

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50546246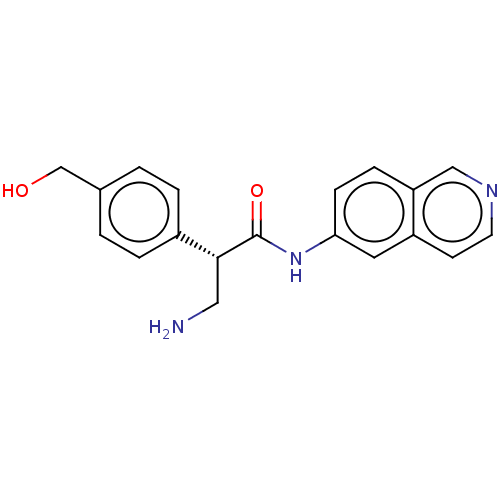 (CHEMBL4753043 | US11608319, Compound AR-13503) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GST-tagged ROCK1 catalytic domain expressed in baculovirus expression system by Kinase-Glo luminescent kinase assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127474 BindingDB Entry DOI: 10.7270/Q25142V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50546246 (CHEMBL4753043 | US11608319, Compound AR-13503) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GST-tagged ROCK2 catalytic domain expressed in baculovirus expression system by Kinase-Glo luminescent kinase assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127474 BindingDB Entry DOI: 10.7270/Q25142V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50546247 (AR-11324 FREE BASE | AR-13324 | Netarsudil | US114...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GST-tagged ROCK1 catalytic domain expressed in baculovirus expression system by Kinase-Glo luminescent kinase assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127474 BindingDB Entry DOI: 10.7270/Q25142V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50546247 (AR-11324 FREE BASE | AR-13324 | Netarsudil | US114...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GST-tagged ROCK2 catalytic domain expressed in baculovirus expression system by Kinase-Glo luminescent kinase assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127474 BindingDB Entry