 Found 119 hits with Last Name = 'schostarez' and Initial = 'hj'
Found 119 hits with Last Name = 'schostarez' and Initial = 'hj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Renin
(Homo sapiens (Human)) | BDBM50368278
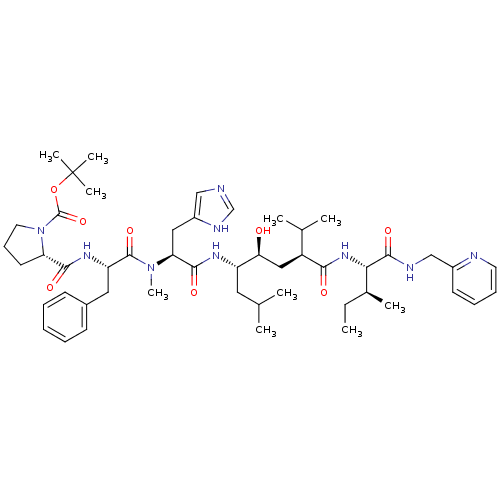
(CHEMBL1790497)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)OC(C)(C)C)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C50H75N9O8/c1-11-33(6)43(47(64)53-29-35-20-15-16-22-52-35)57-44(61)37(32(4)5)27-42(60)38(24-31(2)3)55-46(63)41(26-36-28-51-30-54-36)58(10)48(65)39(25-34-18-13-12-14-19-34)56-45(62)40-21-17-23-59(40)49(66)67-50(7,8)9/h12-16,18-20,22,28,30-33,37-43,60H,11,17,21,23-27,29H2,1-10H3,(H,51,54)(H,53,64)(H,55,63)(H,56,62)(H,57,61)/t33-,37-,38-,39-,40-,41-,42-,43-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition constants using recombinant human renin assay |
J Med Chem 34: 2107-12 (1991)
BindingDB Entry DOI: 10.7270/Q2HQ40J6 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50368274
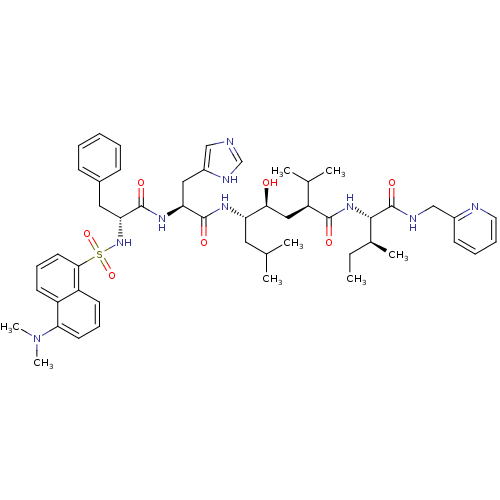
(CHEMBL1790492)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](Cc1ccccc1)NS(=O)(=O)c1cccc2c(cccc12)N(C)C)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C51H69N9O7S/c1-9-34(6)47(51(65)54-30-36-19-13-14-24-53-36)58-48(62)40(33(4)5)28-45(61)41(25-32(2)3)56-49(63)42(27-37-29-52-31-55-37)57-50(64)43(26-35-17-11-10-12-18-35)59-68(66,67)46-23-16-20-38-39(46)21-15-22-44(38)60(7)8/h10-24,29,31-34,40-43,45,47,59,61H,9,25-28,30H2,1-8H3,(H,52,55)(H,54,65)(H,56,63)(H,57,64)(H,58,62)/t34-,40-,41-,42-,43+,45-,47-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition constants using recombinant human renin assay |
J Med Chem 34: 2107-12 (1991)
BindingDB Entry DOI: 10.7270/Q2HQ40J6 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50012322

(CHEMBL264524 | N-(2-Hydroxy-1,1-bis-hydroxymethyl-...