 Found 823 hits with Last Name = 'ryono' and Initial = 'de'
Found 823 hits with Last Name = 'ryono' and Initial = 'de' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-1 angiotensin II receptor B
(RAT) | BDBM50449917
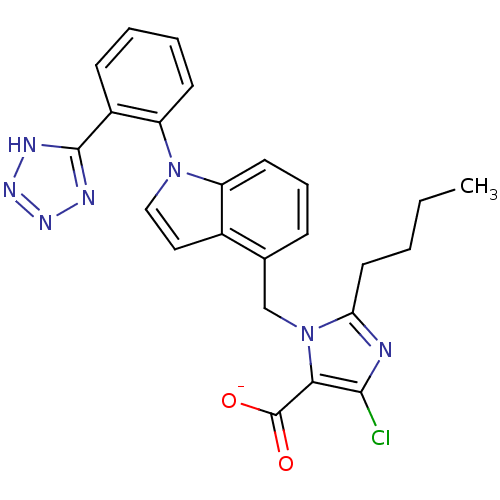
(BMS-180560 | CHEMBL2021417)Show SMILES [Li+].[Li]O.CCCCc1nc(Cl)c(C([O-])=O)n1Cc1cccc2n(ccc12)-c1ccccc1-c1nn[nH]n1 Show InChI InChI=1S/C24H22ClN7O2.2Li.H2O/c1-2-3-11-20-26-22(25)21(24(33)34)32(20)14-15-7-6-10-18-16(15)12-13-31(18)19-9-5-4-8-17(19)23-27-29-30-28-23;;;/h4-10,12-13H,2-3,11,14H2,1H3,(H,33,34)(H,27,28,29,30);;;1H2/q;2*+1;/p-2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes |
Bioorg Med Chem Lett 4: 145-150 (1994)
Article DOI: 10.1016/S0960-894X(01)81137-9
BindingDB Entry DOI: 10.7270/Q2V988KF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282324

(2-Cyclopropyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-...)Show SMILES OC(=O)c1c(Oc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c(nc2cccnc12)C1CC1 Show InChI InChI=1S/C25H18N6O3/c32-25(33)20-22-19(6-3-13-26-22)27-21(15-7-8-15)23(20)34-16-11-9-14(10-12-16)17-4-1-2-5-18(17)24-28-30-31-29-24/h1-6,9-13,15H,7-8H2,(H,32,33)(H,28,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane |
Bioorg Med Chem Lett 4: 195-200 (1994)
Article DOI: 10.1016/S0960-894X(01)81146-X
BindingDB Entry DOI: 10.7270/Q2Z60P13 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50049201

(2-Cyclopropyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-...)Show SMILES OC(=O)c1c(OCc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c(nc2ccccc12)C1CC1 Show InChI InChI=1S/C27H21N5O3/c33-27(34)23-21-7-3-4-8-22(21)28-24(18-13-14-18)25(23)35-15-16-9-11-17(12-10-16)19-5-1-2-6-20(19)26-29-31-32-30-26/h1-12,18H,13-15H2,(H,33,34)(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane |
Bioorg Med Chem Lett 4: 195-200 (1994)
Article DOI: 10.1016/S0960-894X(01)81146-X
BindingDB Entry DOI: 10.7270/Q2Z60P13 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50049201