DOI: 10.7270/Q25142V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50011322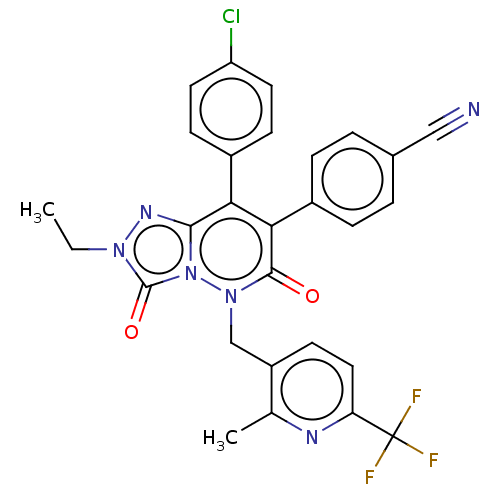 (CHEMBL3260745) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis | J Med Chem 56: 9586-600 (2014) Article DOI: 10.1021/jm4010835 BindingDB Entry DOI: 10.7270/Q24X59B8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50011324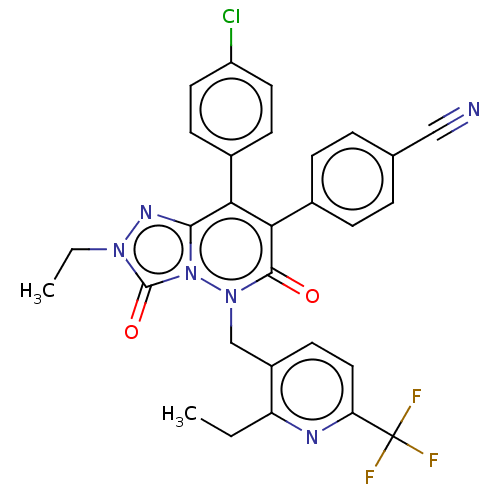 (CHEMBL3260747) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis | J Med Chem 56: 9586-600 (2014) Article DOI: 10.1021/jm4010835 BindingDB Entry DOI: 10.7270/Q24X59B8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50011328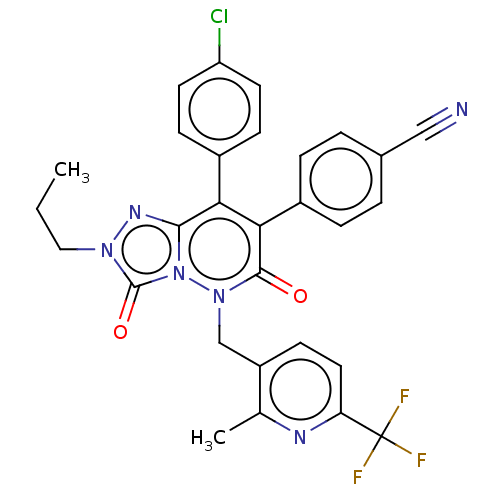 (CHEMBL3259829) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis | J Med Chem 56: 9586-600 (2014) Article DOI: 10.1021/jm4010835 BindingDB Entry DOI: 10.7270/Q24X59B8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50011326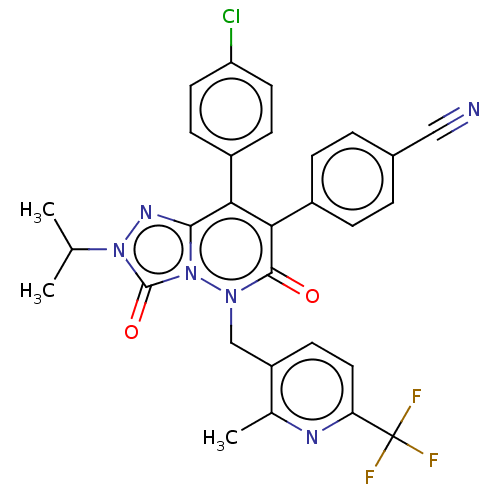 (CHEMBL3260748) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis | J Med Chem 56: 9586-600 (2014) Article DOI: 10.1021/jm4010835 BindingDB Entry DOI: 10.7270/Q24X59B8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50287059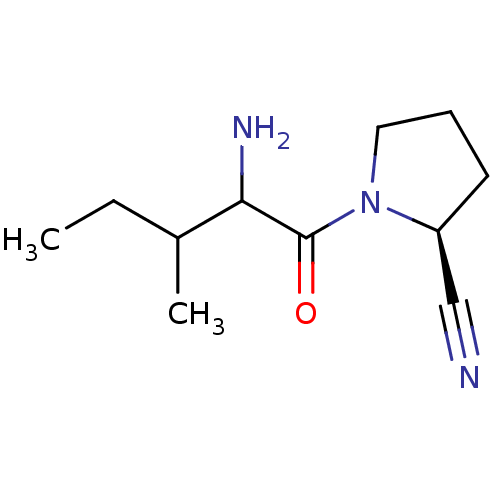 ((S)-1-(2-Amino-3-methyl-pentanoyl)-pyrrolidine-2-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50011330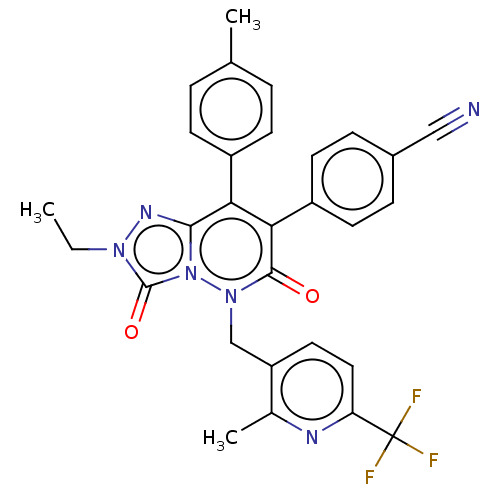 (CHEMBL3260750) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis | J Med Chem 56: 9586-600 (2014) Article DOI: 10.1021/jm4010835 BindingDB Entry