

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21244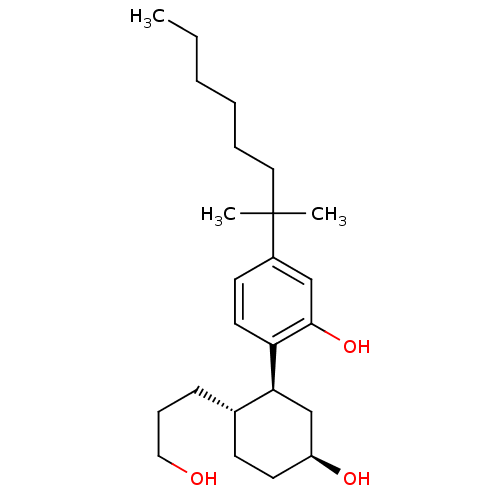 (2-[(1S,2S,5S)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 620-32 (2004) Article DOI: 10.1124/jpet.103.062836 BindingDB Entry DOI: 10.7270/Q2N0154R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21244 (2-[(1S,2S,5S)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 620-32 (2004) Article DOI: 10.1124/jpet.103.062836 BindingDB Entry DOI: 10.7270/Q2N0154R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21244 (2-[(1S,2S,5S)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 620-32 (2004) Article DOI: 10.1124/jpet.103.062836 BindingDB Entry DOI: 10.7270/Q2N0154R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM86515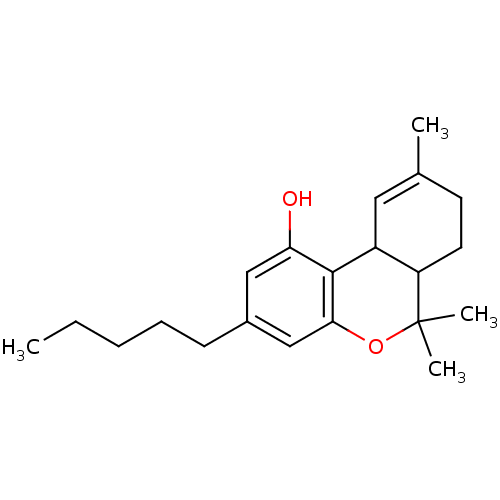 (CAS_1972-08-3 | NSC_16078 | THC, delta 9 | US94161...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 13.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 620-32 (2004) Article DOI: 10.1124/jpet.103.062836 BindingDB Entry DOI: 10.7270/Q2N0154R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM86515 (CAS_1972-08-3 | NSC_16078 | THC, delta 9 | US94161...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 22.