

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM13304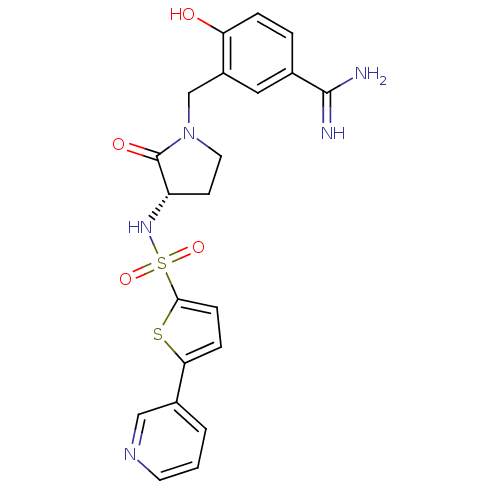 (4-hydroxy-3-[((3S)-2-oxo-3-{[(5-pyridin-3-ylthien-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13306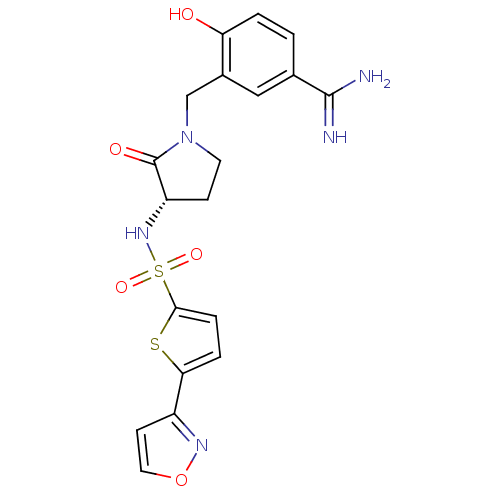 (4-Hydroxy-3-[3-(S)-(5-isoxazol-3-ylthiophene-2-yls...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13286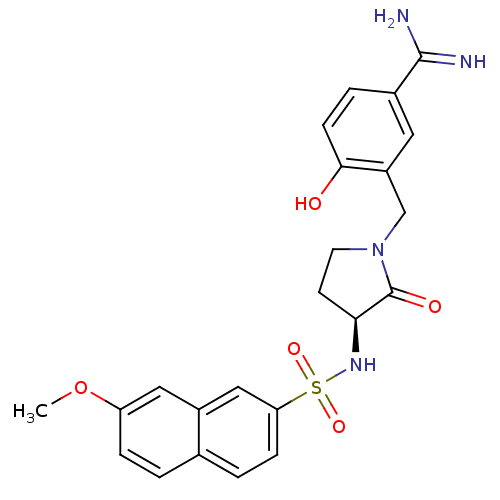 (4-Hydroxy-3-[3-(S)-(7-methoxynaphthalen-2-ylsulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13283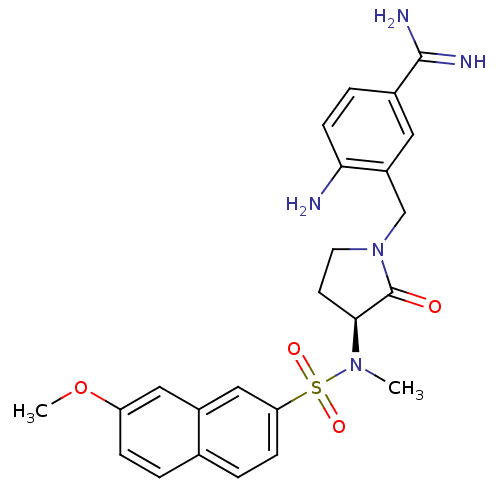 (4-amino-3-({(3S)-3-[[(7-methoxy-2-naphthyl)sulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13303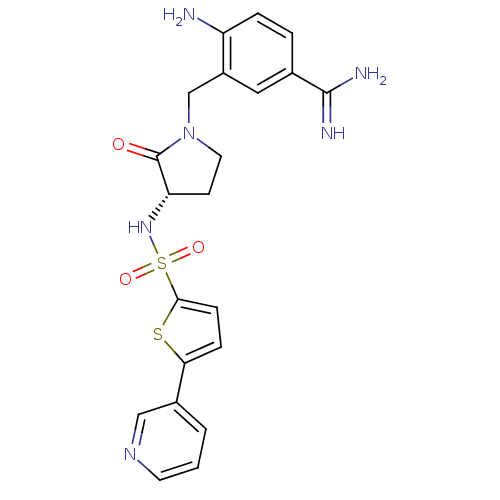 (4-Amino-3-[2-oxo-3-(S)-(5-pyridin-3-ylthiophene-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13281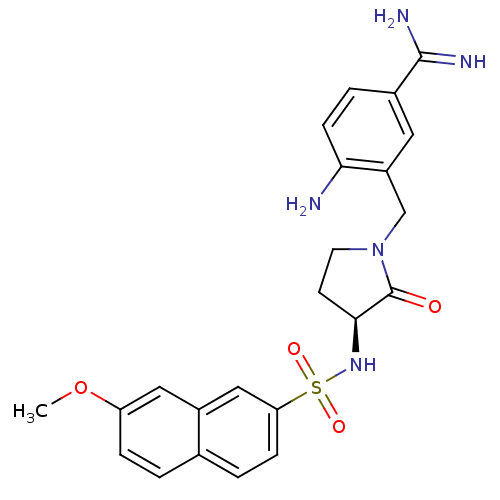 (4-Amino-3-[3-(S)-(7-methoxynaphthalen-2-ylsulfonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13279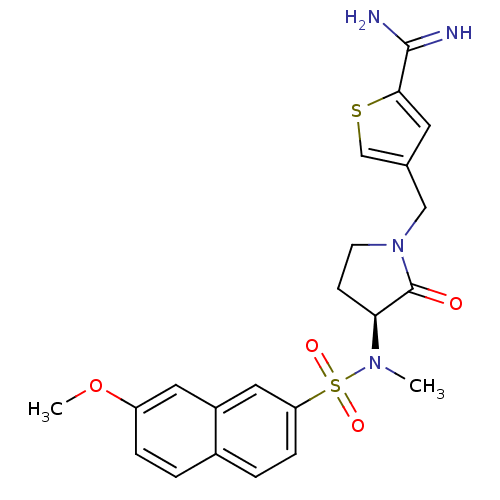 (4-{[(3S)-3-[(7-methoxynaphthalene-2-)(methyl)sulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.90 | -46.