

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor (RABBIT) | BDBM50282276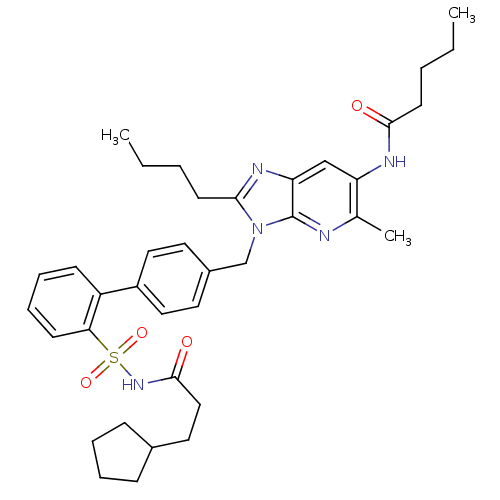 (CHEMBL304947 | Pentanoic acid {2-butyl-3-[2'-(3-cy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity determined by its ability to displace the specific binding ligand [125I]-Sar1, Ile8-AII from Angiotensin II receptor, type ... | Bioorg Med Chem Lett 4: 17-22 (1994) Article DOI: 10.1016/S0960-894X(01)81115-X BindingDB Entry DOI: 10.7270/Q2G73DPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50282274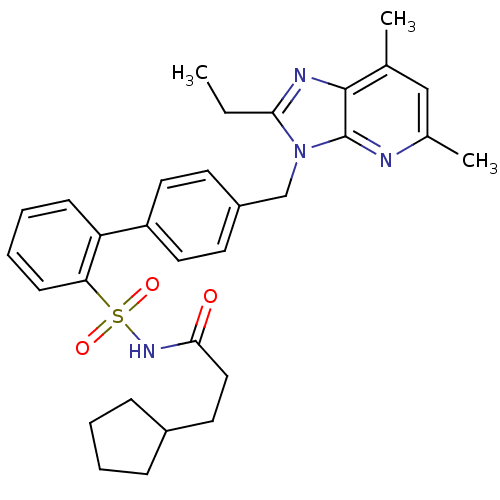 (4'-(2-Ethyl-5,7-dimethyl-imidazo[4,5-b]pyridin-3-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity determined by its ability to displace the specific binding ligand [125I]-Sar1, Ile8-AII from Angiotensin II receptor, type ... | Bioorg Med Chem Lett 4: 17-22 (1994) Article DOI: 10.1016/S0960-894X(01)81115-X BindingDB Entry DOI: 10.7270/Q2G73DPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50282277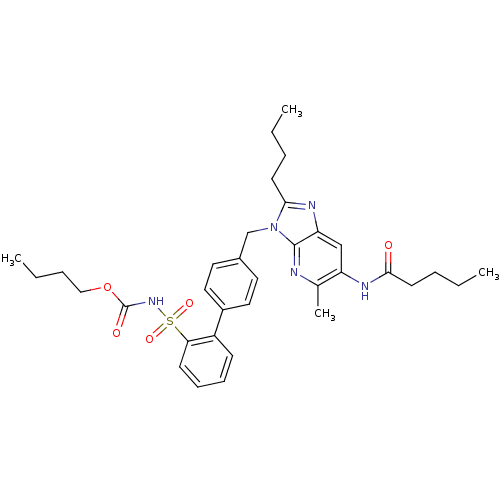 (CHEMBL306407 | Imidazo[4,5-b]pyridine derivative) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity determined by its ability to displace the specific binding ligand [125I]-Sar1, Ile8-AII from Angiotensin II receptor, type ... | Bioorg Med Chem Lett 4: 17-22 (1994) Article DOI: 10.1016/S0960-894X(01)81115-X BindingDB Entry DOI: 10.7270/Q2G73DPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50282281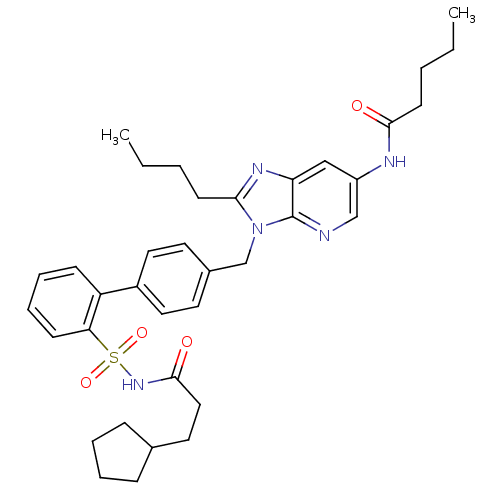 (CHEMBL68618 | Pentanoic acid {2-butyl-3-[2'-(3-cyc...