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)NC(CO)(CO)CO)C(C)C)C(=O)NCc1cccc[n+]1[O-] Show InChI InChI=1S/C50H76N10O11/c1-8-33(6)43(47(68)52-26-36-17-12-13-20-60(36)71)56-44(65)37(32(4)5)24-42(64)38(21-31(2)3)54-46(67)41(23-35-25-51-30-53-35)58(7)48(69)39(22-34-15-10-9-11-16-34)55-45(66)40-18-14-19-59(40)49(70)57-50(27-61,28-62)29-63/h9-13,15-17,20,25,30-33,37-43,61-64H,8,14,18-19,21-24,26-29H2,1-7H3,(H,51,53)(H,52,68)(H,54,67)(H,55,66)(H,56,65)(H,57,70)/t33?,37-,38-,39-,40-,41-,42-,43-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of human renin by radio-immuno assay of angiotensin I (ANG I) |
J Med Chem 34: 2107-12 (1991)
BindingDB Entry DOI: 10.7270/Q2HQ40J6 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50076372
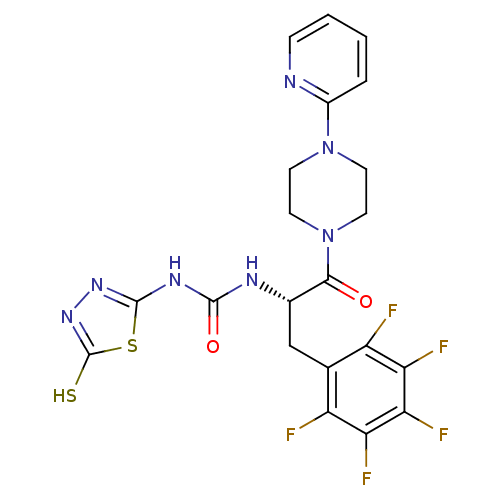
(1-[(S)-2-Oxo-1-pentafluorophenylmethyl-2-(4-pyridi...)Show SMILES Fc1c(F)c(F)c(C[C@H](NC(=O)Nc2nnc(S)s2)C(=O)N2CCN(CC2)c2ccccn2)c(F)c1F Show InChI InChI=1S/C21H18F5N7O2S2/c22-13-10(14(23)16(25)17(26)15(13)24)9-11(28-19(35)29-20-30-31-21(36)37-20)18(34)33-7-5-32(6-8-33)12-3-1-2-4-27-12/h1-4,11H,5-9H2,(H,31,36)(H2,28,29,30,35)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-3 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50076341

((S)-N-Methyl-3-pentafluorophenyl-2-[3-(5-thioxo-4,...)Show SMILES CNC(=O)[C@H](Cc1c(F)c(F)c(F)c(F)c1F)NC(=O)Nc1nnc(S)s1 Show InChI InChI=1S/C13H10F5N5O2S2/c1-19-10(24)4(20-11(25)21-12-22-23-13(26)27-12)2-3-5(14)7(16)9(18)8(17)6(3)15/h4H,2H2,1H3,(H,19,24)(H,23,26)(H2,20,21,22,25)/t4-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-3 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50076338

((S)-3-(4-Fluoro-phenyl)-N-methyl-2-[3-(5-thioxo-4,...)Show SMILES CNC(=O)[C@H](Cc1ccc(F)cc1)NC(=O)Nc1nnc(S)s1 Show InChI InChI=1S/C13H14FN5O2S2/c1-15-10(20)9(6-7-2-4-8(14)5-3-7)16-11(21)17-12-18-19-13(22)23-12/h2-5,9H,6H2,1H3,(H,15,20)(H,19,22)(H2,16,17,18,21)/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-3 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Rattus norvegicus (rat)) | BDBM13614

(2-(carboxymethoxy)-5-[(2S)-2-(pentylcarbamoyl)-2-[...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)c(c1)C(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)Cn1[nH]ncc1=S |r| Show InChI InChI=1S/C30H36N6O8S/c1-2-3-7-12-31-28(40)22(15-20-10-11-24(44-18-27(38)39)21(13-20)30(42)43)34-29(41)23(14-19-8-5-4-6-9-19)33-25(37)17-36-26(45)16-32-35-36/h4-6,8-11,13,16,22-23,35H,2-3,7,12,14-15,17-18H2,1H3,(H,31,40)(H,33,37)(H,34,41)(H,38,39)(H,42,43)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | -39.1 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Pharmacia Corporation