(2-Cyclopropyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-...)Show SMILES OC(=O)c1c(OCc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c(nc2ccccc12)C1CC1 Show InChI InChI=1S/C27H21N5O3/c33-27(34)23-21-7-3-4-8-22(21)28-24(18-13-14-18)25(23)35-15-16-9-11-17(12-10-16)19-5-1-2-6-20(19)26-29-31-32-30-26/h1-12,18H,13-15H2,(H,33,34)(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Receptor binding affinity determined from competitive binding assay using 1251 labelled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes |
Bioorg Med Chem Lett 4: 201-206 (1994)
Article DOI: 10.1016/S0960-894X(01)81147-1
BindingDB Entry DOI: 10.7270/Q2TH8MMS |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282318
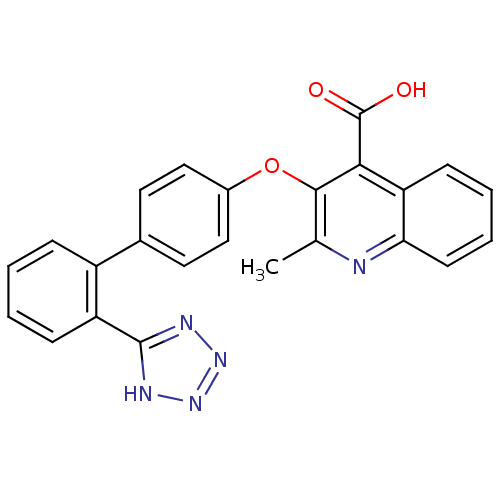
(2-Methyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-yloxy...)Show SMILES Cc1nc2ccccc2c(C(O)=O)c1Oc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H17N5O3/c1-14-22(21(24(30)31)19-8-4-5-9-20(19)25-14)32-16-12-10-15(11-13-16)17-6-2-3-7-18(17)23-26-28-29-27-23/h2-13H,1H3,(H,30,31)(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane |
Bioorg Med Chem Lett 4: 195-200 (1994)
Article DOI: 10.1016/S0960-894X(01)81146-X
BindingDB Entry DOI: 10.7270/Q2Z60P13 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282322
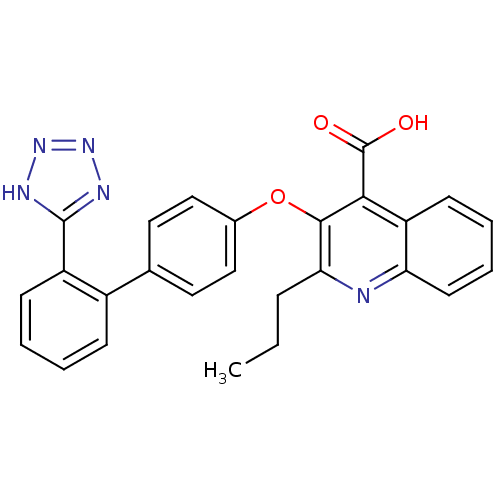
(2-Propyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-yloxy...)Show SMILES CCCc1nc2ccccc2c(C(O)=O)c1Oc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C26H21N5O3/c1-2-7-22-24(23(26(32)33)20-10-5-6-11-21(20)27-22)34-17-14-12-16(13-15-17)18-8-3-4-9-19(18)25-28-30-31-29-25/h3-6,8-15H,2,7H2,1H3,(H,32,33)(H,28,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane |
Bioorg Med Chem Lett 4: 195-200 (1994)
Article DOI: 10.1016/S0960-894X(01)81146-X
BindingDB Entry DOI: 10.7270/Q2Z60P13 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50449914
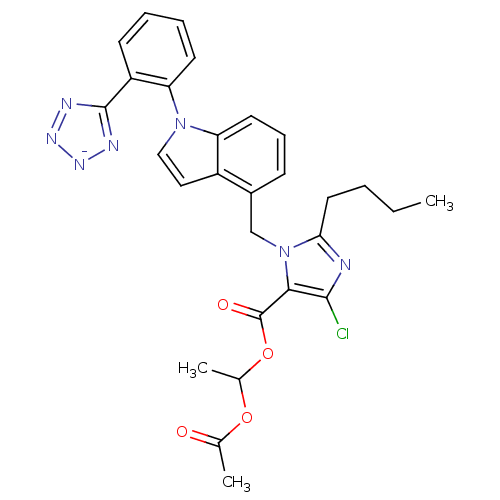
(CHEMBL2079784)Show SMILES [K+].CCCCc1nc(Cl)c(C(=O)OC(C)OC(C)=O)n1Cc1cccc2n(ccc12)-c1ccccc1-c1nn[n-]n1 Show InChI InChI=1S/C28H27ClN7O4.K/c1-4-5-13-24-30-26(29)25(28(38)40-18(3)39-17(2)37)36(24)16-19-9-8-12-22-20(19)14-15-35(22)23-11-7-6-10-21(23)27-31-33-34-32-27;/h6-12,14-15,18H,4-5,13,16H2,1-3H3;/q-1;+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes |
Bioorg Med Chem Lett 4: 145-150 (1994)
Article DOI: 10.1016/S0960-894X(01)81137-9
BindingDB Entry DOI: 10.7270/Q2V988KF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282316
(2-Cyclopropyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-...)Show SMILES OC(=O)c1c(Oc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c(nc2ncccc12)C1CC1 Show InChI InChI=1S/C25H18N6O3/c32-25(33)20-19-6-3-13-26-23(19)27-21(15-7-8-15)22(20)34-16-11-9-14(10-12-16)17-4-1-2-5-18(17)24-28-30-31-29-24/h1-6,9-13,15H,7-8H2,(H,32,33)(H,28,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane |
Bioorg Med Chem Lett 4: 195-200 (1994)
Article DOI: 10.1016/S0960-894X(01)81146-X
BindingDB Entry DOI: 10.7270/Q2Z60P13 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282315
(2-Ethyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-yloxy]...)Show SMILES CCc1nc2ccccc2c(C(O)=O)c1Oc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C25H19N5O3/c1-2-20-23(22(25(31)32)19-9-5-6-10-21(19)26-20)33-16-13-11-15(12-14-16)17-7-3-4-8-18(17)24-27-29-30-28-24/h3-14H,2H2,1H3,(H,31,32)(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane |
Bioorg Med Chem Lett 4: 195-200 (1994)
Article DOI: 10.1016/S0960-894X(01)81146-X
BindingDB Entry DOI: 10.7270/Q2Z60P13 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50366331

(CHEMBL1790055)Show SMILES CCCCc1nc(Cl)c(C[O-])n1Cc1cccc2n(ccc12)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H23ClN7O/c1-2-3-11-22-26-23(25)21(15-33)32(22)14-16-7-6-10-19-17(16)12-13-31(19)20-9-5-4-8-18(20)24-27-29-30-28-24/h4-10,12-13H,2-3,11,14-15H2,1H3,(H,27,28,29,30)/q-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes |
Bioorg Med Chem Lett 4: 145-150 (1994)
Article DOI: 10.1016/S0960-894X(01)81137-9
BindingDB Entry DOI: 10.7270/Q2V988KF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50449919
(CHEMBL2021415)Show SMILES [K+].CCCCc1nc(Cl)c(C(=O)OCC(=O)N(CC)CC)n1Cc1cccc2n(ccc12)-c1ccccc1-c1nn[n-]n1 Show InChI InChI=1S/C30H32ClN8O3.K/c1-4-7-15-25-32-28(31)27(30(41)42-19-26(40)37(5-2)6-3)39(25)18-20-11-10-14-23-21(20)16-17-38(23)24-13-9-8-12-22(24)29-33-35-36-34-29;/h8-14,16-17H,4-7,15,18-19H2,1-3H3;/q-1;+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes |
Bioorg Med Chem Lett 4: 145-150 (1994)
Article DOI: 10.1016/S0960-894X(01)81137-9
BindingDB Entry DOI: 10.7270/Q2V988KF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50449910

(CHEMBL2079782)Show SMILES [K+].CCCCOC(=O)c1c(Cl)nc(CCCC)n1Cc1cccc2n(ccc12)-c1ccccc1-c1nn[n-]n1 Show InChI InChI=1S/C28H29ClN7O2.K/c1-3-5-14-24-30-26(29)25(28(37)38-17-6-4-2)36(24)18-19-10-9-13-22-20(19)15-16-35(22)23-12-8-7-11-21(23)27-31-33-34-32-27;/h7-13,15-16H,3-6,14,17-18H2,1-2H3;/q-1;+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes |
Bioorg Med Chem Lett 4: 145-150 (1994)
Article DOI: 10.1016/S0960-894X(01)81137-9
BindingDB Entry DOI: 10.7270/Q2V988KF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50449912
(CHEMBL2079781)Show SMILES [K+].CCCCc1nc(Cl)c(C(=O)OC(OC(=O)C(C)(C)C)C(C)C)n1Cc1cccc2n(ccc12)-c1ccccc1-c1nn[n-]n1 Show InChI InChI=1S/C33H37ClN7O4.K/c1-7-8-16-26-35-28(34)27(30(42)44-31(20(2)3)45-32(43)33(4,5)6)41(26)19-21-12-11-15-24-22(21)17-18-40(24)25-14-10-9-13-23(25)29-36-38-39-37-29;/h9-15,17-18,20,31H,7-8,16,19H2,1-6H3;/q-1;+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes |
Bioorg Med Chem Lett 4: 145-150 (1994)
Article DOI: 10.1016/S0960-894X(01)81137-9
BindingDB Entry DOI: 10.7270/Q2V988KF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282317