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity determined by its ability to displace the specific binding ligand [125I]-Sar1, Ile8-AII from Angiotensin II receptor, type ... | Bioorg Med Chem Lett 4: 17-22 (1994) Article DOI: 10.1016/S0960-894X(01)81115-X BindingDB Entry DOI: 10.7270/Q2G73DPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123235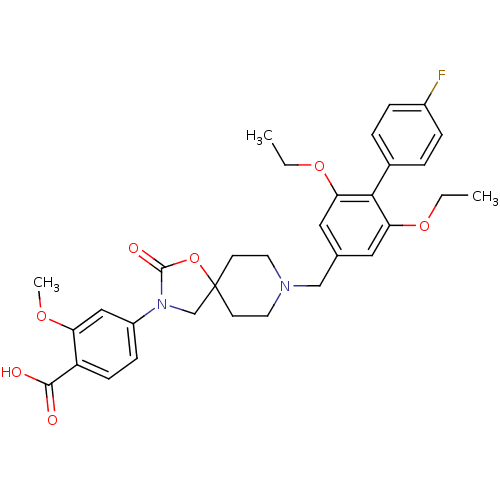 (US8742110, 3-20) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.129 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123247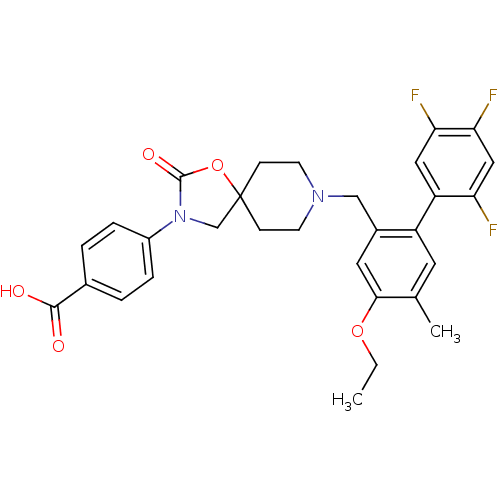 (US8742110, 4-11) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.152 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123253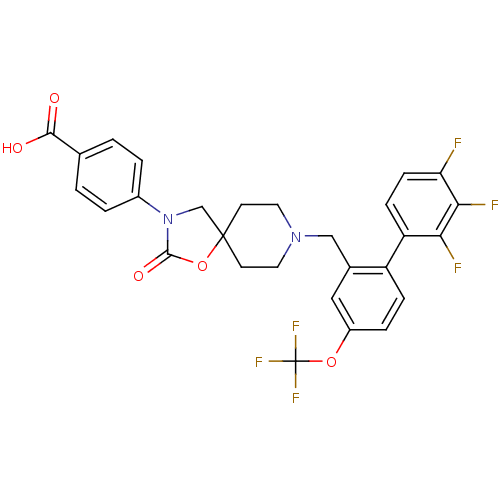 (US8742110, 4-17) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.165 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123270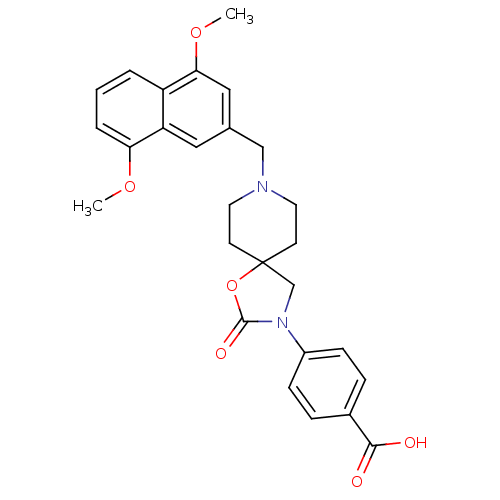 (US8742110, 5-6) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.172 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123248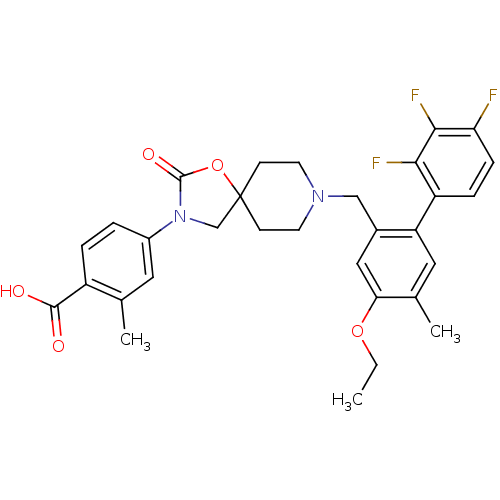 (US8742110, 4-12) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.188 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50229575 (CHEMBL2369937) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Exploratory Chemistry Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin receptor from rabbit aorta | J Med Chem 34: 2919-22 (1991) BindingDB Entry DOI: 10.7270/Q26Q20H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123300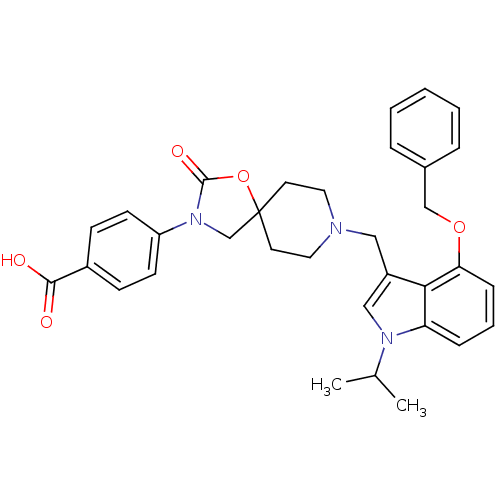 (US8742110, 6-18) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.225 