| Assay Description
Hydrolysis of substrate p-nitrophenyl phosphate was monitored on a 96-well microplate reader. Concentrations of PTP1B inhibitors that resulted in 50%... |
Biochemistry 40: 5642-54 (2001)
Article DOI: 10.1021/bi002865v
BindingDB Entry DOI: 10.7270/Q2D50K6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13611
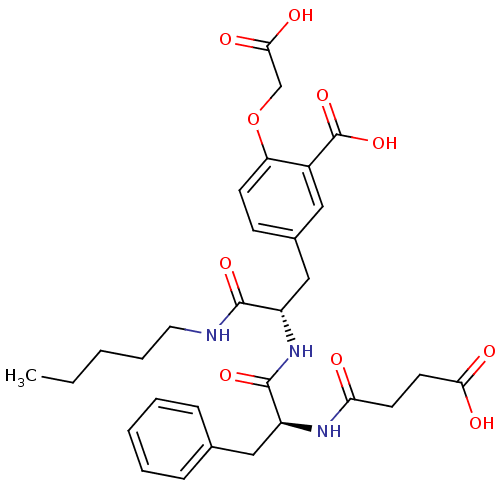
(2-(carboxymethoxy)-5-[(2S)-2-[(2S)-2-(3-formamidop...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)c(c1)C(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)CCC(O)=O |r| Show InChI InChI=1S/C30H37N3O10/c1-2-3-7-14-31-28(39)22(17-20-10-11-24(43-18-27(37)38)21(15-20)30(41)42)33-29(40)23(16-19-8-5-4-6-9-19)32-25(34)12-13-26(35)36/h4-6,8-11,15,22-23H,2-3,7,12-14,16-18H2,1H3,(H,31,39)(H,32,34)(H,33,40)(H,35,36)(H,37,38)(H,41,42)/t22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against human protein tyrosine phosphatase 1B (PTP1B) |
Bioorg Med Chem Lett 13: 971-5 (2003)
BindingDB Entry DOI: 10.7270/Q24M93WC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Rattus norvegicus (rat)) | BDBM13611

(2-(carboxymethoxy)-5-[(2S)-2-[(2S)-2-(3-formamidop...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)c(c1)C(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)CCC(O)=O |r| Show InChI InChI=1S/C30H37N3O10/c1-2-3-7-14-31-28(39)22(17-20-10-11-24(43-18-27(37)38)21(15-20)30(41)42)33-29(40)23(16-19-8-5-4-6-9-19)32-25(34)12-13-26(35)36/h4-6,8-11,15,22-23H,2-3,7,12-14,16-18H2,1H3,(H,31,39)(H,32,34)(H,33,40)(H,35,36)(H,37,38)(H,41,42)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 250 | -37.3 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Pharmacia Corporation
| Assay Description
Hydrolysis of substrate p-nitrophenyl phosphate was monitored on a 96-well microplate reader. Concentrations of PTP1B inhibitors that resulted in 50%... |
Biochemistry 40: 5642-54 (2001)