(2-Cyclopropyl-5-methyl-3-[2'-(2H-tetrazol-5-yl)-bi...)Show SMILES Cc1cccc2nc(C3CC3)c(Oc3ccc(cc3)-c3ccccc3-c3nnn[nH]3)c(C(O)=O)c12 Show InChI InChI=1S/C27H21N5O3/c1-15-5-4-8-21-22(15)23(27(33)34)25(24(28-21)17-9-10-17)35-18-13-11-16(12-14-18)19-6-2-3-7-20(19)26-29-31-32-30-26/h2-8,11-14,17H,9-10H2,1H3,(H,33,34)(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane |
Bioorg Med Chem Lett 4: 195-200 (1994)
Article DOI: 10.1016/S0960-894X(01)81146-X
BindingDB Entry DOI: 10.7270/Q2Z60P13 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50449909
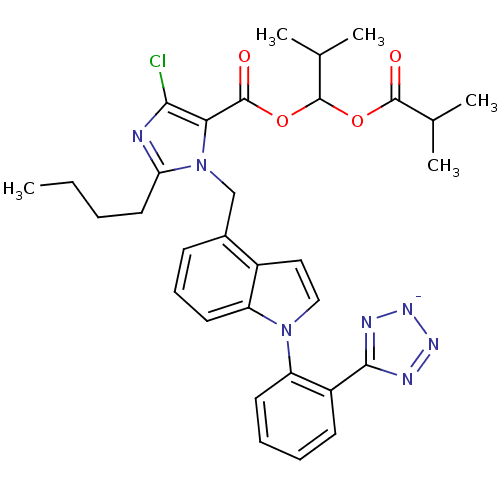
(CHEMBL2079769)Show SMILES [K+].CCCCc1nc(Cl)c(C(=O)OC(OC(=O)C(C)C)C(C)C)n1Cc1cccc2n(ccc12)-c1ccccc1-c1nn[n-]n1 Show InChI InChI=1S/C32H35ClN7O4.K/c1-6-7-15-26-34-28(33)27(31(42)44-32(20(4)5)43-30(41)19(2)3)40(26)18-21-11-10-14-24-22(21)16-17-39(24)25-13-9-8-12-23(25)29-35-37-38-36-29;/h8-14,16-17,19-20,32H,6-7,15,18H2,1-5H3;/q-1;+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes |
Bioorg Med Chem Lett 4: 145-150 (1994)
Article DOI: 10.1016/S0960-894X(01)81137-9
BindingDB Entry DOI: 10.7270/Q2V988KF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282319

(2-Cyclopropyl-6-fluoro-3-[2'-(2H-tetrazol-5-yl)-bi...)Show SMILES OC(=O)c1c(Oc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c(nc2ccc(F)cc12)C1CC1 Show InChI InChI=1S/C26H18FN5O3/c27-16-9-12-21-20(13-16)22(26(33)34)24(23(28-21)15-5-6-15)35-17-10-7-14(8-11-17)18-3-1-2-4-19(18)25-29-31-32-30-25/h1-4,7-13,15H,5-6H2,(H,33,34)(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane |
Bioorg Med Chem Lett 4: 195-200 (1994)
Article DOI: 10.1016/S0960-894X(01)81146-X
BindingDB Entry DOI: 10.7270/Q2Z60P13 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50449918

(BMS-181688 | CHEMBL2021416)Show SMILES [K+].CCCCc1nc(Cl)c(C(=O)OCC)n1Cc1cccc2n(ccc12)-c1ccccc1-c1nn[n-]n1 Show InChI InChI=1S/C26H25ClN7O2.K/c1-3-5-13-22-28-24(27)23(26(35)36-4-2)34(22)16-17-9-8-12-20-18(17)14-15-33(20)21-11-7-6-10-19(21)25-29-31-32-30-25;/h6-12,14-15H,3-5,13,16H2,1-2H3;/q-1;+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes |
Bioorg Med Chem Lett 4: 145-150 (1994)
Article DOI: 10.1016/S0960-894X(01)81137-9
BindingDB Entry DOI: 10.7270/Q2V988KF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50449920

(CHEMBL2079768)Show SMILES [K+].CCCCc1nc(Cl)c(C(=O)OC(OC(=O)CC)C(C)C)n1Cc1cccc2n(ccc12)-c1ccccc1-c1nn[n-]n1 Show InChI InChI=1S/C31H33ClN7O4.K/c1-5-7-15-25-33-28(32)27(30(41)43-31(19(3)4)42-26(40)6-2)39(25)18-20-11-10-14-23-21(20)16-17-38(23)24-13-9-8-12-22(24)29-34-36-37-35-29;/h8-14,16-17,19,31H,5-7,15,18H2,1-4H3;/q-1;+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes |
Bioorg Med Chem Lett 4: 145-150 (1994)
Article DOI: 10.1016/S0960-894X(01)81137-9
BindingDB Entry DOI: 10.7270/Q2V988KF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282321
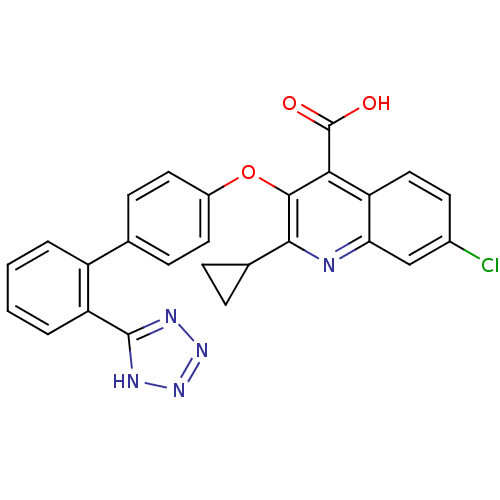
(7-Chloro-2-cyclopropyl-3-[2'-(2H-tetrazol-5-yl)-bi...)Show SMILES OC(=O)c1c(Oc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c(nc2cc(Cl)ccc12)C1CC1 Show InChI InChI=1S/C26H18ClN5O3/c27-16-9-12-20-21(13-16)28-23(15-5-6-15)24(22(20)26(33)34)35-17-10-7-14(8-11-17)18-3-1-2-4-19(18)25-29-31-32-30-25/h1-4,7-13,15H,5-6H2,(H,33,34)(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane |
Bioorg Med Chem Lett 4: 195-200 (1994)
Article DOI: 10.1016/S0960-894X(01)81146-X
BindingDB Entry DOI: 10.7270/Q2Z60P13 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282313