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 620-32 (2004) Article DOI: 10.1124/jpet.103.062836 BindingDB Entry DOI: 10.7270/Q2N0154R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM86514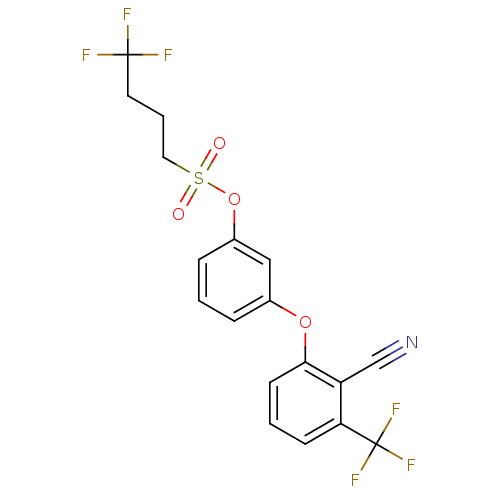 (BAY 59-3074 | CAS_406205-74-1 | CHEMBL1354658) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 45.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 620-32 (2004) Article DOI: 10.1124/jpet.103.062836 BindingDB Entry DOI: 10.7270/Q2N0154R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM86514 (BAY 59-3074 | CAS_406205-74-1 | CHEMBL1354658) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 55.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 620-32 (2004) Article DOI: 10.1124/jpet.103.062836 BindingDB Entry DOI: 10.7270/Q2N0154R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM86515 (CAS_1972-08-3 | NSC_16078 | THC, delta 9 | US94161...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 69.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 620-32 (2004) Article DOI: 10.1124/jpet.103.062836 BindingDB Entry DOI: 10.7270/Q2N0154R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50409115 (LONAPRISAN) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antagonist potency in transactivation assay in neuroblastoma cells expressing human PR-B progesterone receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50409115 (LONAPRISAN) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 