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123254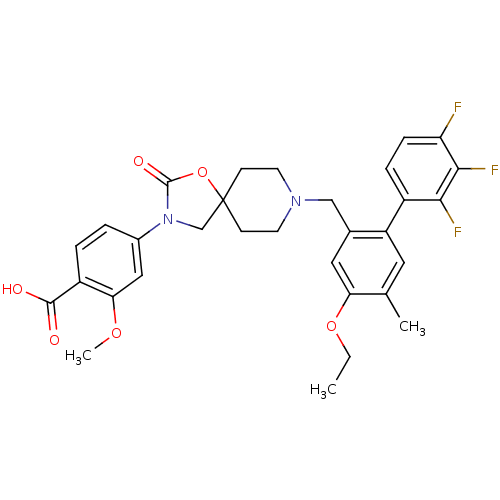 (US8742110, 4-18) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.228 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123271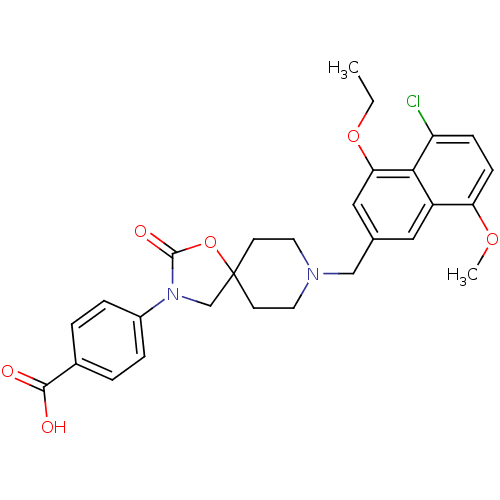 (US8742110, 5-7) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.233 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123255 (US8742110, 4-19) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.243 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50282279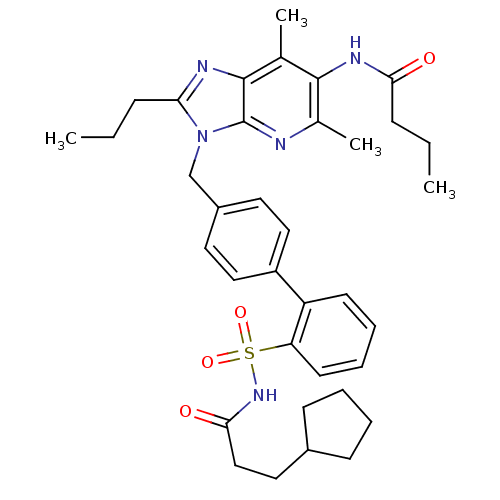 (CHEMBL304946 | N-{3-[2'-(3-Cyclopentyl-propionylsu...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity determined by its ability to displace the specific binding ligand [125I]-Sar1, Ile8-AII from AT2 receptor in rat midbrain m... | Bioorg Med Chem Lett 4: 17-22 (1994) Article DOI: 10.1016/S0960-894X(01)81115-X BindingDB Entry DOI: 10.7270/Q2G73DPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50282279 (CHEMBL304946 | N-{3-[2'-(3-Cyclopentyl-propionylsu...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity determined by its ability to displace the specific binding ligand [125I]-Sar1, Ile8-AII from Angiotensin II receptor, type ... | Bioorg Med Chem Lett 4: 17-22 (1994) Article DOI: 10.1016/S0960-894X(01)81115-X BindingDB Entry DOI: 10.7270/Q2G73DPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123296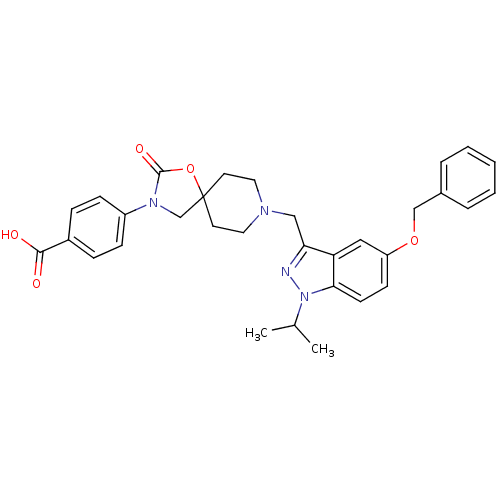 (US8742110, 6-14) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50282286 (CHEMBL71388 | Imidazo[4,5-b]pyridine derivative) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity determined by its ability to displace the specific binding ligand [125I]-Sar1, Ile8-AII from Angiotensin II receptor, type ... | Bioorg Med Chem Lett 4: 17-22 (1994) Article DOI: 10.1016/S0960-894X(01)81115-X BindingDB Entry DOI: 10.7270/Q2G73DPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123252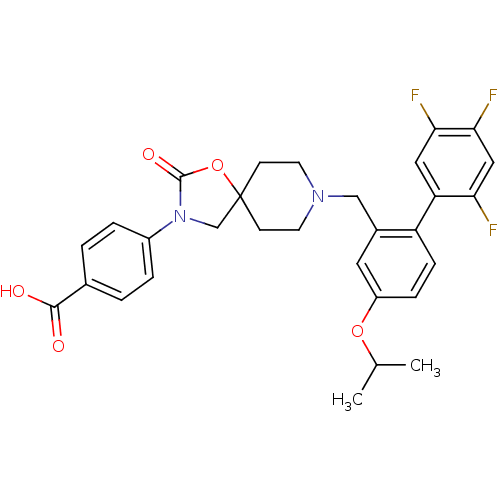 (US8742110, 4-16) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.272 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50229912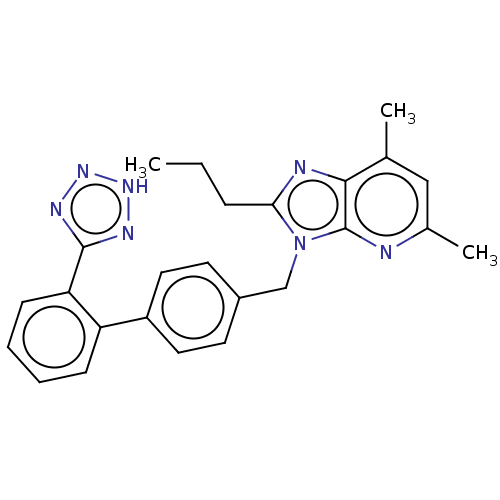 (CHEMBL315104) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Exploratory Chemistry Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin receptor from rabbit aorta | J Med Chem 34: 2919-22 (1991) BindingDB Entry DOI: 10.7270/Q26Q20H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50009718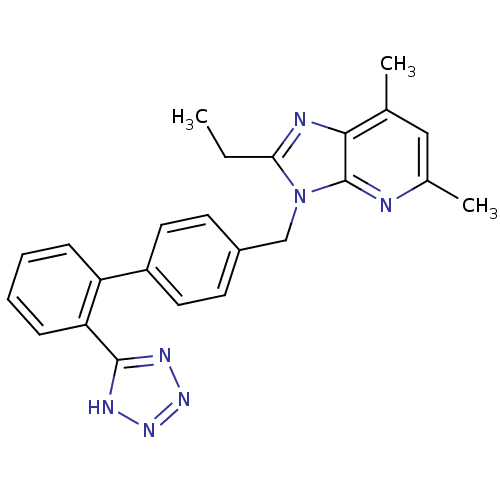 (2-Ethyl-5,7-dimethyl-3-[2'-(1H-tetrazol-5-yl)-biph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Exploratory Chemistry Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin receptor from rabbit aorta | J Med Chem 34: 2919-22 (1991) BindingDB Entry DOI: 10.7270/Q26Q20H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50009718 (2-Ethyl-5,7-dimethyl-3-[2'-(1H-tetrazol-5-yl)-biph...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity determined by its ability to displace the specific binding ligand [125I]-Sar1, Ile8-AII from Angiotensin II receptor, type ... | Bioorg Med Chem Lett 4: 17-22 (1994) Article DOI: 10.1016/S0960-894X(01)81115-X BindingDB Entry DOI: 