(2-Cyclopropyl-6-methoxy-3-[2'-(2H-tetrazol-5-yl)-b...)Show SMILES COc1ccc2nc(C3CC3)c(Oc3ccc(cc3)-c3ccccc3-c3nnn[nH]3)c(C(O)=O)c2c1 Show InChI InChI=1S/C27H21N5O4/c1-35-18-12-13-22-21(14-18)23(27(33)34)25(24(28-22)16-6-7-16)36-17-10-8-15(9-11-17)19-4-2-3-5-20(19)26-29-31-32-30-26/h2-5,8-14,16H,6-7H2,1H3,(H,33,34)(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane |
Bioorg Med Chem Lett 4: 195-200 (1994)
Article DOI: 10.1016/S0960-894X(01)81146-X
BindingDB Entry DOI: 10.7270/Q2Z60P13 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50449911
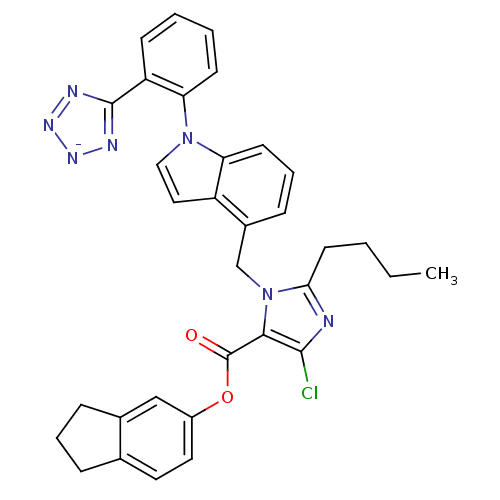
(CHEMBL2079770)Show SMILES [K+].CCCCc1nc(Cl)c(C(=O)Oc2ccc3CCCc3c2)n1Cc1cccc2n(ccc12)-c1ccccc1-c1nn[n-]n1 Show InChI InChI=1S/C33H29ClN7O2.K/c1-2-3-14-29-35-31(34)30(33(42)43-24-16-15-21-8-6-9-22(21)19-24)41(29)20-23-10-7-13-27-25(23)17-18-40(27)28-12-5-4-11-26(28)32-36-38-39-37-32;/h4-5,7,10-13,15-19H,2-3,6,8-9,14,20H2,1H3;/q-1;+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes |
Bioorg Med Chem Lett 4: 145-150 (1994)
Article DOI: 10.1016/S0960-894X(01)81137-9
BindingDB Entry DOI: 10.7270/Q2V988KF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50449915
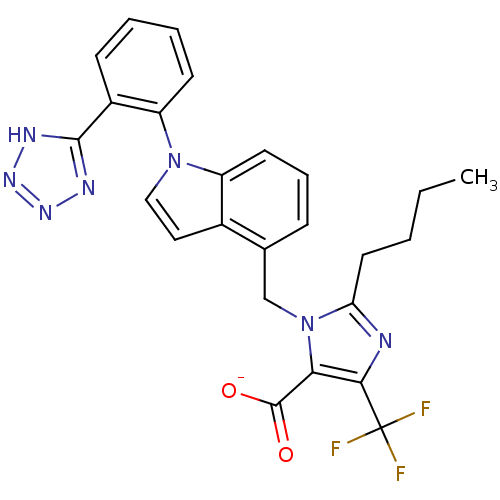
(CHEMBL2021419)Show SMILES [Na+].O[Na].CCCCc1nc(c(C([O-])=O)n1Cc1cccc2n(ccc12)-c1ccccc1-c1nn[nH]n1)C(F)(F)F Show InChI InChI=1S/C25H22F3N7O2.2Na.H2O/c1-2-3-11-20-29-22(25(26,27)28)21(24(36)37)35(20)14-15-7-6-10-18-16(15)12-13-34(18)19-9-5-4-8-17(19)23-30-32-33-31-23;;;/h4-10,12-13H,2-3,11,14H2,1H3,(H,36,37)(H,30,31,32,33);;;1H2/q;2*+1;/p-2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes |
Bioorg Med Chem Lett 4: 145-150 (1994)
Article DOI: 10.1016/S0960-894X(01)81137-9
BindingDB Entry DOI: 10.7270/Q2V988KF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282240
(CHEMBL288691 | Lithium; 2-[4-(2-butyl-5-hydroxymet...)Show SMILES CCCCc1ncc(CO)n1Cc1cccc2n(ccc12)-c1ccccc1C([O-])=O Show InChI InChI=1S/C24H25N3O3/c1-2-3-11-23-25-14-18(16-28)27(23)15-17-7-6-10-21-19(17)12-13-26(21)22-9-5-4-8-20(22)24(29)30/h4-10,12-14,28H,2-3,11,15-16H2,1H3,(H,29,30)/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes |
Bioorg Med Chem Lett 4: 145-150 (1994)
Article DOI: 10.1016/S0960-894X(01)81137-9
BindingDB Entry DOI: 10.7270/Q2V988KF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282323
(2-Cyclopropyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-...)Show SMILES OC(=O)c1cccc2nc(C3CC3)c(Oc3ccc(cc3)-c3ccccc3-c3nnn[nH]3)c(C(O)=O)c12 Show InChI InChI=1S/C27H19N5O5/c33-26(34)19-6-3-7-20-21(19)22(27(35)36)24(23(28-20)15-8-9-15)37-16-12-10-14(11-13-16)17-4-1-2-5-18(17)25-29-31-32-30-25/h1-7,10-13,15H,8-9H2,(H,33,34)(H,35,36)(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane |
Bioorg Med Chem Lett 4: 195-200 (1994)
Article DOI: 10.1016/S0960-894X(01)81146-X
BindingDB Entry DOI: 10.7270/Q2Z60P13 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282248