0.00360 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antagonist potency in transactivation assay in neuroblastoma cells expressing human PR-A progesterone receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antagonist potency in transactivation assay in neuroblastoma cells expressing human PR-B progesterone receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antgonist potency in transactivation assay in neuroblastoma cells expressing human PR-A progesterone receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50327343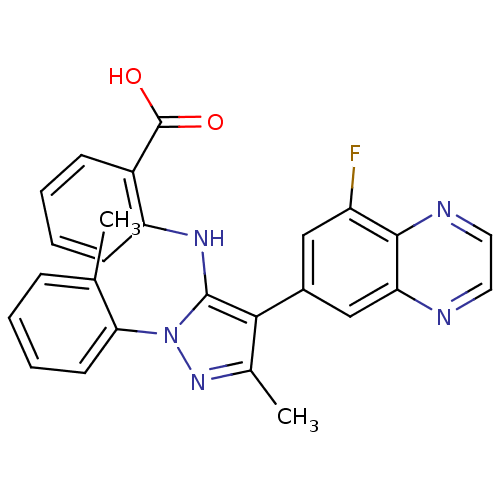 (2-(4-(8-fluoroquinoxalin-6-yl)-3-methyl-1-o-tolyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Schering Pharma AG Curated by ChEMBL | Assay Description Antagonist activity at adenosine A1 receptor by cAMP assay | Bioorg Med Chem Lett 20: 5891-4 (2010) Article DOI: 10.1016/j.bmcl.2010.07.095 BindingDB Entry DOI: 10.7270/Q2FQ9WV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM256036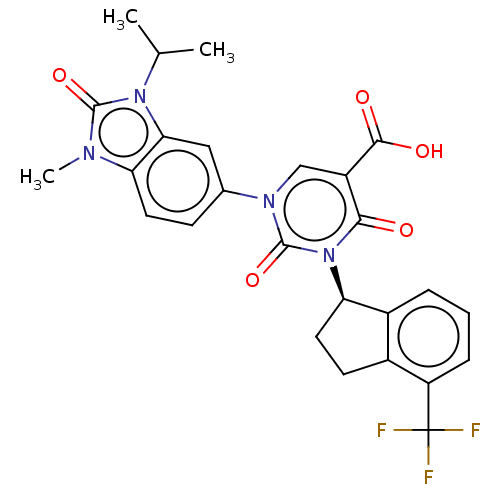 (US9481672, 217) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM256064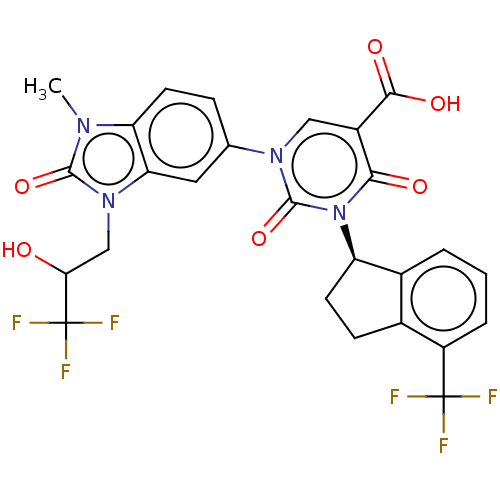 (US9481672, 246) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM256099 (US9481672, 281) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50143581 (7-[4-(4-Fluoro-benzyl)-[1,4]diazepan-1-yl]-2-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AG Curated by ChEMBL | Assay Description Inhibitory activity against human adenosine kinase expressed in E. coli | Bioorg Med Chem Lett 14: 1997-2000 (2004) Article DOI: 10.1016/j.bmcl.2004.01.082 BindingDB Entry DOI: 10.7270/Q2GQ6X6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50143575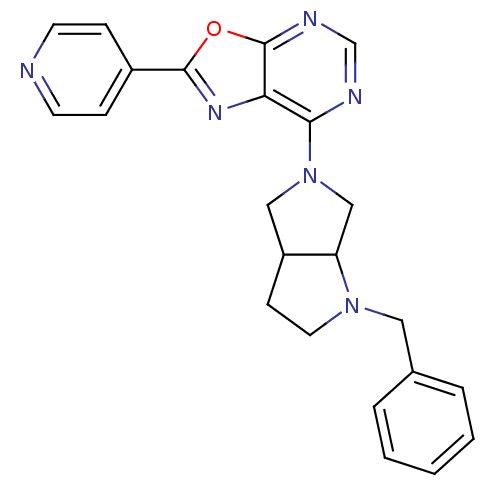 (7-(1-Benzyl-hexahydro-pyrrolo[3,4-b]pyrrol-5-yl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AG Curated by ChEMBL | Assay Description Inhibitory activity against human adenosine kinase expressed in E. coli | Bioorg Med Chem Lett 14: 1997-2000 (2004) Article DOI: 10.1016/j.bmcl.2004.01.082 BindingDB Entry DOI: 10.7270/Q2GQ6X6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50143574 (7-((4aS,7aS)-6-Benzyl-octahydro-pyrrolo[3,4-b]pyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AG Curated by ChEMBL | Assay Description Inhibitory activity against human adenosine kinase expressed in E. coli | Bioorg Med Chem Lett 14: 1997-2000 (2004) Article DOI: 10.1016/j.bmcl.2004.01.082 BindingDB Entry DOI: 10.7270/Q2GQ6X6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM255968 (US9481672, 146) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM255964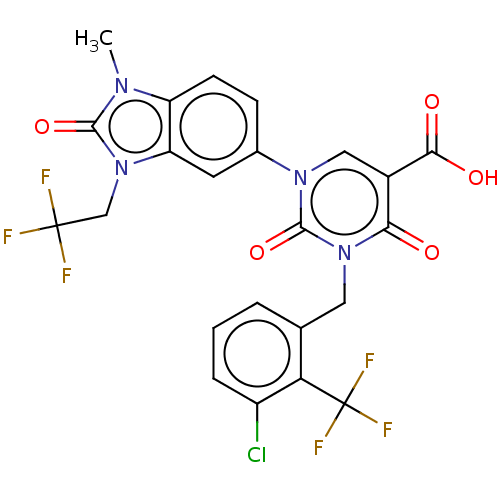 (US9481672, 142) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM256065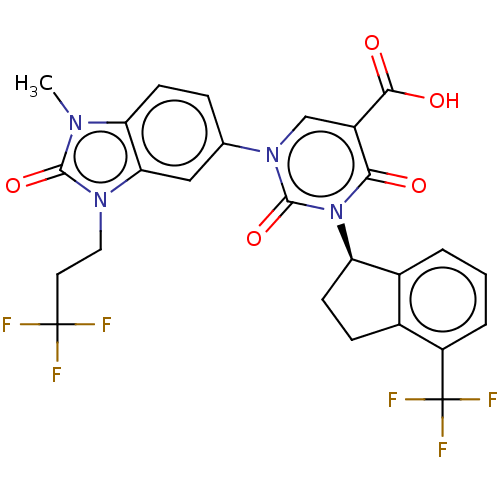 (US9481672, 247) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM255962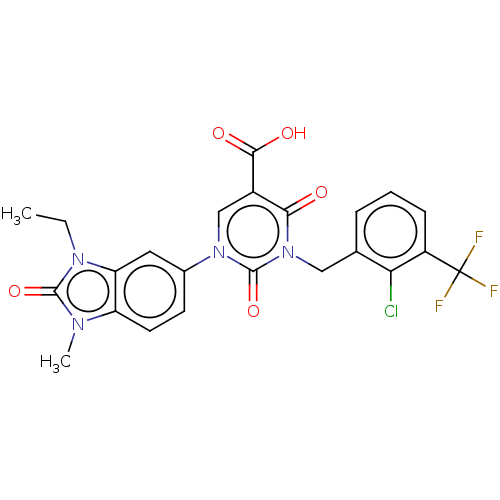 (US9481672, 140) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium channel subfamily K member 9 (Homo sapiens (Human)) | BDBM458963 ((4-{[2-(4-Bromophenyl)imidazo[,2-a]pyridin-3-yl]me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.04 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The investigations on the inhibition of the recombinant TASK-1 and TASK-3 channels were carried out using stably transfected CHO cells. Here, the com... | US Patent US10759794 (2020) BindingDB Entry DOI: 10.7270/Q2KK9FW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50327327 (2-(4-(4-fluorophenyl)-3-methyl-1-o-tolyl-1H-pyrazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.39 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Schering Pharma AG Curated by ChEMBL | Assay Description Antagonist activity at adenosine A1 receptor by cAMP assay | Bioorg Med Chem Lett 20: 5891-4 (2010) Article DOI: 10.1016/j.bmcl.2010.07.095 BindingDB Entry DOI: 10.7270/Q2FQ9WV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50143592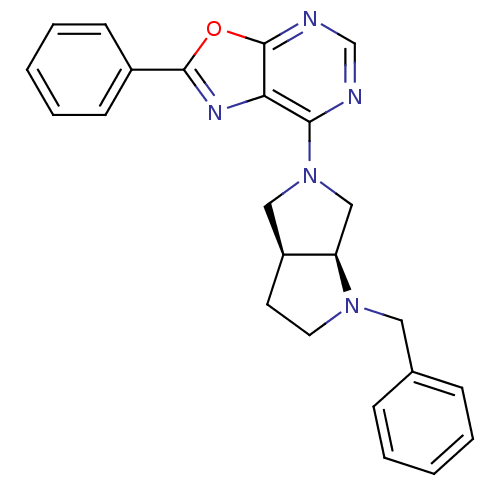 (7-((3aS,6aS)-1-Benzyl-hexahydro-pyrrolo[3,4-b]pyrr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AG