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13305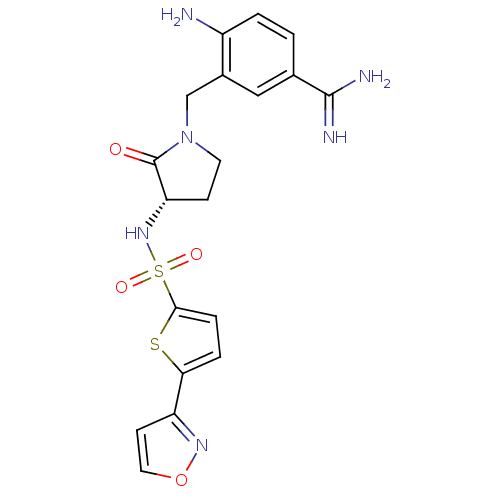 (4-Amino-3-[3-(S)-(5-isoxazol-3-ylthiophene-2-ylsul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13288 (Sulfonamidopyrrolidinone 27 | methyl 2-(4-carbamim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM13288 (Sulfonamidopyrrolidinone 27 | methyl 2-(4-carbamim...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | -44.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13282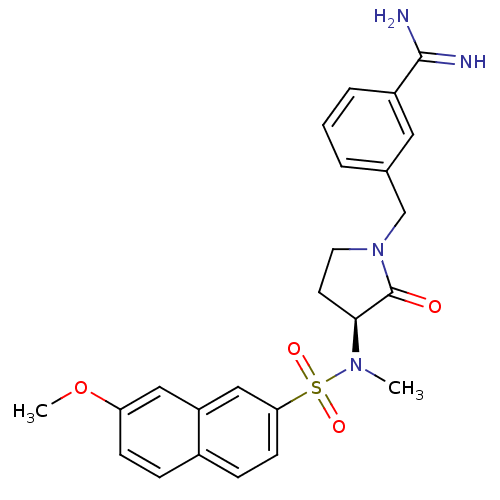 (3-{[(3S)-3-[(7-methoxynaphthalene-2-)(methyl)sulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | -43.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13298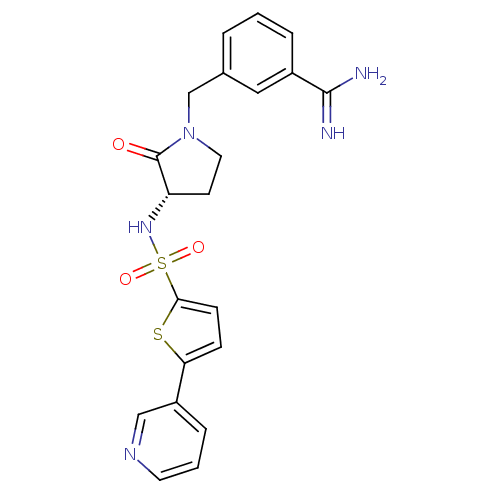 (3-[2-Oxo-3-(S)-(5-pyridin-3-ylthiophene-2-ylsulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13280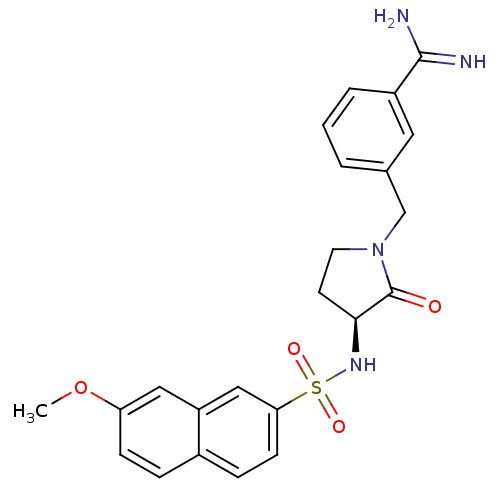 (3-{[(3S)-3-[(7-methoxynaphthalene-2-)sulfonamido]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 47 | -41.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13301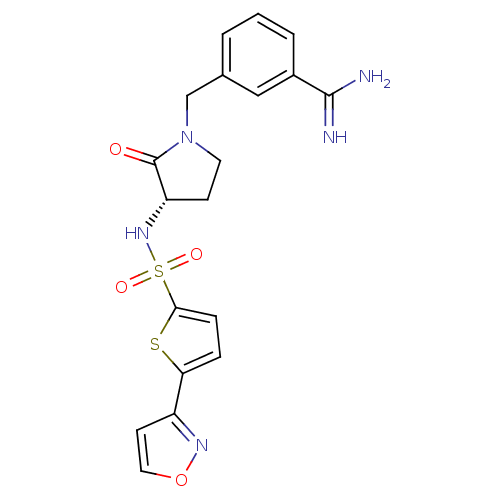 (3-[3-(S)-(5-Isoxazol-3-ylthiophene-2-ylsulfonylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13299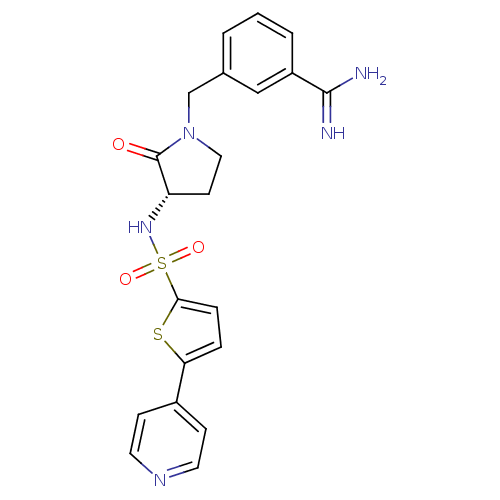 (3-[2-Oxo-3-(S)-(5-pyridin-4-ylthiophene-2-ylsulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50328879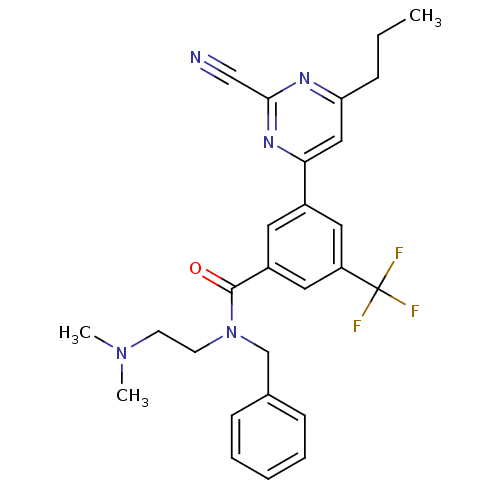 (CHEMBL1234898 | N-benzyl-3-(2-cyano-6-propylpyrimi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells | Bioorg Med Chem Lett 20: 6237-41 (2010) Article DOI: 10.1016/j.bmcl.2010.08.101 BindingDB Entry DOI: 10.7270/Q2RR1ZFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13293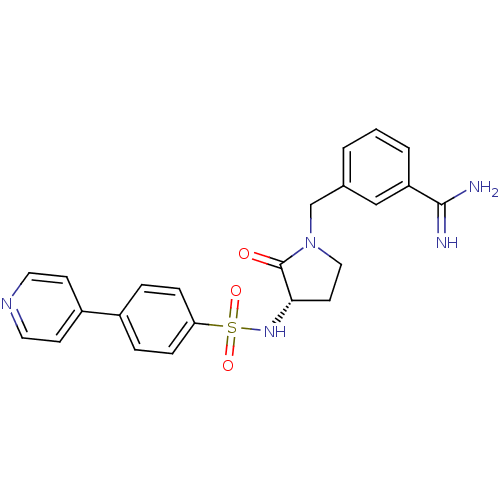 (3-{[(3S)-2-oxo-3-{[4-(pyridin-4-yl)benzene]sulfona...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13302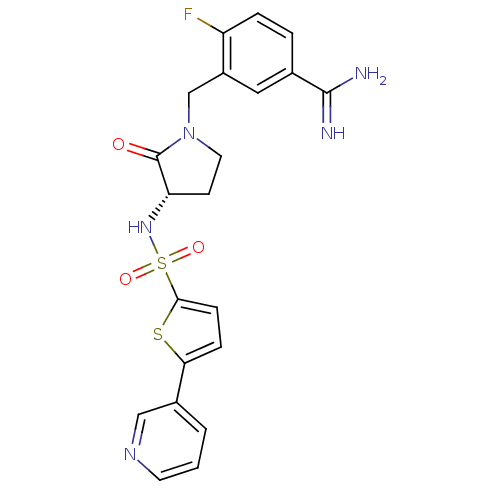 (4-fluoro-3-[((3S)-2-oxo-3-{[(5-pyridin-3-ylthien-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13297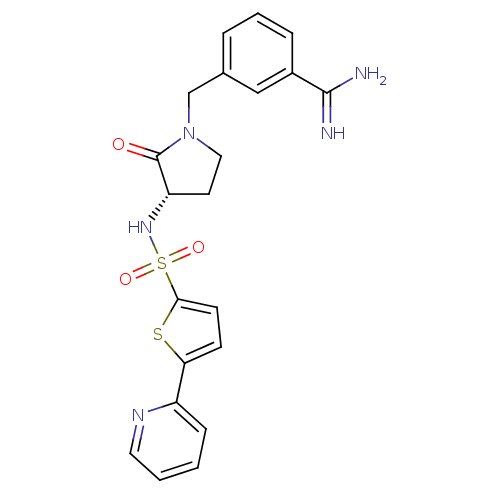 (3-{[(3S)-2-oxo-3-{[5-(pyridin-2-yl)thiophene-2-]su...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13291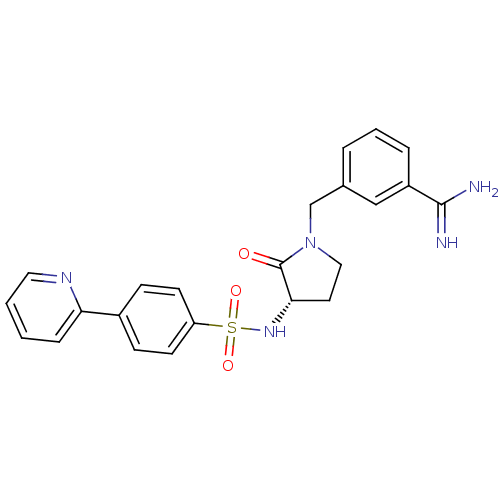 (3-{[(3S)-2-oxo-3-{[4-(pyridin-2-yl)benzene]sulfona...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 150 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13300 (3-{[(3S)-3-{[5-(2-methoxypyrimidin-4-yl)thiophene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13290 (3-{[(3S)-2-oxo-3-[(4-phenylbenzene)sulfonamido]pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 160 | -38.