10.7270/Q2G73DPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50282276 (CHEMBL304947 | Pentanoic acid {2-butyl-3-[2'-(3-cy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity determined by its ability to displace the specific binding ligand [125I]-Sar1, Ile8-AII from AT2 receptor in rat midbrain m... | Bioorg Med Chem Lett 4: 17-22 (1994) Article DOI: 10.1016/S0960-894X(01)81115-X BindingDB Entry DOI: 10.7270/Q2G73DPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123277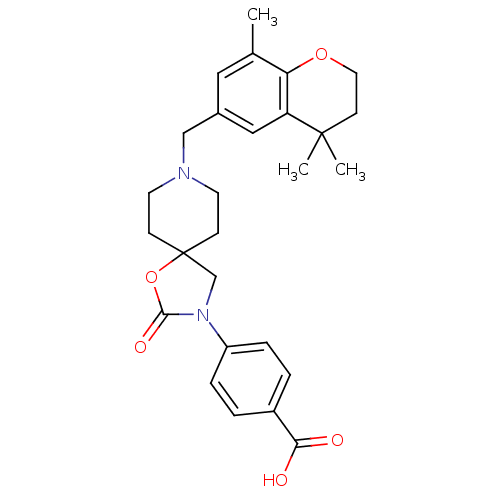 (US8742110, 5-13) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.317 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123227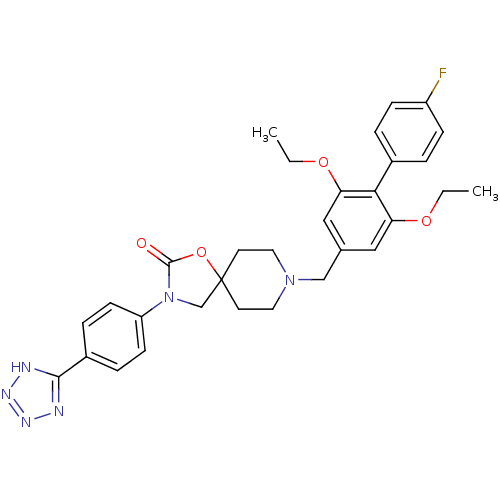 (US8742110, 3-12) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.323 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50049185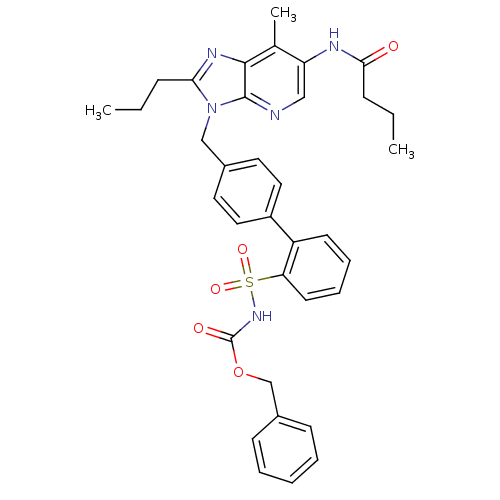 (4'-[2-Butyl-6-(3-isopropyl-3-methyl-ureido)-4-oxo-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity determined by its ability to displace the specific binding ligand [125I]-Sar1, Ile8-AII from Angiotensin II receptor, type ... | Bioorg Med Chem Lett 4: 17-22 (1994) Article DOI: 10.1016/S0960-894X(01)81115-X BindingDB Entry DOI: 10.7270/Q2G73DPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123306 (US8742110, 6-24) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.342 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123234 (US8742110, 3-19) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.359 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50282283 (CHEMBL67801 | Pentanoic acid [2-butyl-3-(2'-hexano...