O)=O)c3cc2[nH]1 Show InChI InChI=1S/C23H25N5O5/c24-23-26-17-10-15-2-1-9-28(19(15)11-18(17)27-23)12-13-3-5-14(6-4-13)21(31)25-16(22(32)33)7-8-20(29)30/h3-6,10-11,16H,1-2,7-9,12H2,(H,25,31)(H,29,30)(H,32,33)(H3,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase (TS) |
J Med Chem 35: 847-58 (1992)
BindingDB Entry DOI: 10.7270/Q2X0660R |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50055212
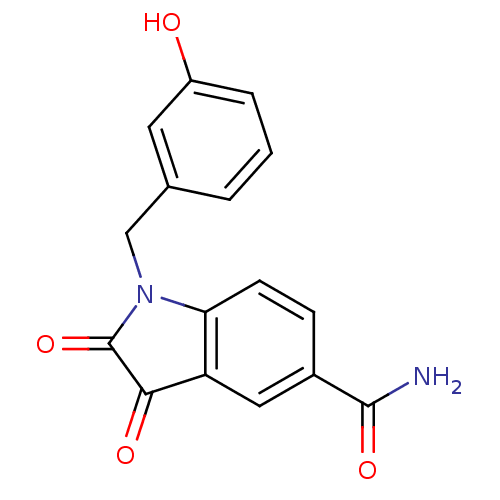
(1-(3-Hydroxy-benzyl)-2,3-dioxo-2,3-dihydro-1H-indo...)Show InChI InChI=1S/C16H12N2O4/c17-15(21)10-4-5-13-12(7-10)14(20)16(22)18(13)8-9-2-1-3-11(19)6-9/h1-7,19H,8H2,(H2,17,21) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human rhinovirus 3C protease |
J Med Chem 39: 5072-82 (1997)
Article DOI: 10.1021/jm960603e
BindingDB Entry DOI: 10.7270/Q2K936MP |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM50005329

(5-[4-(Morpholine-4-sulfonyl)-benzyl]-5,6,7,8-tetra...)Show SMILES Nc1nc2cc3CCCN(Cc4ccc(cc4)S(=O)(=O)N4CCOCC4)c3cc2[nH]1 Show InChI InChI=1S/C21H25N5O3S/c22-21-23-18-12-16-2-1-7-25(20(16)13-19(18)24-21)14-15-3-5-17(6-4-15)30(27,28)26-8-10-29-11-9-26/h3-6,12-13H,1-2,7-11,14H2,(H3,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli Thymidylate synthase (TS) |
J Med Chem 35: 847-58 (1992)
BindingDB Entry DOI: 10.7270/Q2X0660R |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50046646
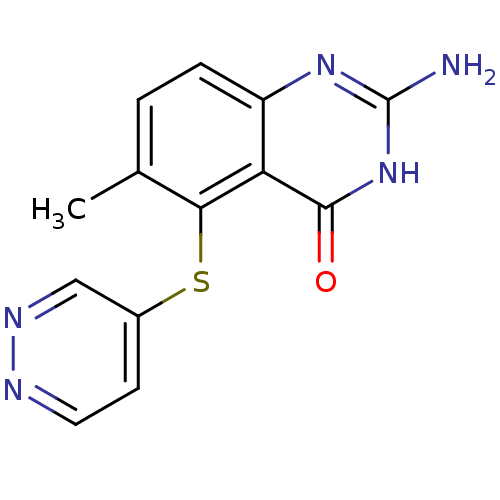
(2-Amino-6-methyl-5-(pyridazin-4-ylsulfanyl)-3H-qui...)Show InChI InChI=1S/C13H11N5OS/c1-7-2-3-9-10(12(19)18-13(14)17-9)11(7)20-8-4-5-15-16-6-8/h2-6H,1H3,(H3,14,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase |
J Med Chem 36: 733-46 (1993)
BindingDB Entry DOI: 10.7270/Q2HM57JF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50011244

(6-{[(4-Benzenesulfonyl-phenyl)-prop-2-ynyl-amino]-...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(cc3)S(=O)(=O)c3ccccc3)cc2c(=O)[nH]1 Show InChI InChI=1S/C25H21N3O3S/c1-3-15-28(17-19-9-14-24-23(16-19)25(29)27-18(2)26-24)20-10-12-22(13-11-20)32(30,31)21-7-5-4-6-8-21/h1,4-14,16H,15,17H2,2H3,(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against recombinant human thymidylate synthase |
J Med Chem 40: 677-83 (1997)
Article DOI: 10.1021/jm960613f
BindingDB Entry DOI: 10.7270/Q25X2810 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50065599

(CHEMBL94652 | {(S)-1-[(S)-1-((S)-2-Benzoylamino-1-...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CNC(=O)c1ccccc1)C=O Show InChI InChI=1S/C33H38N4O6/c1-23(2)18-28(37-33(42)43-22-25-14-8-4-9-15-25)32(41)36-29(19-24-12-6-3-7-13-24)31(40)35-27(21-38)20-34-30(39)26-16-10-5-11-17-26/h3-17,21,23,27-29H,18-20,22H2,1-2H3,(H,34,39)(H,35,40)(H,36,41)(H,37,42)/t27-,28-,29-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against HRV-14 3C protease. |
J Med Chem 41: 2786-805 (1998)
Article DOI: 10.1021/jm980071x
BindingDB Entry DOI: 10.7270/Q2F76BPF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50011244