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50313476 (4-(3-(piperidin-1-yl)propyl)-6-(3-(trifluoromethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells | Bioorg Med Chem Lett 20: 6237-41 (2010) Article DOI: 10.1016/j.bmcl.2010.08.101 BindingDB Entry DOI: 10.7270/Q2RR1ZFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM13283 (4-amino-3-({(3S)-3-[[(7-methoxy-2-naphthyl)sulfony...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 189 | -38.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13292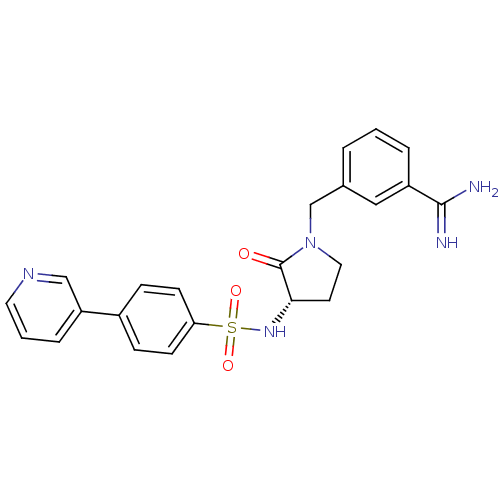 (3-{[(3S)-2-oxo-3-{[4-(pyridin-3-yl)benzene]sulfona...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM13281 (4-Amino-3-[3-(S)-(7-methoxynaphthalen-2-ylsulfonyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 299 | -36.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM13286 (4-Hydroxy-3-[3-(S)-(7-methoxynaphthalen-2-ylsulfon...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 305 | -36.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50313477 (4-(3-(pentan-3-ylamino)propyl)-6-(3-(trifluorometh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells | Bioorg Med Chem Lett 20: 6237-41 (2010) Article DOI: 10.1016/j.bmcl.2010.08.101 BindingDB Entry DOI: 10.7270/Q2RR1ZFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50328887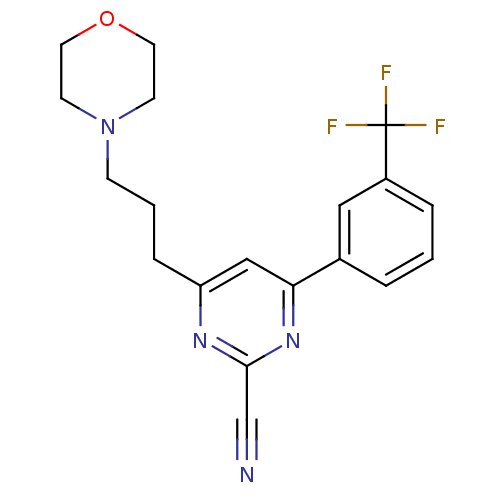 (4-(3-morpholinopropyl)-6-(3-(trifluoromethyl)pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells | Bioorg Med Chem Lett 20: 6237-41 (2010) Article DOI: 10.1016/j.bmcl.2010.08.101 BindingDB Entry DOI: 10.7270/Q2RR1ZFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13295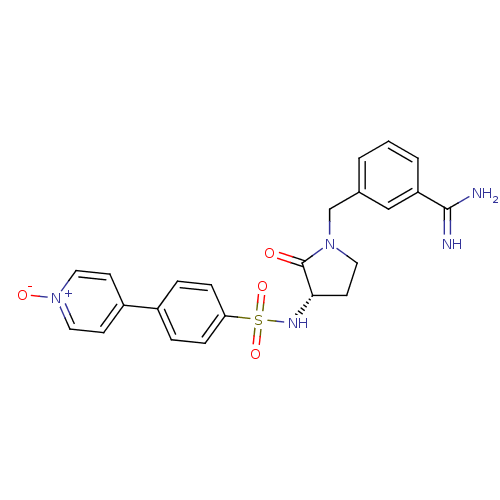 (4-(4-{[(3S)-1-[(3-carbamimidoylphenyl)methyl]-2-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50328880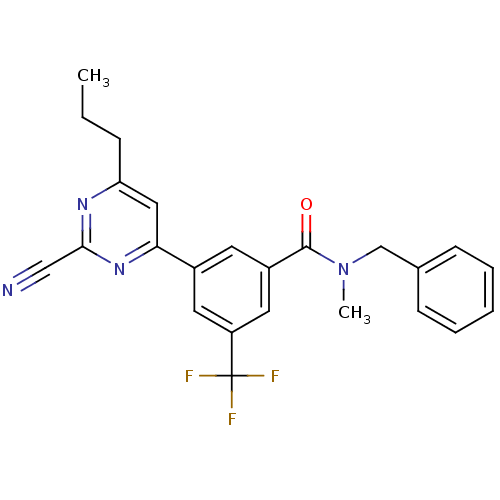 (CHEMBL1271174 | N-benzyl-3-(2-cyano-6-propylpyrimi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells | Bioorg Med Chem Lett 20: 6237-41 (2010) Article DOI: 10.1016/j.bmcl.2010.08.101 BindingDB Entry DOI: 10.7270/Q2RR1ZFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50328890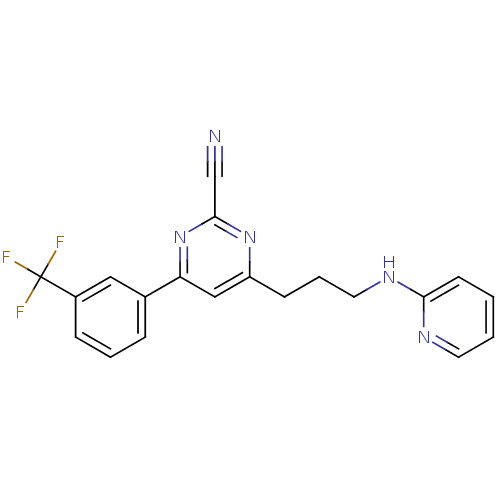 (4-(3-(pyridin-2-ylamino)propyl)-6-(3-(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells | Bioorg Med Chem Lett 20: 6237-41 (2010) Article DOI: 10.1016/j.bmcl.2010.08.101 BindingDB Entry DOI: 10.7270/Q2RR1ZFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50328885 (4-(3-(2,2,2-trifluoroethylamino)propyl)-6-(3-(trif...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells | Bioorg Med Chem Lett 20: 6237-41 (2010) Article DOI: 10.1016/j.bmcl.2010.08.101 BindingDB Entry DOI: 10.7270/Q2RR1ZFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50328888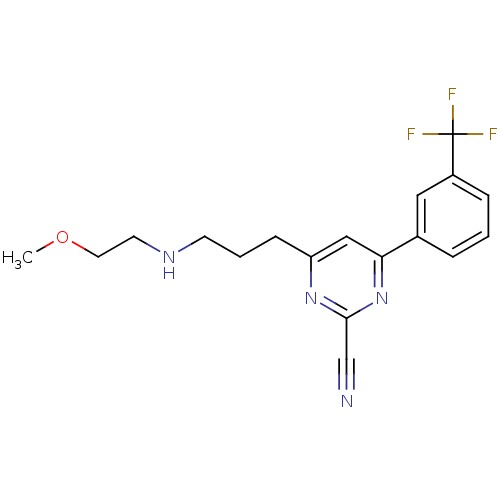 (4-(3-(2-methoxyethylamino)propyl)-6-(3-(trifluorom...