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity determined by its ability to displace the specific binding ligand [125I]-Sar1, Ile8-AII from Angiotensin II receptor, type ... | Bioorg Med Chem Lett 4: 17-22 (1994) Article DOI: 10.1016/S0960-894X(01)81115-X BindingDB Entry DOI: 10.7270/Q2G73DPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123264 (US8742110, 4-28) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.395 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123257 (US8742110, 4-21) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.397 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123250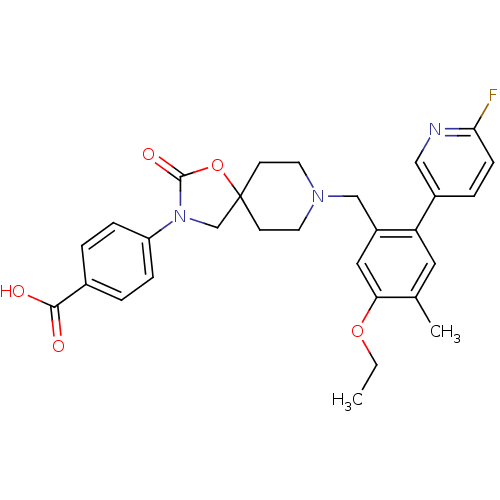 (US8742110, 4-14) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.407 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123243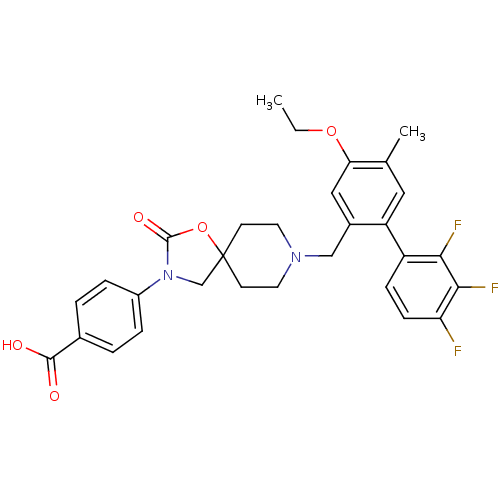 (US8742110, 4-7) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.409 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123179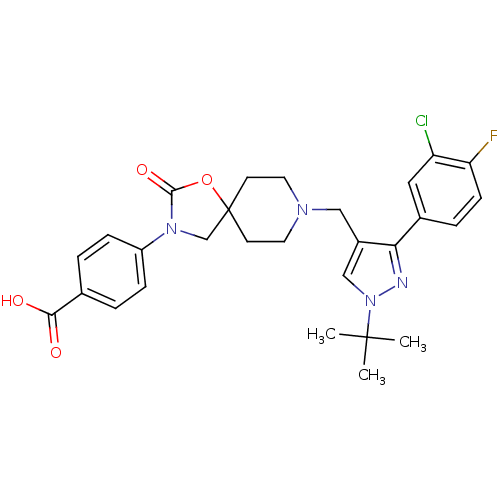 (US8742110, 1-28) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.421 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123272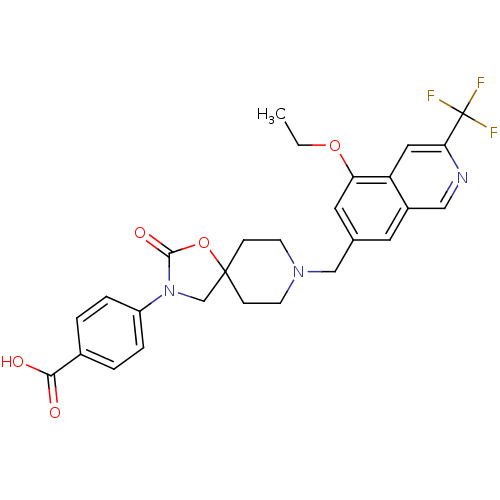 (US8742110, 5-8) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.424 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123236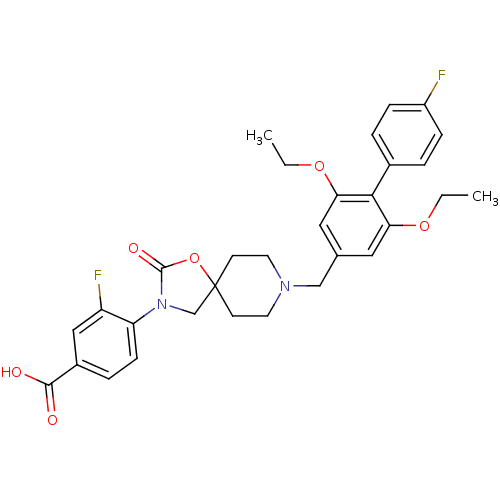 (US8742110, 3-21) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.437 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123245 (US8742110, 4-9) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.439 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50282281 (CHEMBL68618 | Pentanoic acid {2-butyl-3-[2'-(3-cyc...