(CHEMBL287065 | Lithium; 2-[5-(2-butyl-5-hydroxymet...)Show SMILES CCCCc1ncc(CO)n1Cc1ccc2n(ccc2c1)-c1ccccc1C([O-])=O Show InChI InChI=1S/C24H25N3O3/c1-2-3-8-23-25-14-19(16-28)27(23)15-17-9-10-21-18(13-17)11-12-26(21)22-7-5-4-6-20(22)24(29)30/h4-7,9-14,28H,2-3,8,15-16H2,1H3,(H,29,30)/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes |
Bioorg Med Chem Lett 4: 145-150 (1994)
Article DOI: 10.1016/S0960-894X(01)81137-9
BindingDB Entry DOI: 10.7270/Q2V988KF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50449916
(CHEMBL2021418)Show SMILES [Li+].[Li]O.CCCCc1nc(Cl)c(C([O-])=O)n1Cc1cccc2n(c(Br)c(Br)c12)-c1ccccc1-c1nn[nH]n1 |(16.45,-8.34,;18.81,-10.28,;20.35,-10.28,;2.91,-6.88,;4.05,-7.9,;5.51,-7.43,;6.65,-8.47,;8.11,-7.99,;8.6,-6.52,;10.13,-6.52,;11.04,-5.28,;10.62,-7.99,;12.08,-8.47,;13.22,-7.44,;12.38,-9.97,;9.36,-8.89,;10.46,-9.98,;10.46,-11.52,;9.11,-12.27,;9.09,-13.81,;10.43,-14.59,;11.78,-13.81,;13.29,-14.15,;14.12,-13.05,;15.59,-12.58,;13.24,-11.8,;13.71,-10.34,;11.78,-12.28,;13.33,-15.7,;12.01,-16.51,;12.06,-18.05,;13.4,-18.78,;14.7,-17.97,;14.66,-16.44,;15.98,-15.62,;16.1,-14.08,;17.58,-13.72,;18.4,-15.03,;17.4,-16.21,)| Show InChI InChI=1S/C24H20Br2ClN7O2.2Li.H2O/c1-2-3-11-17-28-22(27)20(24(35)36)33(17)12-13-7-6-10-16-18(13)19(25)21(26)34(16)15-9-5-4-8-14(15)23-29-31-32-30-23;;;/h4-10H,2-3,11-12H2,1H3,(H,35,36)(H,29,30,31,32);;;1H2/q;2*+1;/p-2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes |
Bioorg Med Chem Lett 4: 145-150 (1994)
Article DOI: 10.1016/S0960-894X(01)81137-9
BindingDB Entry DOI: 10.7270/Q2V988KF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282320
(2-Cyclopropyl-8-methyl-3-[2'-(2H-tetrazol-5-yl)-bi...)Show SMILES Cc1cccc2c(C(O)=O)c(Oc3ccc(cc3)-c3ccccc3-c3nnn[nH]3)c(nc12)C1CC1 Show InChI InChI=1S/C27H21N5O3/c1-15-5-4-8-21-22(27(33)34)25(24(17-9-10-17)28-23(15)21)35-18-13-11-16(12-14-18)19-6-2-3-7-20(19)26-29-31-32-30-26/h2-8,11-14,17H,9-10H2,1H3,(H,33,34)(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane |
Bioorg Med Chem Lett 4: 195-200 (1994)
Article DOI: 10.1016/S0960-894X(01)81146-X
BindingDB Entry DOI: 10.7270/Q2Z60P13 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282244

(2-Butyl-3-{1-[2-(2H-tetrazol-5-yl)-phenyl]-1H-indo...)Show SMILES CCCCc1ncc(C(O)=O)n1Cc1cccc2n(ccc12)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H23N7O2/c1-2-3-11-22-25-14-21(24(32)33)31(22)15-16-7-6-10-19-17(16)12-13-30(19)20-9-5-4-8-18(20)23-26-28-29-27-23/h4-10,12-14H,2-3,11,15H2,1H3,(H,32,33)(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes |
Bioorg Med Chem Lett 4: 145-150 (1994)
Article DOI: 10.1016/S0960-894X(01)81137-9
BindingDB Entry DOI: 10.7270/Q2V988KF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282254

(CHEMBL36259 | Potassium; 2-[6-(2-butyl-4-chloro-5-...)Show SMILES CCCCc1nc(Cl)c(C=O)n1Cc1ccc2nc(oc2c1)-c1ccccc1C([O-])=O Show InChI InChI=1S/C23H20ClN3O4/c1-2-3-8-20-26-21(24)18(13-28)27(20)12-14-9-10-17-19(11-14)31-22(25-17)15-6-4-5-7-16(15)23(29)30/h4-7,9-11,13H,2-3,8,12H2,1H3,(H,29,30)/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes |
Bioorg Med Chem Lett 4: 145-150 (1994)
Article DOI: 10.1016/S0960-894X(01)81137-9
BindingDB Entry DOI: 10.7270/Q2V988KF |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50449917

(BMS-180560 | CHEMBL2021417)Show SMILES [Li+].[Li]O.CCCCc1nc(Cl)c(C([O-])=O)n1Cc1cccc2n(ccc12)-c1ccccc1-c1nn[nH]n1 Show InChI InChI=1S/C24H22ClN7O2.2Li.H2O/c1-2-3-11-20-26-22(25)21(24(33)34)32(20)14-15-7-6-10-18-16(15)12-13-31(18)19-9-5-4-8-17(19)23-27-29-30-28-23;;;/h4-10,12-13H,2-3,11,14H2,1H3,(H,33,34)(H,27,28,29,30);;;1H2/q;2*+1;/p-2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Receptor binding affinity for Angiotensin II receptor, type 2 determined |
Bioorg Med Chem Lett 4: 145-150 (1994)
Article DOI: 10.1016/S0960-894X(01)81137-9
BindingDB Entry DOI: 10.7270/Q2V988KF |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18867
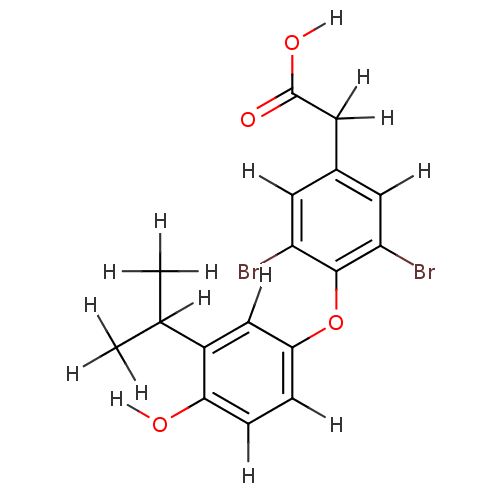
(2-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)phenoxy...)Show InChI InChI=1S/C17H16Br2O4/c1-9(2)12-8-11(3-4-15(12)20)23-17-13(18)5-10(6-14(17)19)7-16(21)22/h3-6,8-9,20H,7H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.0950 | n/a | 0.200 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to r... |
Bioorg Med Chem Lett 17: 4131-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.049
BindingDB Entry DOI: 10.7270/Q2VT1QB6 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18867