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells | Bioorg Med Chem Lett 20: 6237-41 (2010) Article DOI: 10.1016/j.bmcl.2010.08.101 BindingDB Entry DOI: 10.7270/Q2RR1ZFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50328884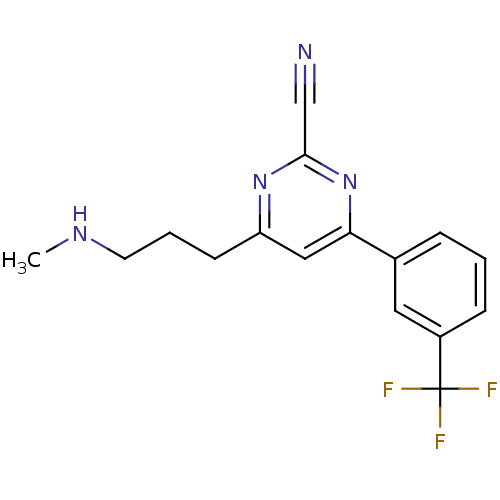 (4-(3-(methylamino)propyl)-6-(3-(trifluoromethyl)ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells | Bioorg Med Chem Lett 20: 6237-41 (2010) Article DOI: 10.1016/j.bmcl.2010.08.101 BindingDB Entry DOI: 10.7270/Q2RR1ZFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50313479 (4-cycloheptyl-6-(3-(piperidin-1-yl)propyl)pyrimidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells | Bioorg Med Chem Lett 20: 6237-41 (2010) Article DOI: 10.1016/j.bmcl.2010.08.101 BindingDB Entry DOI: 10.7270/Q2RR1ZFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM13282 (3-{[(3S)-3-[(7-methoxynaphthalene-2-)(methyl)sulfo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 704 | -34.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50328878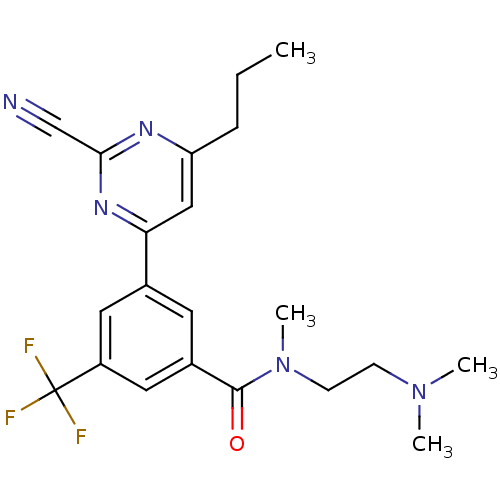 (3-(2-cyano-6-propylpyrimidin-4-yl)-N-(2-(dimethyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells | Bioorg