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity determined by its ability to displace the specific binding ligand [125I]-Sar1, Ile8-AII from AT2 receptor in rat midbrain m... | Bioorg Med Chem Lett 4: 17-22 (1994) Article DOI: 10.1016/S0960-894X(01)81115-X BindingDB Entry DOI: 10.7270/Q2G73DPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123258 (US8742110, 4-22) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.468 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50282277 (CHEMBL306407 | Imidazo[4,5-b]pyridine derivative) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity determined by its ability to displace the specific binding ligand [125I]-Sar1, Ile8-AII from AT2 receptor in rat midbrain m... | Bioorg Med Chem Lett 4: 17-22 (1994) Article DOI: 10.1016/S0960-894X(01)81115-X BindingDB Entry DOI: 10.7270/Q2G73DPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123244 (US8742110, 4-8) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.474 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123239 (US8742110, 4-3) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50282282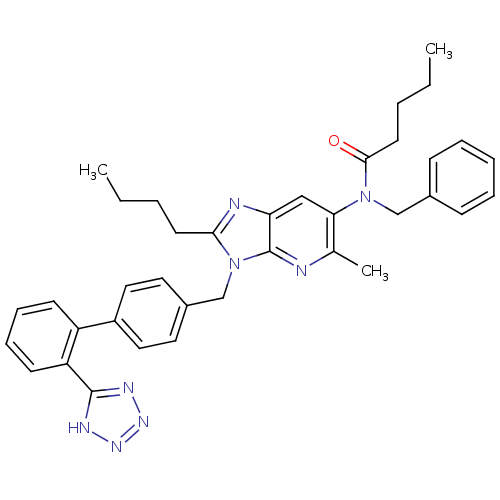 (CHEMBL71253 | Pentanoic acid benzyl-{2-butyl-5-met...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity determined by its ability to displace the specific binding ligand [125I]-Sar1, Ile8-AII from Angiotensin II receptor, type ... | Bioorg Med Chem Lett 4: 17-22 (1994) Article DOI: 10.1016/S0960-894X(01)81115-X BindingDB Entry DOI: 