(2-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)phenoxy...)Show InChI InChI=1S/C17H16Br2O4/c1-9(2)12-8-11(3-4-15(12)20)23-17-13(18)5-10(6-14(17)19)7-16(21)22/h3-6,8-9,20H,7H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18913
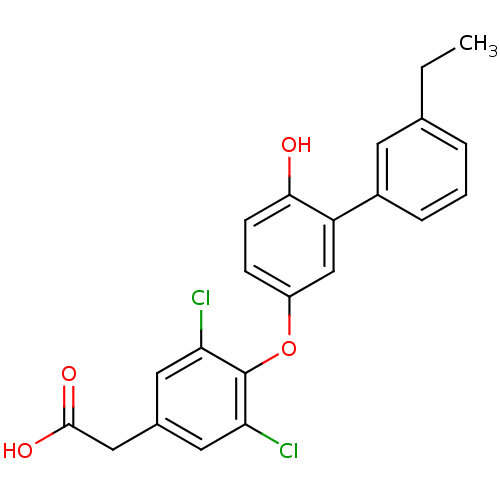
(2-{3,5-dichloro-4-[3-(3-ethylphenyl)-4-hydroxyphen...)Show SMILES CCc1cccc(c1)-c1cc(Oc2c(Cl)cc(CC(O)=O)cc2Cl)ccc1O Show InChI InChI=1S/C22H18Cl2O4/c1-2-13-4-3-5-15(8-13)17-12-16(6-7-20(17)25)28-22-18(23)9-14(10-19(22)24)11-21(26)27/h3-10,12,25H,2,11H2,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18860
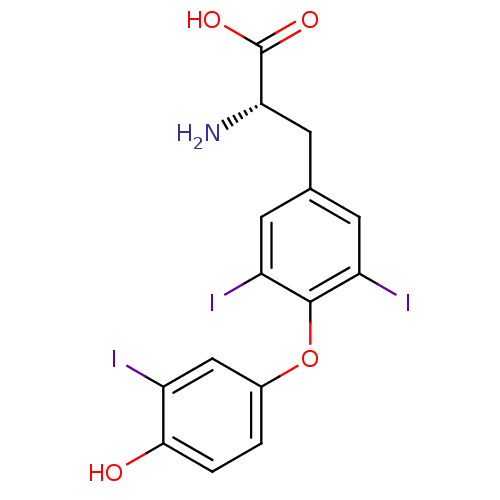
((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against cloned human thyroid hormone receptor alpha 1 |
Bioorg Med Chem Lett 15: 4579-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.093
BindingDB Entry DOI: 10.7270/Q2GB23MG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. EC50 is the concentration of compound required to ... |
Bioorg Med Chem Lett 17: 4131-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.049
BindingDB Entry DOI: 10.7270/Q2VT1QB6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against cloned human thyroid hormone receptor beta 1 |
Bioorg Med Chem Lett 15: 4579-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.093
BindingDB Entry DOI: 10.7270/Q2GB23MG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to r... |
Bioorg Med Chem Lett 17: 4131-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.049
BindingDB Entry DOI: 10.7270/Q2VT1QB6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18874

((2S)-2-(1-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl...)Show SMILES CC(C)[C@H](NC(=O)Cc1cc(Br)c(Oc2ccc(O)c(c2)C(C)C)c(Br)c1)C(O)=O |r| Show InChI InChI=1S/C22H25Br2NO5/c1-11(2)15-10-14(5-6-18(15)26)30-21-16(23)7-13(8-17(21)24)9-19(27)25-20(12(3)4)22(28)29/h5-8,10-12,20,26H,9H2,1-4H3,(H,25,27)(H,28,29)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | 0.770 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to r... |
Bioorg Med Chem Lett 17: 4131-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.049
BindingDB Entry DOI: 10.7270/Q2VT1QB6 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50171808
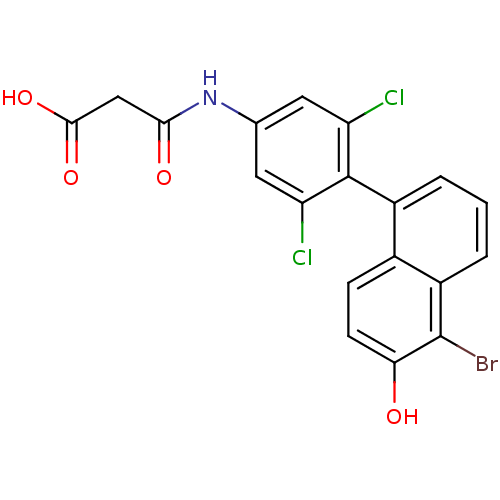
(CHEMBL194242 | N-[4-(5-Bromo-6-hydroxy-naphthalen-...)Show SMILES OC(=O)CC(=O)Nc1cc(Cl)c(c(Cl)c1)-c1cccc2c(Br)c(O)ccc12 |(13.88,-4.76,;13.87,-3.22,;15.2,-2.45,;12.54,-2.46,;12.53,-.92,;13.87,-.14,;11.2,-.15,;9.85,-.92,;9.87,-2.46,;8.52,-3.24,;8.52,-4.78,;7.19,-2.47,;7.19,-.93,;5.86,-.16,;8.52,-.16,;5.86,-3.25,;5.86,-4.78,;4.53,-5.56,;3.2,-4.79,;3.2,-3.25,;1.87,-2.48,;.52,-3.25,;1.87,-.93,;.54,-.17,;3.2,-.16,;4.53,-.93,;4.53,-2.48,)| Show InChI InChI=1S/C19H12BrCl2NO4/c20-19-12-3-1-2-11(10(12)4-5-15(19)24)18-13(21)6-9(7-14(18)22)23-16(25)8-17(26)27/h1-7,24H,8H2,(H,23,25)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against cloned human thyroid hormone receptor beta 1 |
Bioorg Med Chem Lett 15: 4579-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.093
BindingDB Entry DOI: 10.7270/Q2GB23MG |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50171801
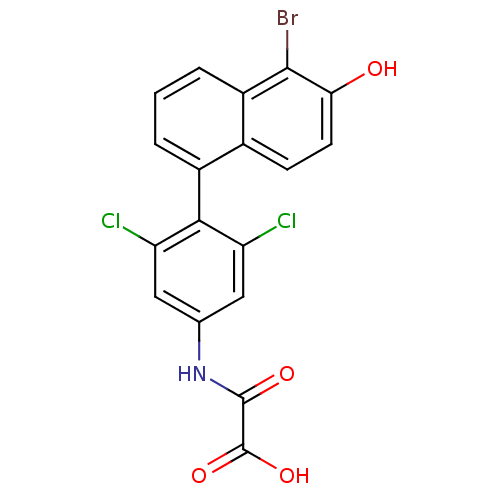
(CHEMBL198367 | N-[4-(5-Bromo-6-hydroxy-naphthalen-...)Show SMILES OC(=O)C(=O)Nc1cc(Cl)c(c(Cl)c1)-c1cccc2c(Br)c(O)ccc12 |(11.21,-4.35,;12.54,-3.57,;13.87,-4.32,;12.53,-2.03,;13.87,-1.24,;11.2,-1.26,;9.85,-2.04,;8.52,-1.27,;7.19,-2.04,;5.86,-1.27,;7.19,-3.58,;8.52,-4.35,;8.52,-5.89,;9.87,-3.58,;5.86,-4.36,;5.86,-5.89,;4.53,-6.67,;3.2,-5.9,;3.2,-4.36,;1.87,-3.59,;.52,-4.36,;1.87,-2.05,;.54,-1.28,;3.2,-1.27,;4.53,-2.04,;4.53,-3.59,)| Show InChI InChI=1S/C18H10BrCl2NO4/c19-16-11-3-1-2-10(9(11)4-5-14(16)23)15-12(20)6-8(7-13(15)21)22-17(24)18(25)26/h1-7,23H,(H,22,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against cloned human thyroid hormone receptor beta 1 |
Bioorg Med Chem Lett 15: 4579-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.093
BindingDB Entry DOI: 10.7270/Q2GB23MG |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50171802