Article DOI: 10.1021/bi002865v
BindingDB Entry DOI: 10.7270/Q2D50K6S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50076367
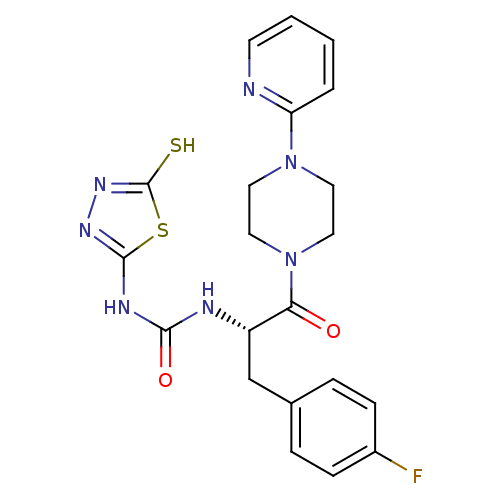
(1-[(S)-1-(4-Fluoro-benzyl)-2-oxo-2-(4-pyridin-2-yl...)Show SMILES Fc1ccc(C[C@H](NC(=O)Nc2nnc(S)s2)C(=O)N2CCN(CC2)c2ccccn2)cc1 Show InChI InChI=1S/C21H22FN7O2S2/c22-15-6-4-14(5-7-15)13-16(24-19(31)25-20-26-27-21(32)33-20)18(30)29-11-9-28(10-12-29)17-3-1-2-8-23-17/h1-8,16H,9-13H2,(H,27,32)(H2,24,25,26,31)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-3 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50076357
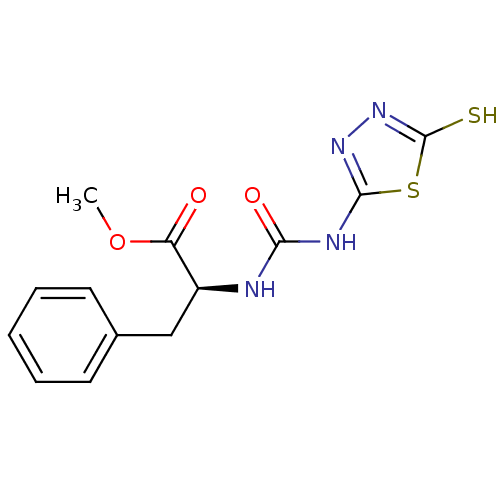
((S)-3-Phenyl-2-[3-(5-thioxo-4,5-dihydro-[1,3,4]thi...)Show InChI InChI=1S/C13H14N4O3S2/c1-20-10(18)9(7-8-5-3-2-4-6-8)14-11(19)15-12-16-17-13(21)22-12/h2-6,9H,7H2,1H3,(H,17,21)(H2,14,15,16,19)/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-3 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50076366
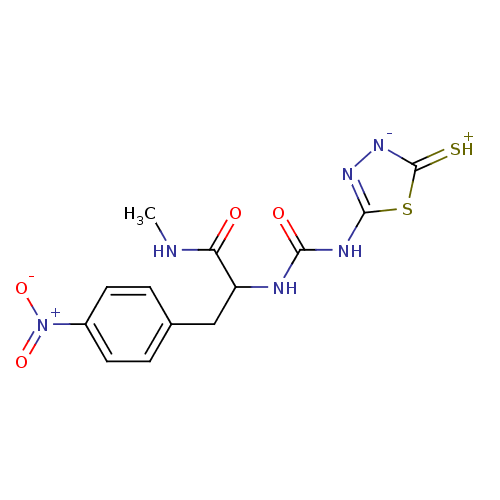
((S)-N-Methyl-3-(4-nitro-phenyl)-2-[3-(5-thioxo-4,5...)Show SMILES CNC(=O)C(Cc1ccc(cc1)[N+]([O-])=O)NC(=O)Nc1n[n-]c(=[SH+])s1 Show InChI InChI=1S/C13H14N6O4S2/c1-14-10(20)9(6-7-2-4-8(5-3-7)19(22)23)15-11(21)16-12-17-18-13(24)25-12/h2-5,9H,6H2,1H3,(H4,14,15,16,17,18,20,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-3 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50076374
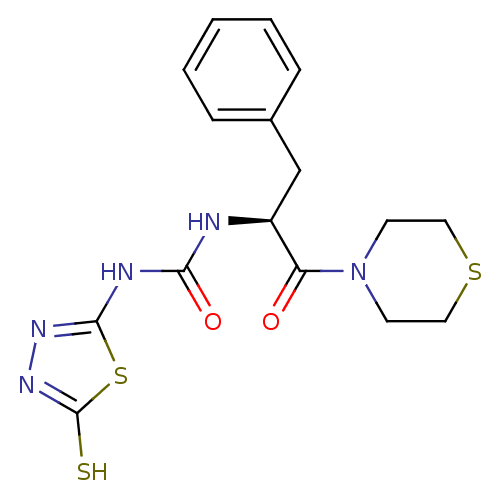
(1-((S)-1-Benzyl-2-oxo-2-thiomorpholin-4-yl-ethyl)-...)Show SMILES Sc1nnc(NC(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCSCC2)s1 Show InChI InChI=1S/C16H19N5O2S3/c22-13(21-6-8-25-9-7-21)12(10-11-4-2-1-3-5-11)17-14(23)18-15-19-20-16(24)26-15/h1-5,12H,6-10H2,(H,20,24)(H2,17,18,19,23)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-3 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50076339
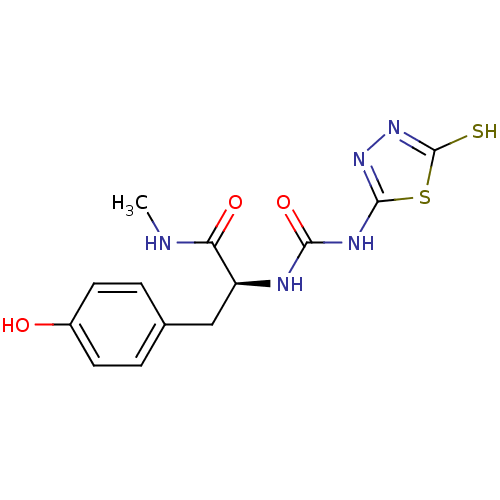
((S)-3-(4-Hydroxy-phenyl)-N-methyl-2-[3-(5-thioxo-4...)Show SMILES CNC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)Nc1nnc(S)s1 Show InChI InChI=1S/C13H15N5O3S2/c1-14-10(20)9(6-7-2-4-8(19)5-3-7)15-11(21)16-12-17-18-13(22)23-12/h2-5,9,19H,6H2,1H3,(H,14,20)(H,18,22)(H2,15,16,17,21)/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-3 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50076336