10.7270/Q2G73DPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50229914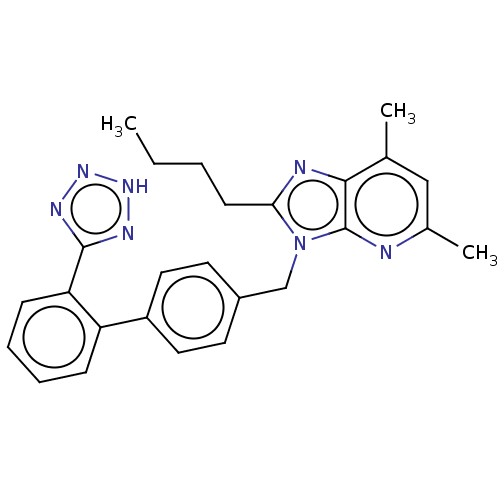 (CHEMBL98907) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exploratory Chemistry Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin receptor from rabbit aorta | J Med Chem 34: 2919-22 (1991) BindingDB Entry DOI: 10.7270/Q26Q20H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123240 (US8742110, 4-4) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.502 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50282273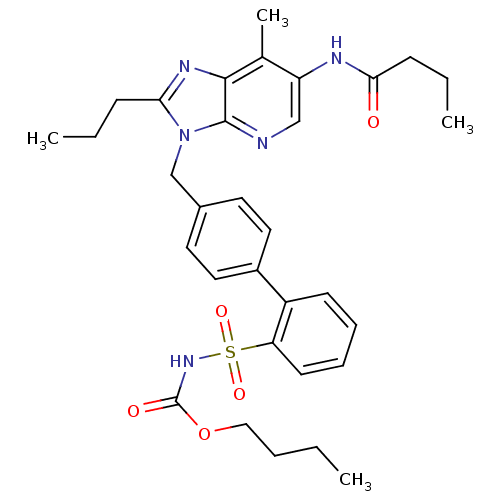 (CHEMBL67921 | Imidazo[4,5-b]pyridine derivative) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity determined by its ability to displace the specific binding ligand [125I]-Sar1, Ile8-AII from Angiotensin II receptor, type ... | Bioorg Med Chem Lett 4: 17-22 (1994) Article DOI: 10.1016/S0960-894X(01)81115-X BindingDB Entry DOI: 10.7270/Q2G73DPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123242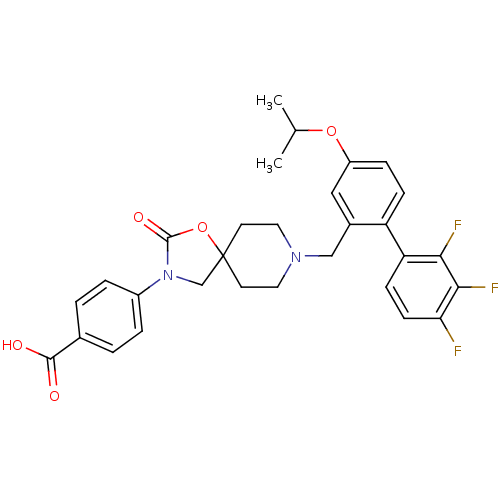 (US8742110, 4-6) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.546 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123307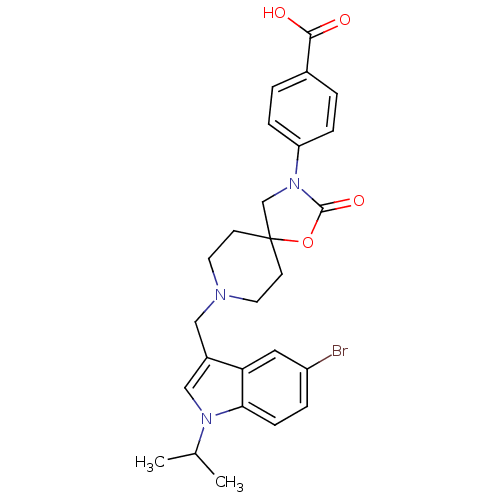 (US8742110, 6-25) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123241 (US8742110, 4-5) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.556 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123180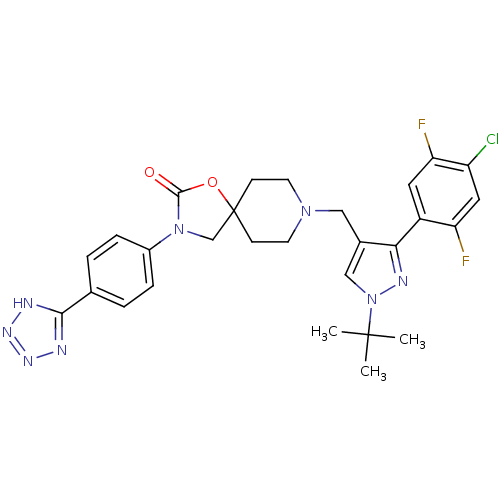 (US8742110, 1-29) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.582 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 589 total ) | Next | Last >> |