(CHEMBL196642 | N-[3,5-Dichloro-4-(5-isopropyl-6-me...)Show SMILES COc1ccc2c(cccc2c1C(C)C)-c1c(Cl)cc(NC(=O)CC(O)=O)cc1Cl |(1.71,1.66,;1.71,.12,;3.04,-.65,;4.37,.13,;5.72,-.64,;5.72,-2.19,;7.05,-2.96,;7.05,-4.49,;5.7,-5.27,;4.37,-4.5,;4.37,-2.96,;3.04,-2.19,;1.71,-2.96,;.38,-2.19,;1.71,-4.5,;8.38,-2.18,;9.71,-2.95,;9.71,-4.49,;11.04,-2.18,;11.04,-.64,;12.37,.14,;13.7,-.63,;15.05,.16,;13.72,-2.17,;15.06,-2.92,;15.06,-4.46,;16.39,-2.15,;9.71,.13,;8.38,-.64,;7.05,.13,)| Show InChI InChI=1S/C23H21Cl2NO4/c1-12(2)22-15-5-4-6-16(14(15)7-8-19(22)30-3)23-17(24)9-13(10-18(23)25)26-20(27)11-21(28)29/h4-10,12H,11H2,1-3H3,(H,26,27)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against cloned human thyroid hormone receptor beta 1 |
Bioorg Med Chem Lett 15: 4579-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.093
BindingDB Entry DOI: 10.7270/Q2GB23MG |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50281226