DOI: 10.7270/Q24X59B8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50011323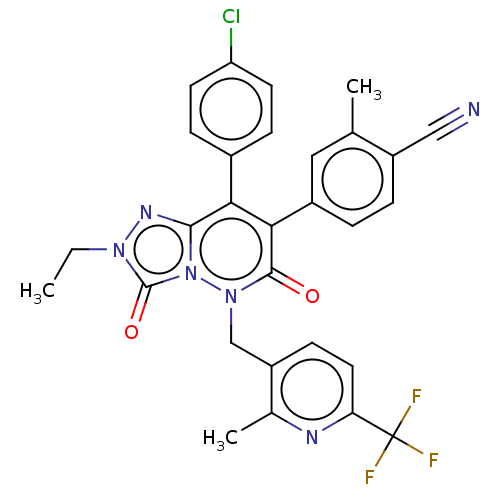 (CHEMBL3260746) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis | J Med Chem 56: 9586-600 (2014) Article DOI: 10.1021/jm4010835 BindingDB Entry DOI: 10.7270/Q24X59B8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50011334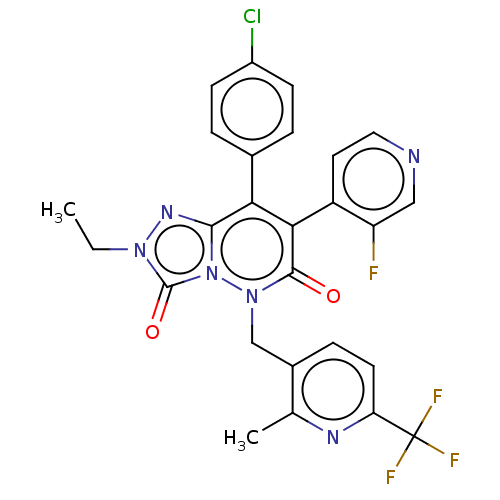 (CHEMBL3260754) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis | J Med Chem 56: 9586-600 (2014) Article DOI: 10.1021/jm4010835 BindingDB Entry DOI: 10.7270/Q24X59B8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM11928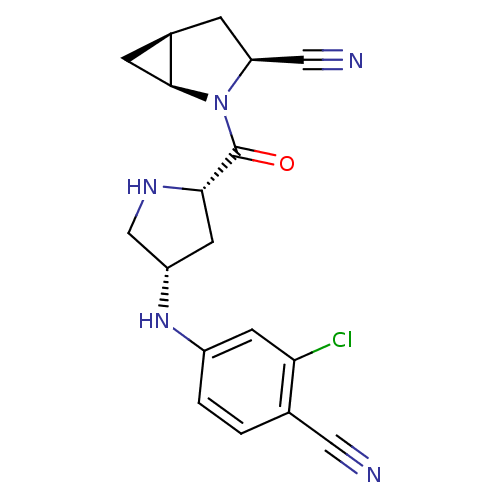 ((1S,3S,5S)-2-{[(2S,4S)-4-[(3-chloro-4-cyanophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.60 | -47.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | Bioorg Med Chem Lett 15: 3992-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.043 BindingDB Entry DOI: 10.7270/Q2CN7259 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50145999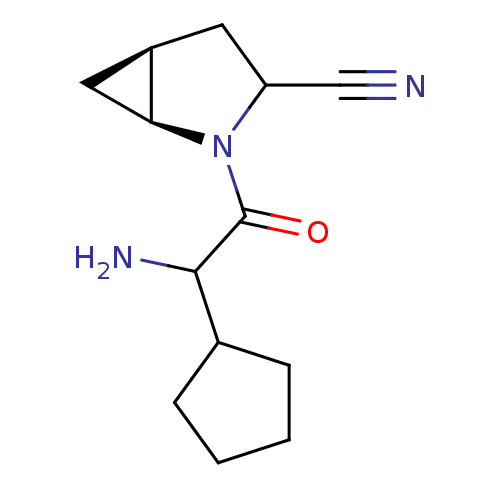 ((1S,5S)-2-(2-Amino-2-cyclopentyl-acetyl)-2-aza-bic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50011310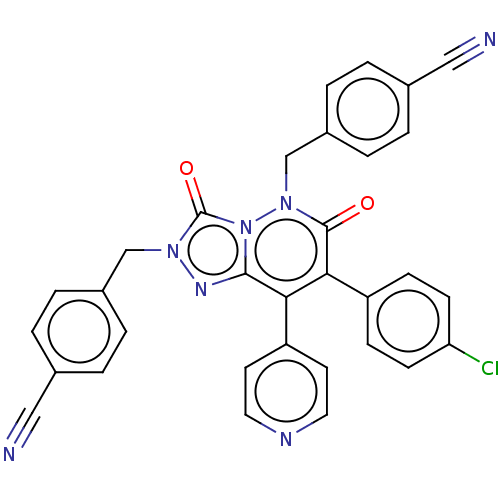 (CHEMBL3260737) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis | J Med Chem 56: 9586-600 (2014) Article DOI: 10.1021/jm4010835 BindingDB Entry DOI: 10.7270/Q24X59B8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50011316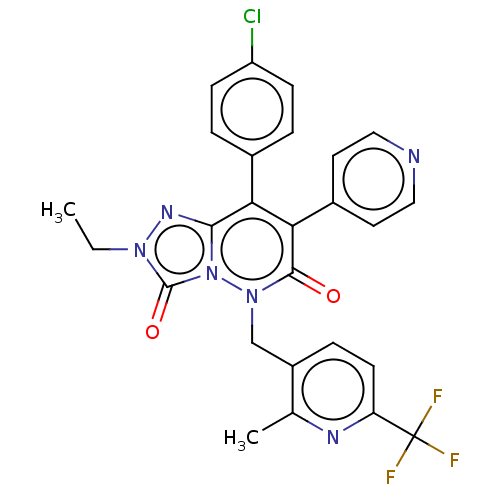 (CHEMBL3260743) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis | J Med Chem 56: 9586-600 (2014) Article DOI: 10.1021/jm4010835 BindingDB Entry DOI: 10.7270/Q24X59B8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50011333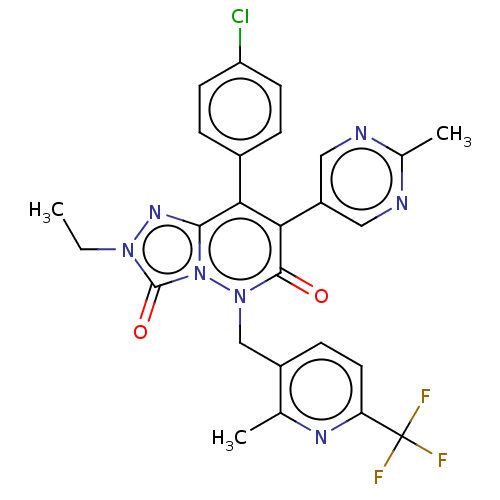 (CHEMBL3260753) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis | J Med Chem 56: 9586-600 (2014) Article DOI: 10.1021/jm4010835 BindingDB Entry DOI: 10.7270/Q24X59B8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358883 (CHEMBL1923468) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | 4.71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a by Michaelis Menten equation analysis | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50011331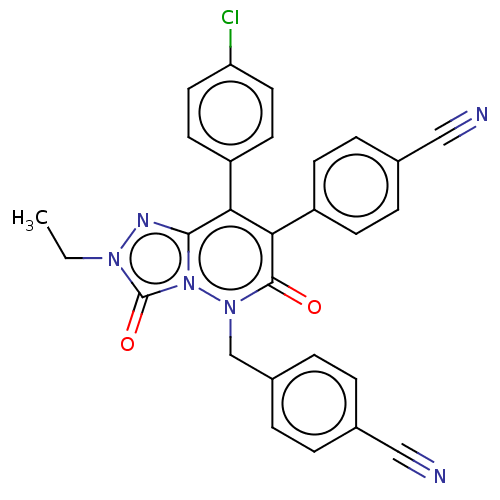 (CHEMBL3260751) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis | J Med Chem 56: 9586-600 (2014) Article DOI: 10.1021/jm4010835 BindingDB Entry DOI: 10.7270/Q24X59B8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50011332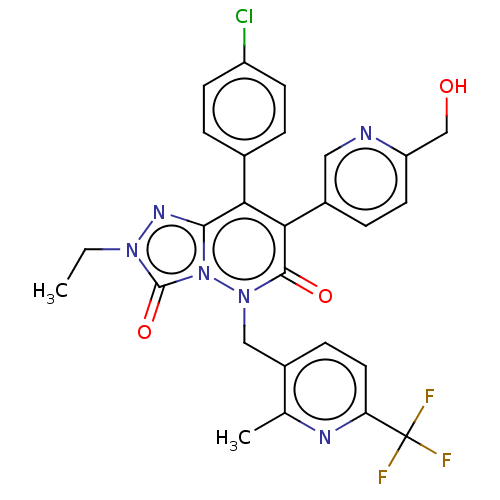 (CHEMBL3260752) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis | J Med Chem 56: 9586-600 (2014) Article DOI: 10.1021/jm4010835 BindingDB Entry DOI: 10.7270/Q24X59B8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50146002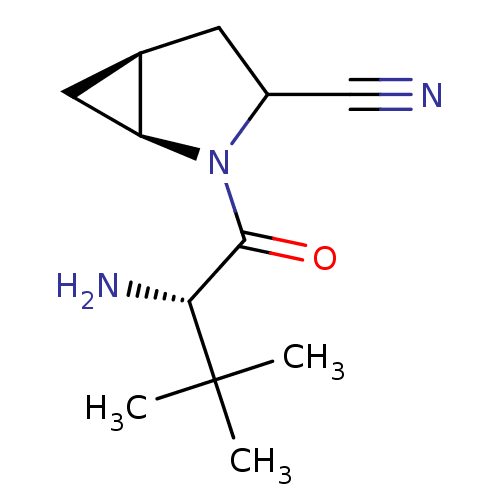 ((S)-2-((S)-2-Amino-3-methyl-3-(S)-methyl-butyryl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50146015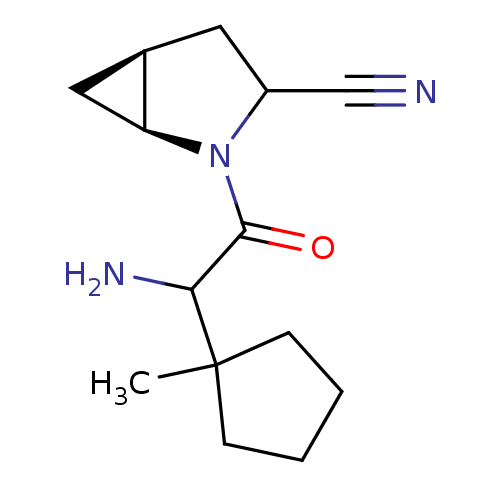 ((1S,5S)-2-[2-Amino-2-(1-methyl-cyclopentyl)-acetyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50146009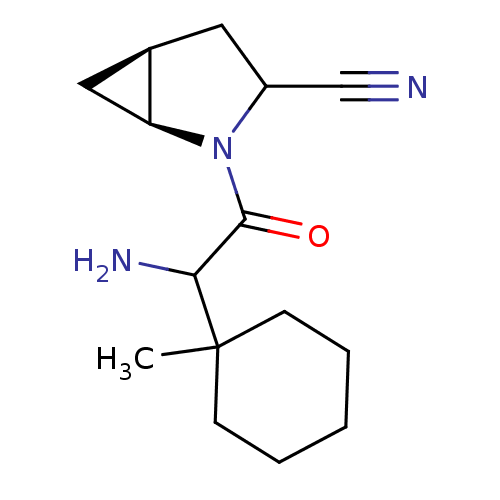 ((1S,5S)-2-[2-Amino-2-(1-methyl-cyclohexyl)-acetyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50151003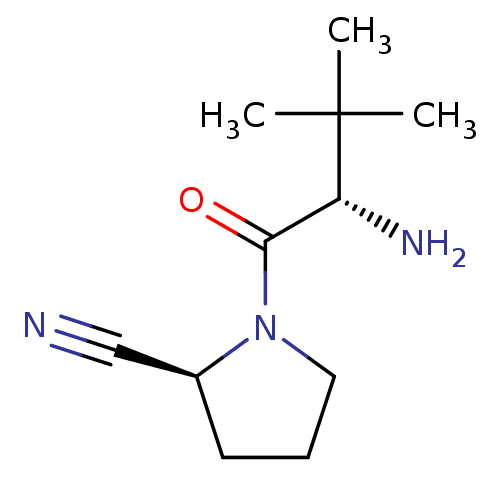 ((S)-1-((S)-2-Amino-3,3-dimethyl-butyryl)-pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50011317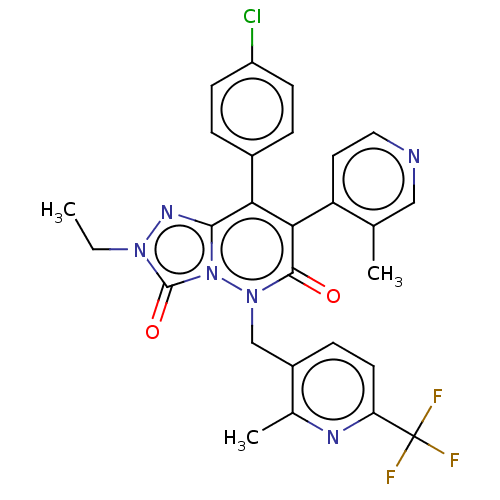 (CHEMBL3260744) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis | J Med Chem 56: 9586-600 (2014) Article DOI: 10.1021/jm4010835 BindingDB Entry DOI: 10.7270/Q24X59B8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50011314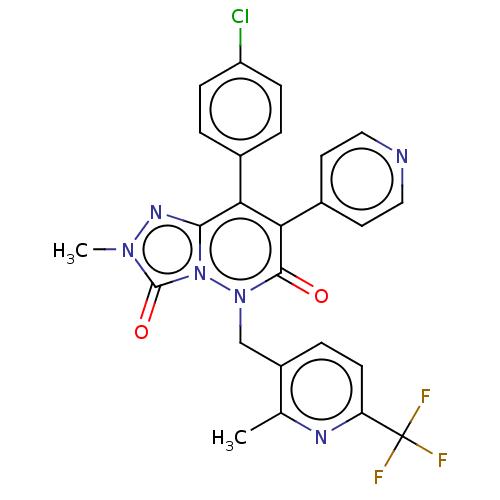 (CHEMBL3260741) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis | J Med Chem 56: 9586-600 (2014) Article DOI: 10.1021/jm4010835 BindingDB Entry DOI: 10.7270/Q24X59B8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50011307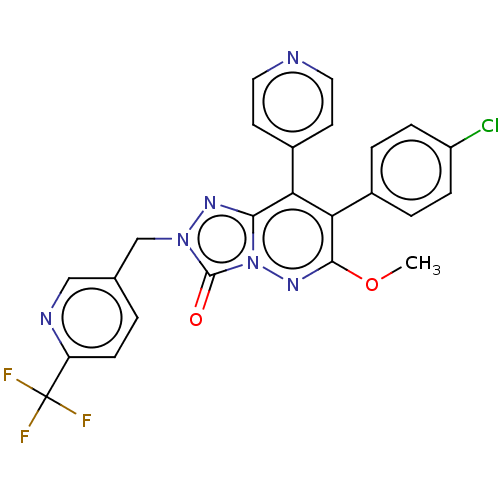 (CHEMBL3260734) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis | J Med Chem 56: 9586-600 (2014) Article DOI: 10.1021/jm4010835 BindingDB Entry DOI: 10.7270/Q24X59B8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267028 (CHEMBL4073525) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at human GPR40 expressed in CHO-A12 cells assessed as increase in intracellular calcium flux measured for 100 secs by FLIPR assay | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50146010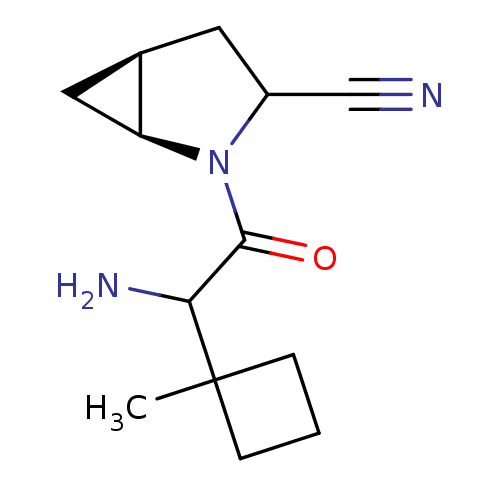 ((1S,5S)-2-[2-Amino-2-(1-methyl-cyclobutyl)-acetyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50146007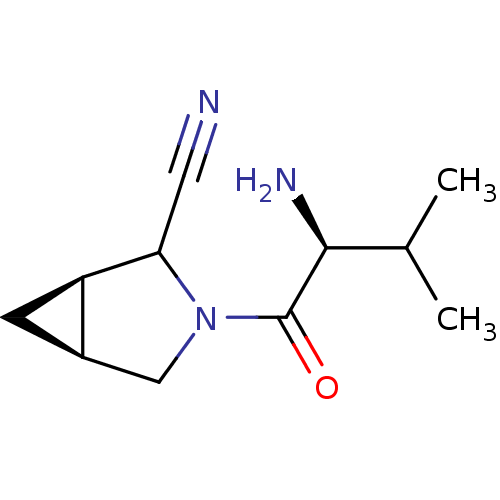 ((1R,5S)-3-((S)-2-Amino-3-methyl-butyryl)-3-aza-bic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50145997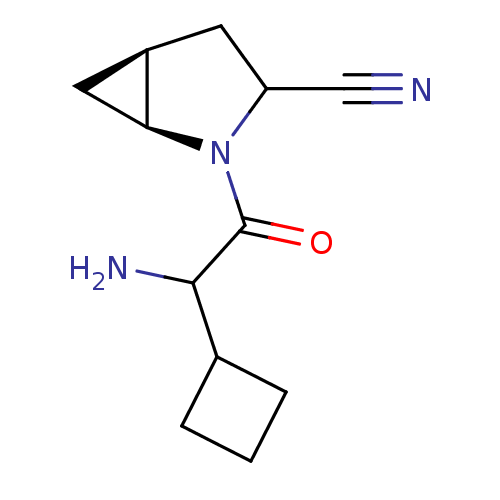 ((1S,5S)-2-(2-Amino-2-cyclobutyl-acetyl)-2-aza-bicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50175075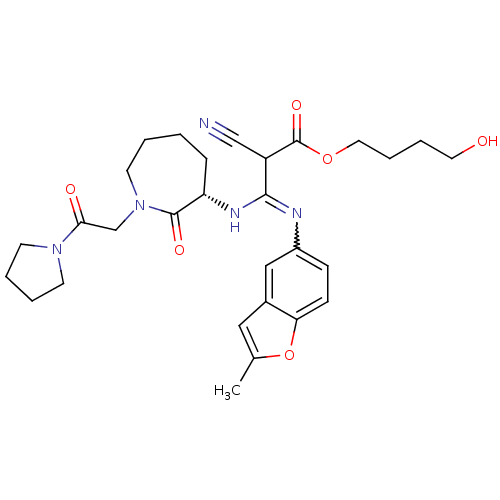 ((S,Z)-4-hydroxybutyl 2-cyano-3-(2-methylbenzofuran...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human FXa | Bioorg Med Chem Lett 15: 5453-8 (2005) Article DOI: 10.1016/j.bmcl.2005.08.107 BindingDB Entry DOI: 10.7270/Q2KH0MW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50011329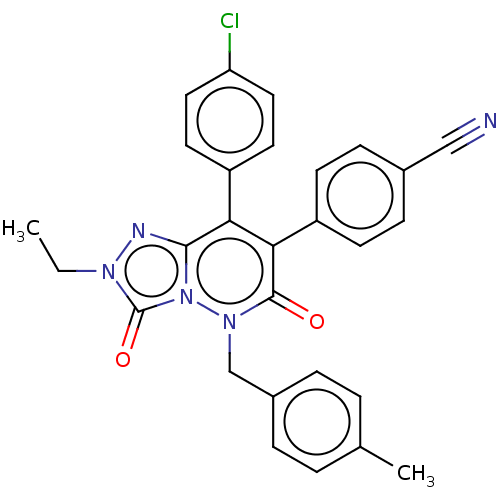 (CHEMBL3260749) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis | J Med Chem 56: 9586-600 (2014) Article DOI: 10.1021/jm4010835 BindingDB Entry DOI: 10.7270/Q24X59B8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50356013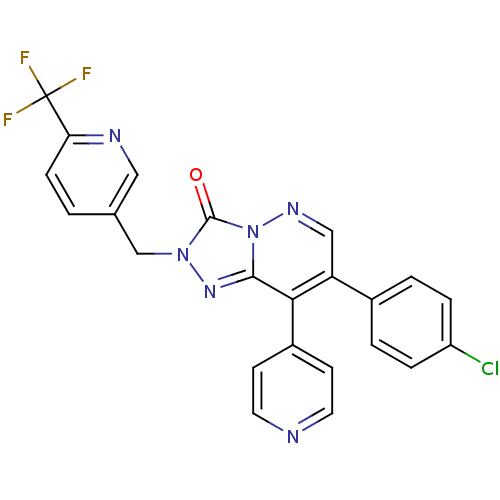 (CHEMBL1911374 | CHEMBL1911375) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis | J Med Chem 56: 9586-600 (2014) Article DOI: 10.1021/jm4010835 BindingDB Entry DOI: 10.7270/Q24X59B8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267031 (CHEMBL4079930) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at human GPR40 expressed in CHO-A12 cells assessed as increase in intracellular calcium flux measured for 100 secs by FLIPR assay | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50146013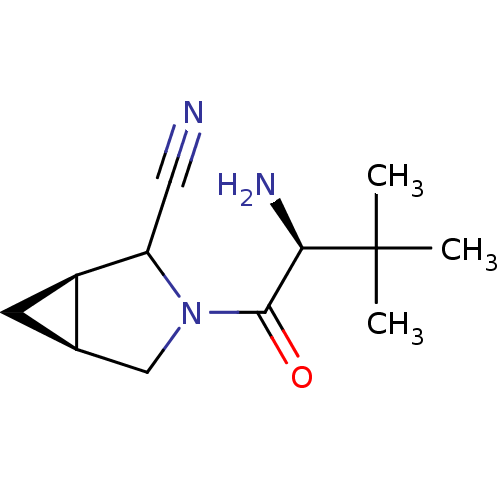 ((1R,5S)-3-((S)-2-Amino-3,3-dimethyl-butyryl)-3-aza...