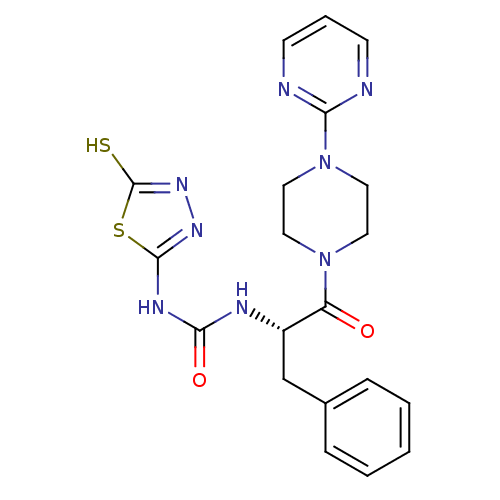
(1-[(S)-1-Benzyl-2-oxo-2-(4-pyrimidin-2-yl-piperazi...)Show SMILES Sc1nnc(NC(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCN(CC2)c2ncccn2)s1 Show InChI InChI=1S/C20H22N8O2S2/c29-16(27-9-11-28(12-10-27)17-21-7-4-8-22-17)15(13-14-5-2-1-3-6-14)23-18(30)24-19-25-26-20(31)32-19/h1-8,15H,9-13H2,(H,26,31)(H2,23,24,25,30)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-3 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50076351

((S)-3-(4-Methoxy-phenyl)-N-methyl-2-[3-(5-thioxo-4...)Show SMILES CNC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)Nc1nnc(S)s1 Show InChI InChI=1S/C14H17N5O3S2/c1-15-11(20)10(7-8-3-5-9(22-2)6-4-8)16-12(21)17-13-18-19-14(23)24-13/h3-6,10H,7H2,1-2H3,(H,15,20)(H,19,23)(H2,16,17,18,21)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-3 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50076334
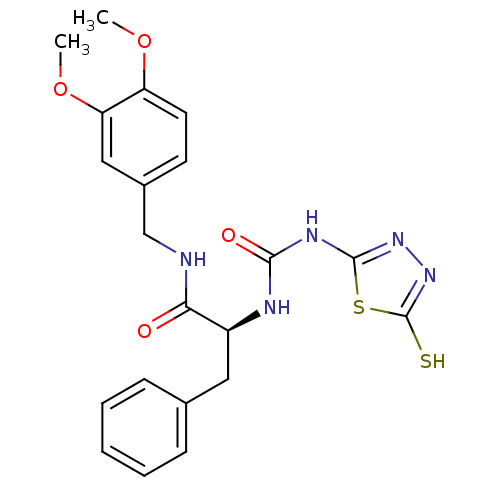
((S)-N-(3,4-Dimethoxy-benzyl)-3-phenyl-2-[3-(5-thio...)Show SMILES COc1ccc(CNC(=O)[C@H](Cc2ccccc2)NC(=O)Nc2nnc(S)s2)cc1OC Show InChI InChI=1S/C21H23N5O4S2/c1-29-16-9-8-14(11-17(16)30-2)12-22-18(27)15(10-13-6-4-3-5-7-13)23-19(28)24-20-25-26-21(31)32-20/h3-9,11,15H,10,12H2,1-2H3,(H,22,27)(H,26,31)(H2,23,24,25,28)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-3 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50076382
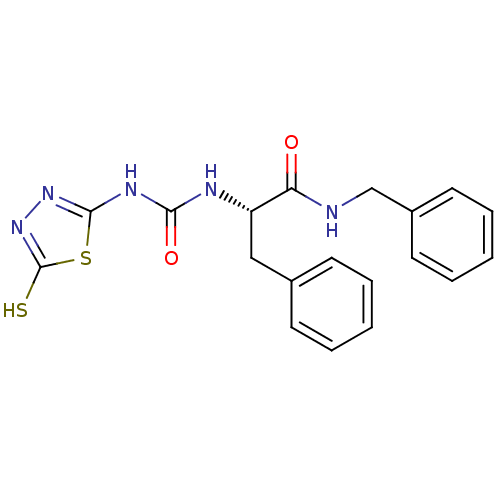
((S)-N-Benzyl-3-phenyl-2-[3-(5-thioxo-4,5-dihydro-[...)Show SMILES Sc1nnc(NC(=O)N[C@@H](Cc2ccccc2)C(=O)NCc2ccccc2)s1 Show InChI InChI=1S/C19H19N5O2S2/c25-16(20-12-14-9-5-2-6-10-14)15(11-13-7-3-1-4-8-13)21-17(26)22-18-23-24-19(27)28-18/h1-10,15H,11-12H2,(H,20,25)(H,24,27)(H2,21,22,23,26)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-3 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50076342