(6-{[(4-Benzenesulfonyl-phenyl)-prop-2-ynyl-amino]-...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(cc3)S(=O)(=O)c3ccccc3)cc2c(=O)[nH]1 Show InChI InChI=1S/C25H21N3O3S/c1-3-15-28(17-19-9-14-24-23(16-19)25(29)27-18(2)26-24)20-10-12-22(13-11-20)32(30,31)21-7-5-4-6-8-21/h1,4-14,16H,15,17H2,2H3,(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against recombinant human thymidylate synthase |
J Med Chem 40: 677-83 (1997)
Article DOI: 10.1021/jm960613f
BindingDB Entry DOI: 10.7270/Q25X2810 |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Mus musculus (Mouse)) | BDBM50019848

(2-(2,3-dihydro-1,4-benzodioxin-2-yl)-4,5-dihydro-1...)Show InChI InChI=1S/C11H12N2O2/c1-2-4-9-8(3-1)14-7-10(15-9)11-12-5-6-13-11/h1-4,10H,5-7H2,(H,12,13) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 236: 90-6 (1986)
BindingDB Entry DOI: 10.7270/Q2T72FXQ |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(RAT) | BDBM50026636

(17alpha-hydroxy-20alpha-yohimban-16beta-carboxylic...)Show SMILES COC(=O)[C@@H]1[C@@H](O)CC[C@@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15+,17+,18+,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 236: 90-6 (1986)
BindingDB Entry DOI: 10.7270/Q2T72FXQ |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(GUINEA PIG) | BDBM66983
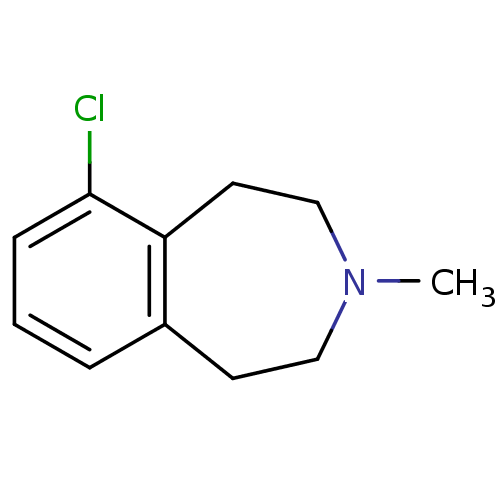
(6-chloranyl-3-methyl-1,2,4,5-tetrahydro-3-benzazep...)Show InChI InChI=1S/C11H14ClN/c1-13-7-5-9-3-2-4-11(12)10(9)6-8-13/h2-4H,5-8H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 236: 90-6 (1986)
BindingDB Entry DOI: 10.7270/Q2T72FXQ |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50005330
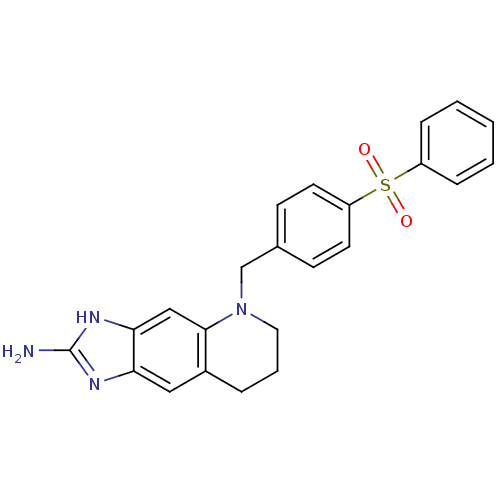
(5-(4-Benzenesulfonyl-benzyl)-5,6,7,8-tetrahydro-1H...)Show SMILES Nc1nc2cc3CCCN(Cc4ccc(cc4)S(=O)(=O)c4ccccc4)c3cc2[nH]1 Show InChI InChI=1S/C23H22N4O2S/c24-23-25-20-13-17-5-4-12-27(22(17)14-21(20)26-23)15-16-8-10-19(11-9-16)30(28,29)18-6-2-1-3-7-18/h1-3,6-11,13-14H,4-5,12,15H2,(H3,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase (TS) |
J Med Chem 35: 847-58 (1992)
BindingDB Entry DOI: 10.7270/Q2X0660R |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(GUINEA PIG) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 236: 90-6 (1986)
BindingDB Entry DOI: 10.7270/Q2T72FXQ |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50046657
(2-Amino-6-methyl-5-(pyridin-4-ylsulfanyl)-3H-quina...)Show InChI InChI=1S/C14H12N4OS/c1-8-2-3-10-11(13(19)18-14(15)17-10)12(8)20-9-4-6-16-7-5-9/h2-7H,1H3,(H3,15,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase |
J Med Chem 36: 733-46 (1993)
BindingDB Entry DOI: 10.7270/Q2HM57JF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50046657

(2-Amino-6-methyl-5-(pyridin-4-ylsulfanyl)-3H-quina...)Show InChI InChI=1S/C14H12N4OS/c1-8-2-3-10-11(13(19)18-14(15)17-10)12(8)20-9-4-6-16-7-5-9/h2-7H,1H3,(H3,15,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase |
J Med Chem 36: 733-46 (1993)
BindingDB Entry DOI: 10.7270/Q2HM57JF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data