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50146005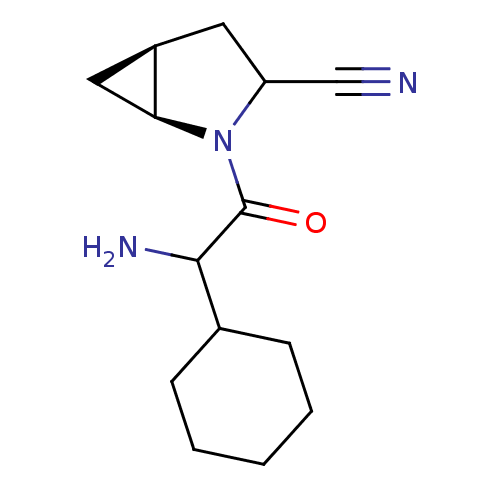 ((1S,5S)-2-(2-Amino-2-cyclohexyl-acetyl)-2-aza-bicy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50011315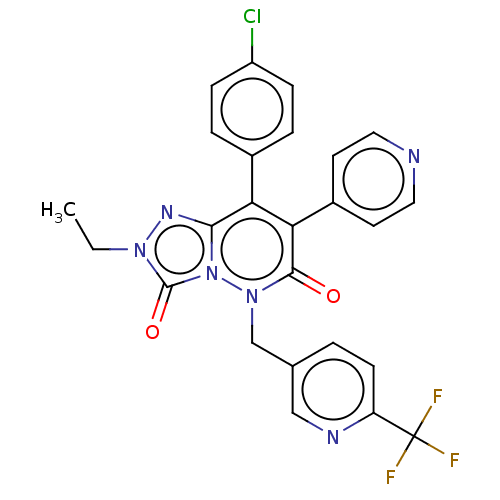 (CHEMBL3260742) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis | J Med Chem 56: 9586-600 (2014) Article DOI: 10.1021/jm4010835 BindingDB Entry DOI: 10.7270/Q24X59B8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50145996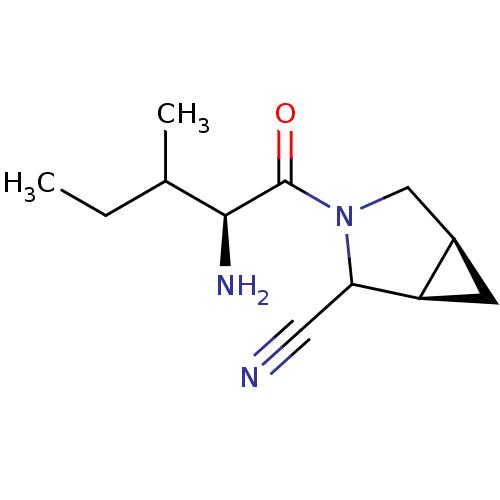 ((1R,5S)-3-((S)-2-Amino-3-methyl-pentanoyl)-3-aza-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50011309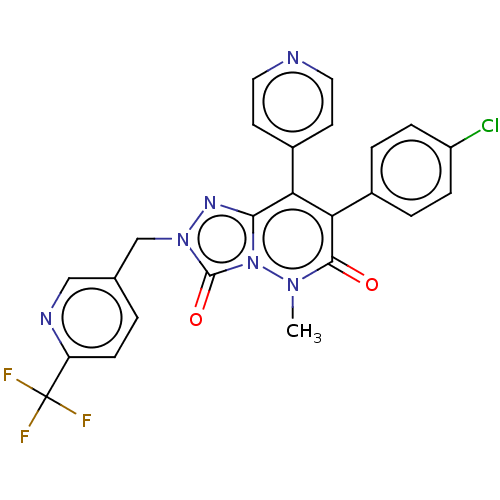 (CHEMBL3260736) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis | J Med Chem 56: 9586-600 (2014) Article DOI: 10.1021/jm4010835 BindingDB Entry DOI: 10.7270/Q24X59B8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50146000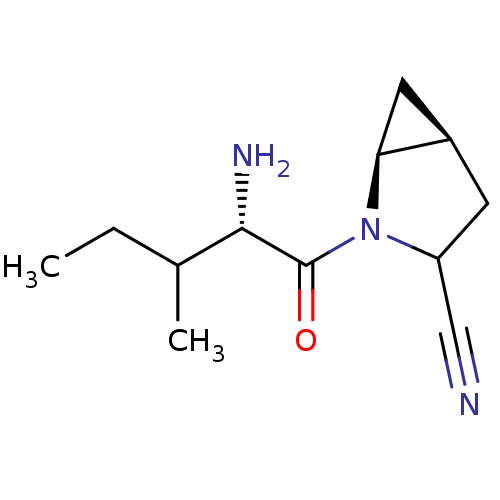 ((1S,5S)-2-((S)-2-Amino-3-methyl-pentanoyl)-2-aza-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50011313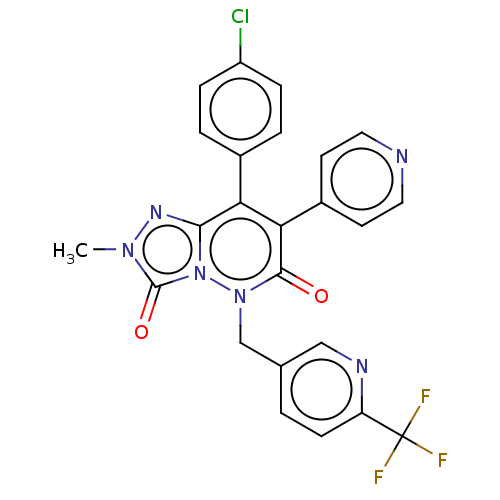 (CHEMBL3260740) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis | J Med Chem 56: 9586-600 (2014) Article DOI: 10.1021/jm4010835 BindingDB Entry DOI: 10.7270/Q24X59B8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50146004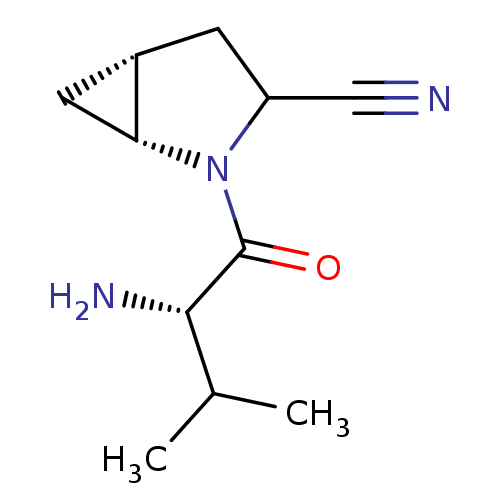 ((1R,5R)-2-((S)-2-Amino-3-methyl-butyryl)-2-aza-bic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50011312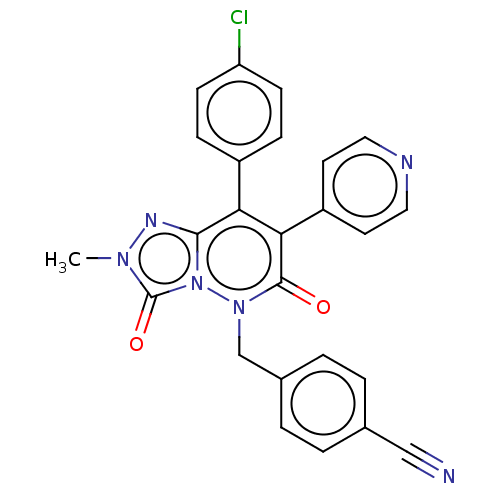 (CHEMBL3260739) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor expressed in CHO cells by radioligand displacement based scintillation counting analysis | J Med Chem 56: 9586-600 (2014) Article DOI: 10.1021/jm4010835 BindingDB Entry DOI: 10.7270/Q24X59B8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267009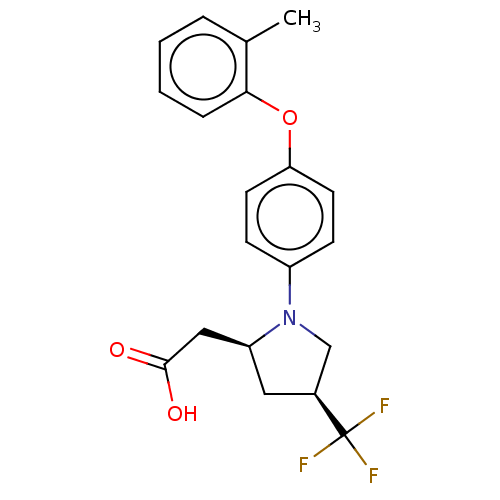 (CHEMBL4089171) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM11920 ((1S,3S,5S)-2-{[(2S)-3,3-dimethylpyrrolidin-2-yl]ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 37 | -42.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | Bioorg Med Chem Lett 15: 3992-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.043 BindingDB Entry DOI: 10.7270/Q2CN7259 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267029 (CHEMBL4069191) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM11927 (4-chloro-N-[(3S,5S)-5-{[(1S,3S,5S)-3-cyano-2-azabi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 39 | -41.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | Bioorg Med Chem Lett 15: 3992-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.043 BindingDB Entry DOI: 10.7270/Q2CN7259 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267010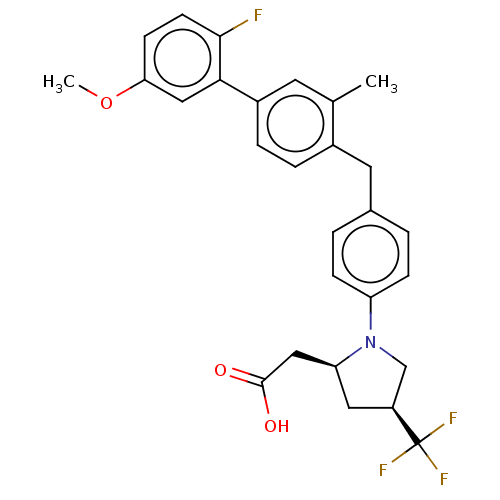 (CHEMBL4082395) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267117 (CHEMBL4060499) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1409 total ) | Next | Last >> |