Curated by ChEMBL | Assay Description Inhibitory activity against human adenosine kinase expressed in E. coli | Bioorg Med Chem Lett 14: 1997-2000 (2004) Article DOI: 10.1016/j.bmcl.2004.01.082 BindingDB Entry DOI: 10.7270/Q2GQ6X6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50344356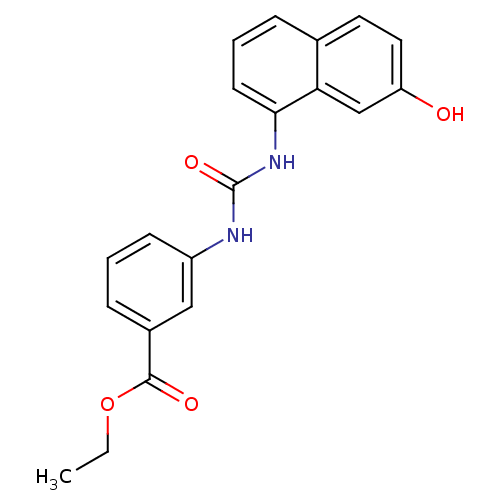 (CHEMBL1779676 | ethyl 3-(3-(7-hydroxynaphthalen-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at rat TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin-i... | Bioorg Med Chem Lett 21: 3354-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.013 BindingDB Entry DOI: 10.7270/Q2NG4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50344356 (CHEMBL1779676 | ethyl 3-(3-(7-hydroxynaphthalen-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin... | Bioorg Med Chem Lett 21: 3354-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.013 BindingDB Entry DOI: 10.7270/Q2NG4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM88376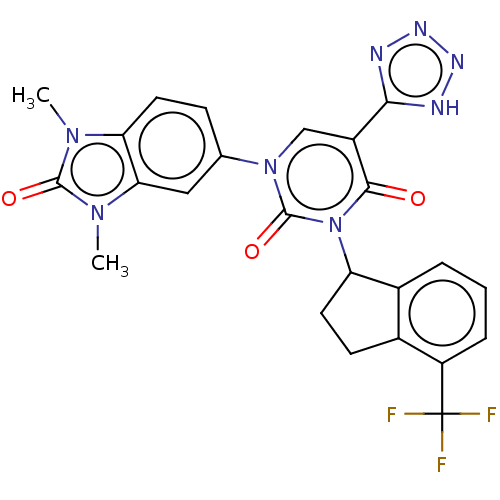 (US9695131, 13) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9695131 (2017) BindingDB Entry DOI: 10.7270/Q2ZP448Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50344367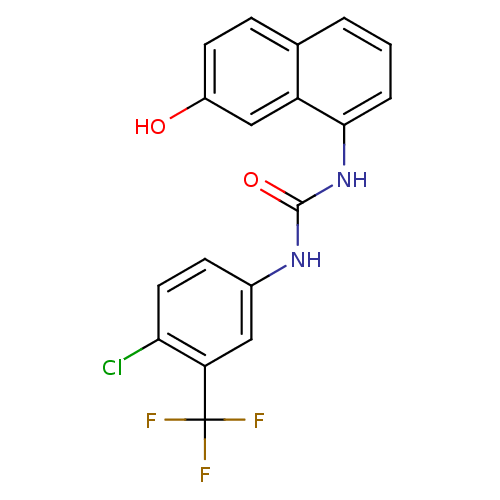 (1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(7-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at rat TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin-i... | Bioorg Med Chem Lett 21: 3354-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.013 BindingDB Entry DOI: 10.7270/Q2NG4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM256043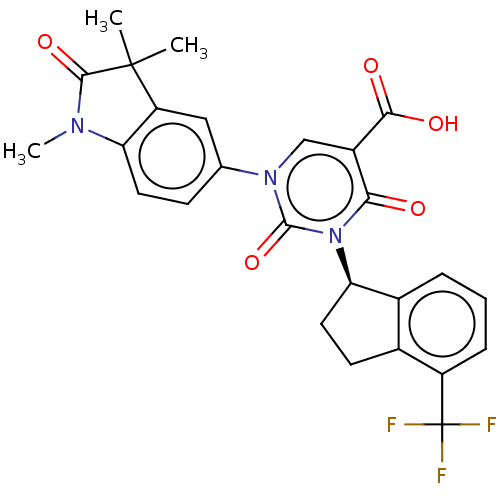 (US9481672, 224) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM256046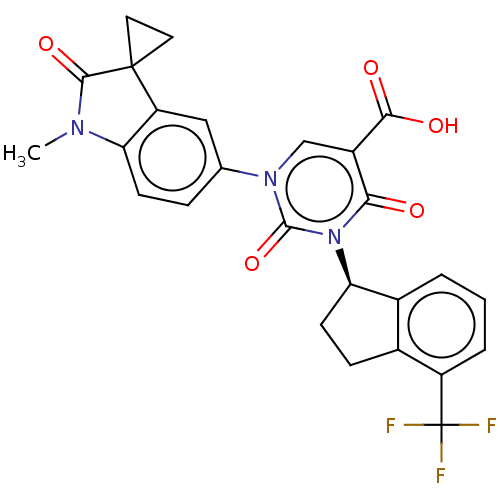 (US9481672, 227) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM256116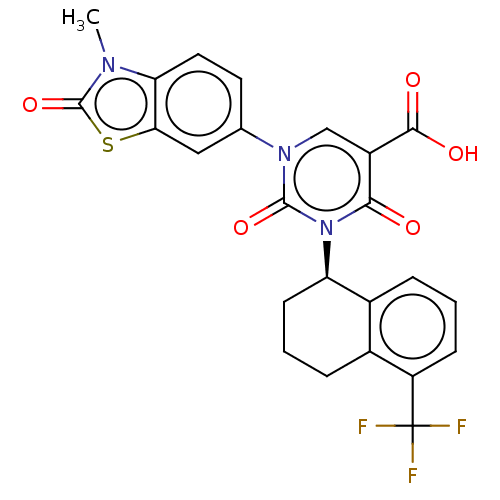 (US9481672, 300) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM256111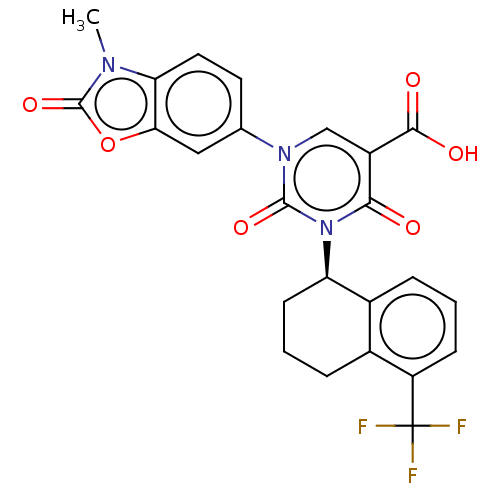 (US9481672, 294) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM256095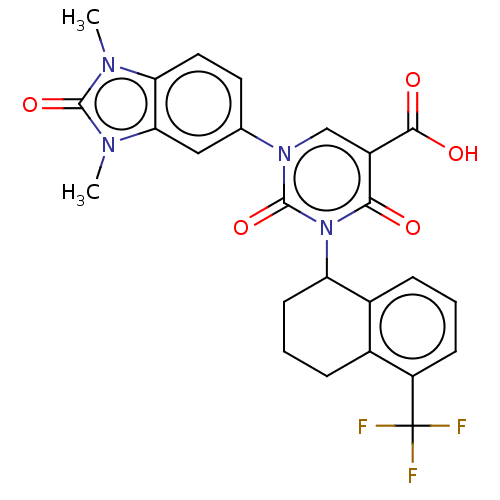 (US9481672, 277) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM256088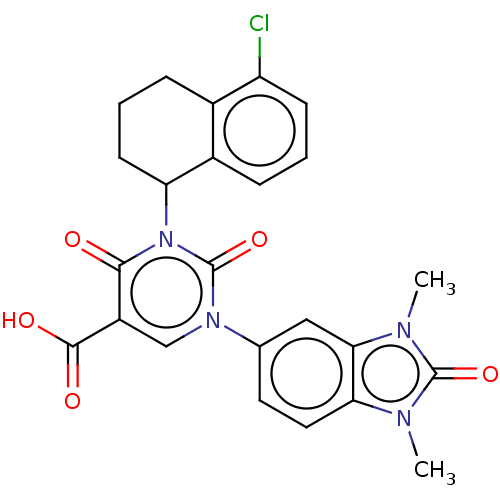 (BDBM256092 | BDBM256093 | US9481672, 270) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM255973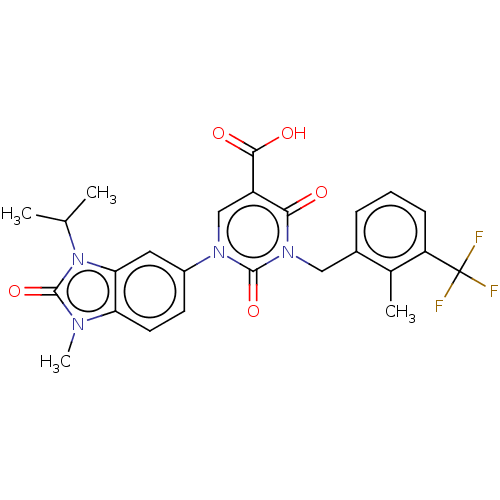 (US9481672, 151) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM255971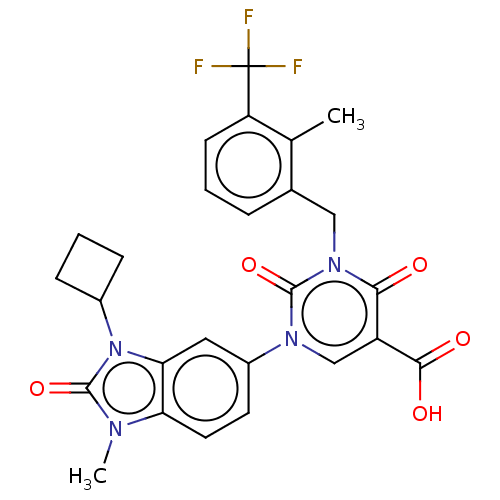 (US9481672, 149) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM255969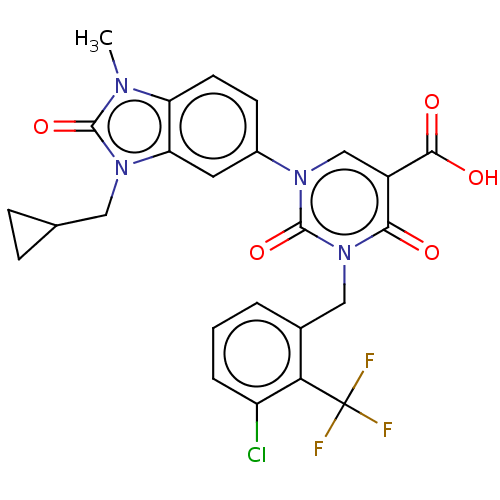 (US9481672, 147) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM255967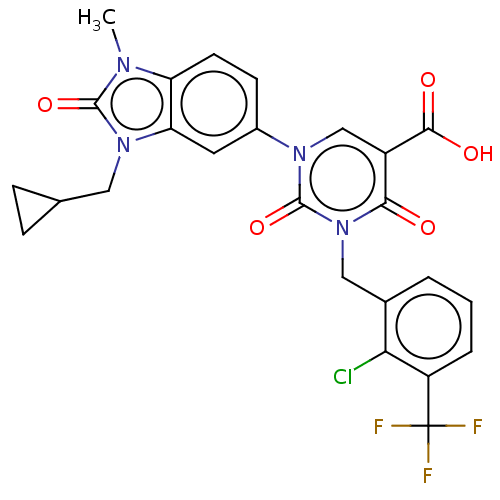 (US9481672, 145) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM256059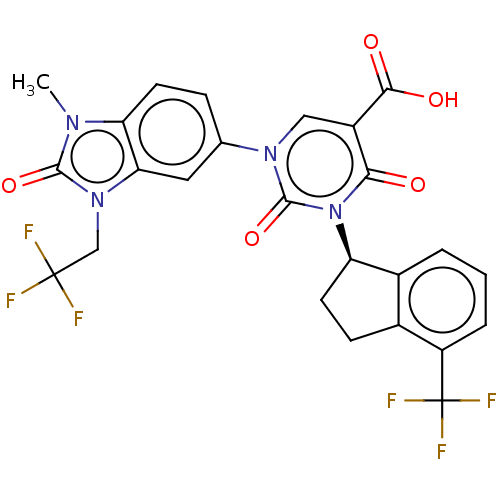 (US9481672, 241) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM256062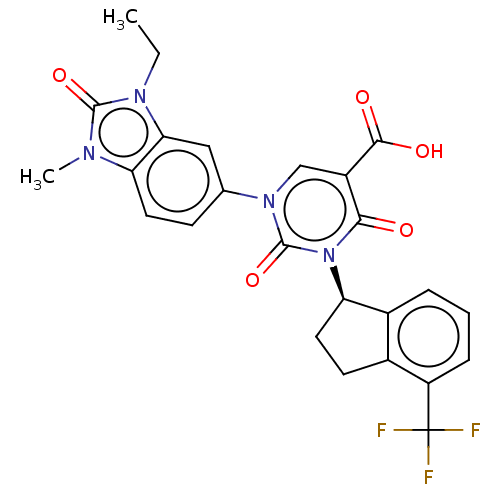 (US9481672, 244) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM256066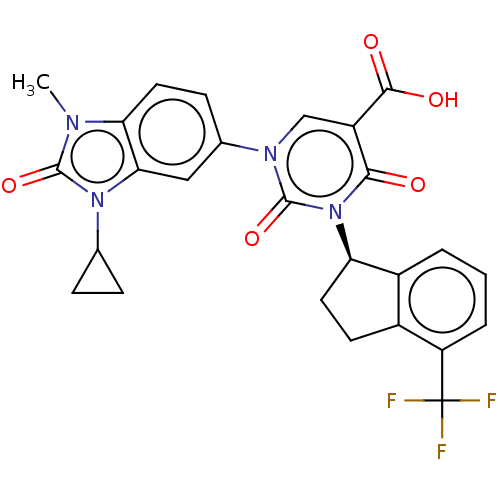 (US9481672, 248) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM256083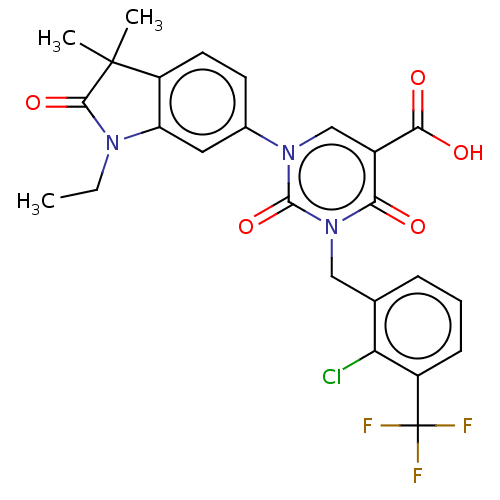 (US9481672, 265) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM255927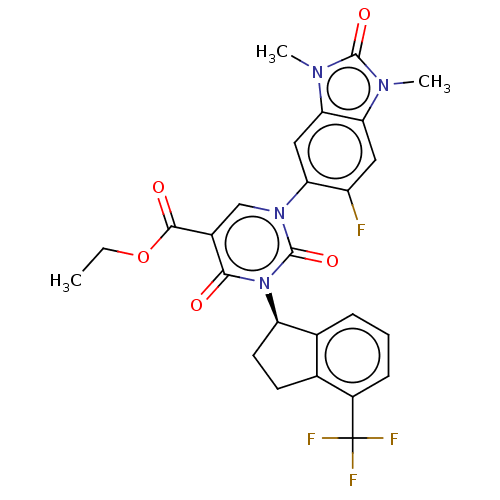 (US9481672, 104) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM255944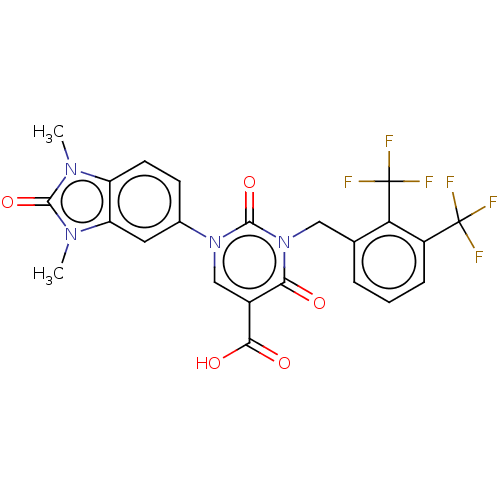 (US9481672, 122) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM255954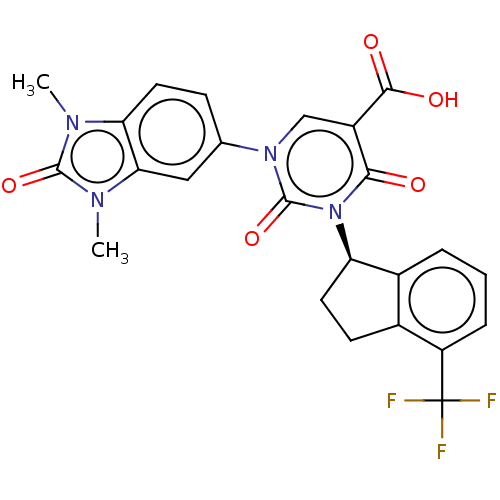 (US9481672, 132) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM255956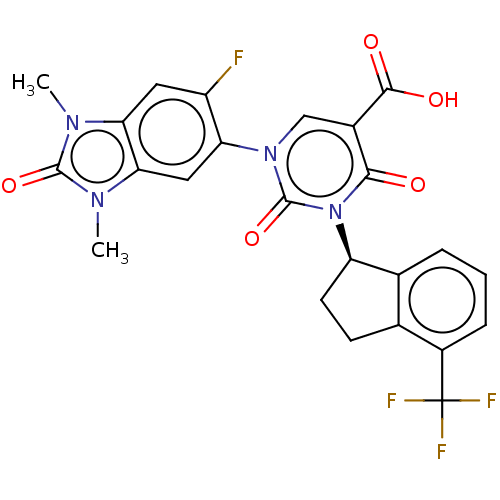 (US9481672, 134) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM255958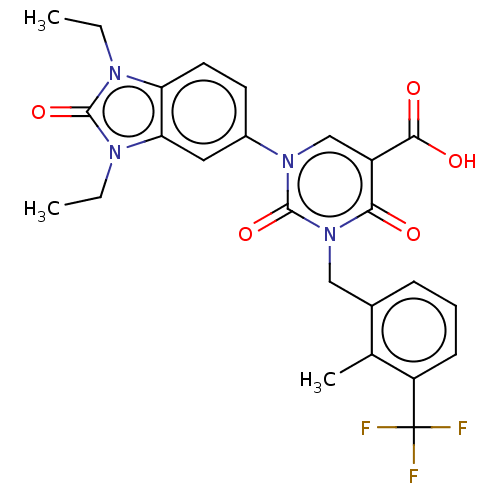 (US9481672, 136) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM255961 (US9481672, 139) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 930 total ) | Next | Last >> |