(CHEMBL328736 | N-[(S)-1-[(2R,3S)-4-((R)-Butylsulfa...)Show SMILES CCCCNS(=O)(=O)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)CS(=O)(=O)C(C)(C)C Show InChI InChI=1S/C35H57N5O8S2/c1-5-6-17-38-50(47,48)23-31(41)32(42)29(19-26-15-11-8-12-16-26)39-34(44)30(20-28-21-36-24-37-28)40-33(43)27(18-25-13-9-7-10-14-25)22-49(45,46)35(2,3)4/h7,9-10,13-14,21,24,26-27,29-32,38,41-42H,5-6,8,11-12,15-20,22-23H2,1-4H3,(H,36,37)(H,39,44)(H,40,43)/t27-,29+,30+,31+,32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | 7.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested for its inhibitory activity against human renal renin at pH 7 |
Bioorg Med Chem Lett 3: 2739-2744 (1993)
Article DOI: 10.1016/S0960-894X(01)80755-1
BindingDB Entry DOI: 10.7270/Q2Q52PJ2 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18954
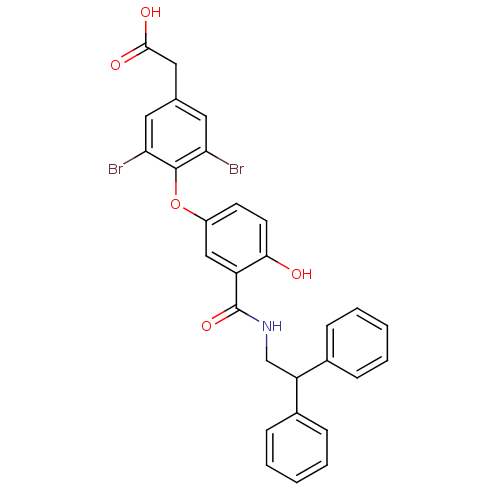
(2-(3,5-dibromo-4-{3-[(2,2-diphenylethyl)carbamoyl]...)Show SMILES OC(=O)Cc1cc(Br)c(Oc2ccc(O)c(c2)C(=O)NCC(c2ccccc2)c2ccccc2)c(Br)c1 Show InChI InChI=1S/C29H23Br2NO5/c30-24-13-18(15-27(34)35)14-25(31)28(24)37-21-11-12-26(33)22(16-21)29(36)32-17-23(19-7-3-1-4-8-19)20-9-5-2-6-10-20/h1-14,16,23,33H,15,17H2,(H,32,36)(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18877
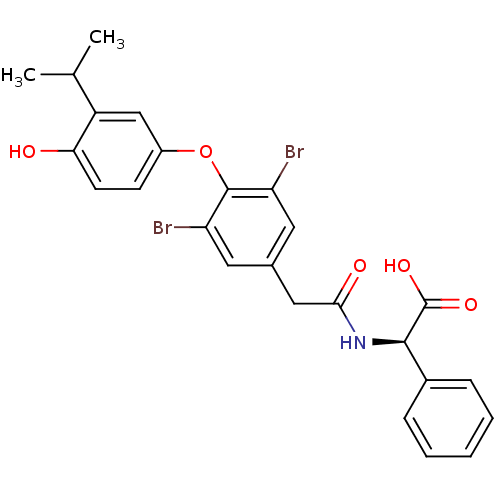
((2R)-2-(1-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl...)Show SMILES CC(C)c1cc(Oc2c(Br)cc(CC(=O)N[C@@H](C(O)=O)c3ccccc3)cc2Br)ccc1O |r| Show InChI InChI=1S/C25H23Br2NO5/c1-14(2)18-13-17(8-9-21(18)29)33-24-19(26)10-15(11-20(24)27)12-22(30)28-23(25(31)32)16-6-4-3-5-7-16/h3-11,13-14,23,29H,12H2,1-2H3,(H,28,30)(H,31,32)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | 0.730 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to r... |
Bioorg Med Chem Lett 17: 4131-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.049
BindingDB Entry DOI: 10.7270/Q2VT1QB6 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18948
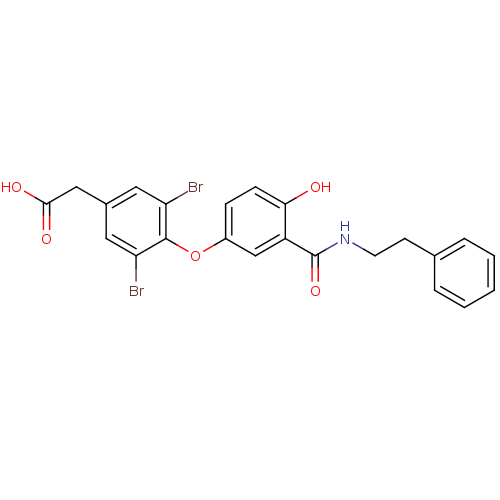
(2-(3,5-dibromo-4-{4-hydroxy-3-[(2-phenylethyl)carb...)Show SMILES OC(=O)Cc1cc(Br)c(Oc2ccc(O)c(c2)C(=O)NCCc2ccccc2)c(Br)c1 Show InChI InChI=1S/C23H19Br2NO5/c24-18-10-15(12-21(28)29)11-19(25)22(18)31-16-6-7-20(27)17(13-16)23(30)26-9-8-14-4-2-1-3-5-14/h1-7,10-11,13,27H,8-9,12H2,(H,26,30)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18875
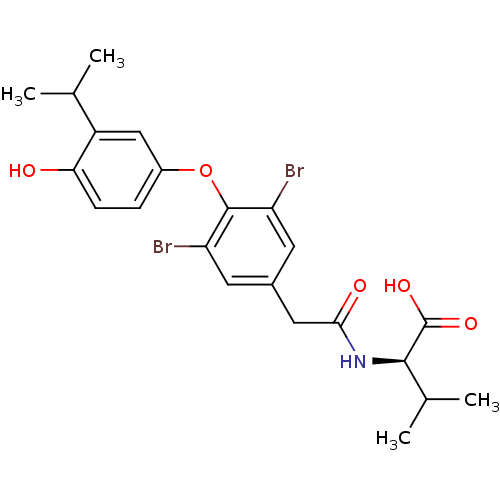
((2R)-2-(1-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl...)Show SMILES CC(C)[C@@H](NC(=O)Cc1cc(Br)c(Oc2ccc(O)c(c2)C(C)C)c(Br)c1)C(O)=O |r| Show InChI InChI=1S/C22H25Br2NO5/c1-11(2)15-10-14(5-6-18(15)26)30-21-16(23)7-13(8-17(21)24)9-19(27)25-20(12(3)4)22(28)29/h5-8,10-12,20,26H,9H2,1-4H3,(H,25,27)(H,28,29)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.710 | n/a | 1.20 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to r... |
Bioorg Med Chem Lett 17: 4131-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.049
BindingDB Entry DOI: 10.7270/Q2VT1QB6 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18927

(2-(3,5-dichloro-4-{3-[3-(difluoromethoxy)phenyl]-4...)Show SMILES OC(=O)Cc1cc(Cl)c(Oc2ccc(O)c(c2)-c2cccc(OC(F)F)c2)c(Cl)c1 Show InChI InChI=1S/C21H14Cl2F2O5/c22-16-6-11(8-19(27)28)7-17(23)20(16)29-14-4-5-18(26)15(10-14)12-2-1-3-13(9-12)30-21(24)25/h1-7,9-10,21,26H,8H2,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
Bioorg Med Chem Lett 14: 3549-53 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.032
BindingDB Entry DOI: 10.7270/Q2736P5M |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18876
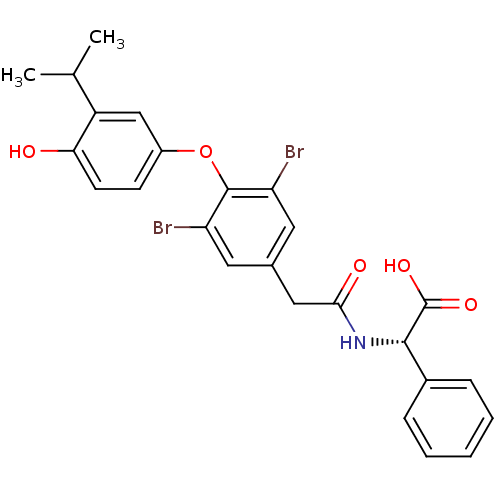
((2S)-2-(1-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl...)Show SMILES CC(C)c1cc(Oc2c(Br)cc(CC(=O)N[C@H](C(O)=O)c3ccccc3)cc2Br)ccc1O |r| Show InChI InChI=1S/C25H23Br2NO5/c1-14(2)18-13-17(8-9-21(18)29)33-24-19(26)10-15(11-20(24)27)12-22(30)28-23(25(31)32)16-6-4-3-5-7-16/h3-11,13-14,23,29H,12H2,1-2H3,(H,28,30)(H,31,32)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to r... |
Bioorg Med Chem Lett 17: 4131-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.049
BindingDB Entry DOI: 10.7270/Q2VT1QB6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data