Med Chem Lett 20: 6237-41 (2010) Article DOI: 10.1016/j.bmcl.2010.08.101 BindingDB Entry DOI: 10.7270/Q2RR1ZFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM13280 (3-{[(3S)-3-[(7-methoxynaphthalene-2-)sulfonamido]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 853 | -34.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13284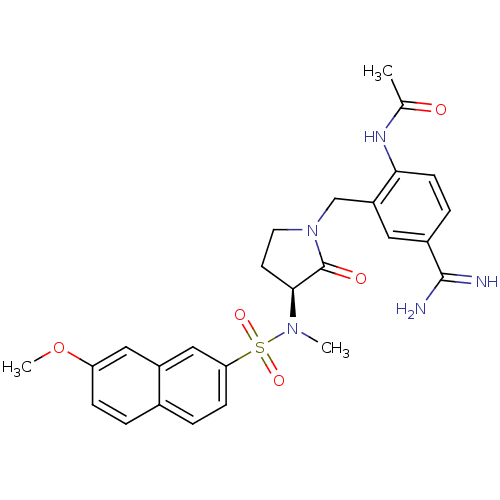 (N-(4-carbamimidoyl-2-{[(3S)-3-[(7-methoxynaphthale...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 919 | -34.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13287 (2-Hydroxy-5-[3-(S)-(7-methoxynaphthalen-2-ylsulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 983 | -33.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13294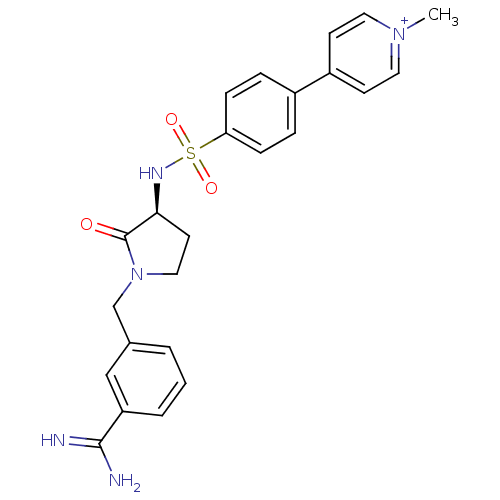 (4-(4-{[(3S)-1-[(3-carbamimidoylphenyl)methyl]-2-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50328886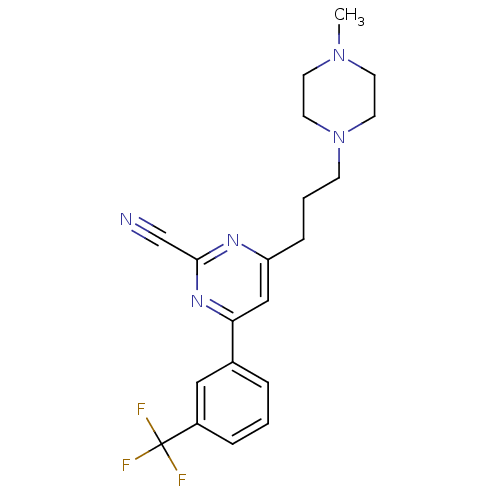 (4-(3-(4-methylpiperazin-1-yl)propyl)-6-(3-(trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells | Bioorg Med Chem Lett 20: 6237-41 (2010) Article DOI: 10.1016/j.bmcl.2010.08.101 BindingDB Entry DOI: 10.7270/Q2RR1ZFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50328889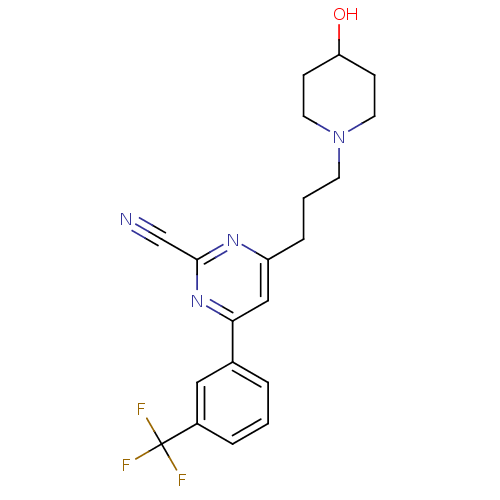 (4-(3-(4-hydroxypiperidin-1-yl)propyl)-6-(3-(triflu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells | Bioorg Med Chem Lett 20: 6237-41 (2010) Article DOI: 10.1016/j.bmcl.2010.08.101 BindingDB Entry DOI: 10.7270/Q2RR1ZFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13285 (3-Amino-5-[3-(S)-(7-methoxynaphthalen-2-ylsulfonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20E+3 | -33.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13289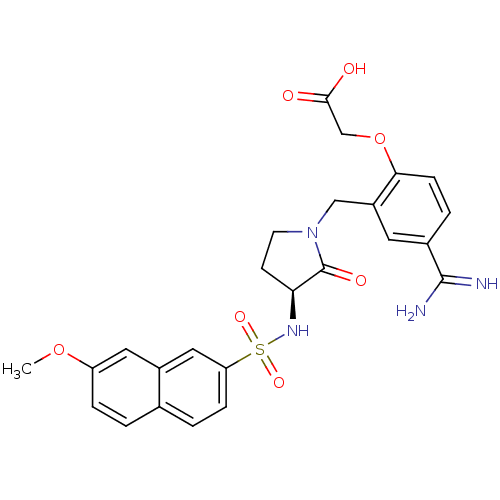 (2-(4-carbamimidoyl-2-{[(3S)-3-[(7-methoxynaphthale...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | -33.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM13303 (4-Amino-3-[2-oxo-3-(S)-(5-pyridin-3-ylthiophene-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50328895 (1-(3-(2-cyano-6-(3-(trifluoromethyl)phenyl)pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells | Bioorg Med Chem Lett 20: 6237-41 (2010) Article DOI: 10.1016/j.bmcl.2010.08.101 BindingDB Entry DOI: 10.7270/Q2RR1ZFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM13305 (4-Amino-3-[3-(S)-(5-isoxazol-3-ylthiophene-2-ylsul...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM13301 (3-[3-(S)-(5-Isoxazol-3-ylthiophene-2-ylsulfonylami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 326 total ) | Next | Last >> |