(1-[(S)-1-Benzyl-2-oxo-2-(4-pyridin-2-yl-piperazin-...)Show SMILES Sc1nnc(NC(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCN(CC2)c2ccccn2)s1 Show InChI InChI=1S/C21H23N7O2S2/c29-18(28-12-10-27(11-13-28)17-8-4-5-9-22-17)16(14-15-6-2-1-3-7-15)23-19(30)24-20-25-26-21(31)32-20/h1-9,16H,10-14H2,(H,26,31)(H2,23,24,25,30)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-3 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50124517
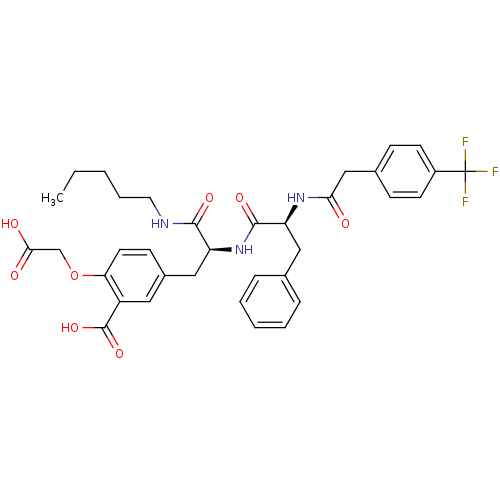
(2-Carboxymethoxy-5-(2-pentylcarbamoyl-2-{(S)-(S)-3...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)c(c1)C(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C35H38F3N3O8/c1-2-3-7-16-39-32(45)27(19-24-12-15-29(49-21-31(43)44)26(17-24)34(47)48)41-33(46)28(18-22-8-5-4-6-9-22)40-30(42)20-23-10-13-25(14-11-23)35(36,37)38/h4-6,8-15,17,27-28H,2-3,7,16,18-21H2,1H3,(H,39,45)(H,40,42)(H,41,46)(H,43,44)(H,47,48)/t27-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against human protein tyrosine phosphatase 1B (PTP1B) |
Bioorg Med Chem Lett 13: 971-5 (2003)
BindingDB Entry DOI: 10.7270/Q24M93WC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50076346

(1-((S)-1-Benzyl-2-oxo-2-pyrrolidin-1-yl-ethyl)-3-(...)Show SMILES Sc1nnc(NC(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCCC2)s1 Show InChI InChI=1S/C16H19N5O2S2/c22-13(21-8-4-5-9-21)12(10-11-6-2-1-3-7-11)17-14(23)18-15-19-20-16(24)25-15/h1-3,6-7,12H,4-5,8-10H2,(H,20,24)(H2,17,18,19,23)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-3 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50124511

(2-Carboxymethoxy-5-(2-{(S)-(S)-2-[2-(4-methoxy-phe...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)c(c1)C(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)Cc1ccc(OC)cc1 Show InChI InChI=1S/C35H41N3O9/c1-3-4-8-17-36-33(42)28(20-25-13-16-30(47-22-32(40)41)27(18-25)35(44)45)38-34(43)29(19-23-9-6-5-7-10-23)37-31(39)21-24-11-14-26(46-2)15-12-24/h5-7,9-16,18,28-29H,3-4,8,17,19-22H2,1-2H3,(H,36,42)(H,37,39)(H,38,43)(H,40,41)(H,44,45)/t28-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against human protein tyrosine phosphatase 1B (PTP1B) |
Bioorg Med Chem Lett 13: 971-5 (2003)
BindingDB Entry DOI: 10.7270/Q24M93WC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50124514
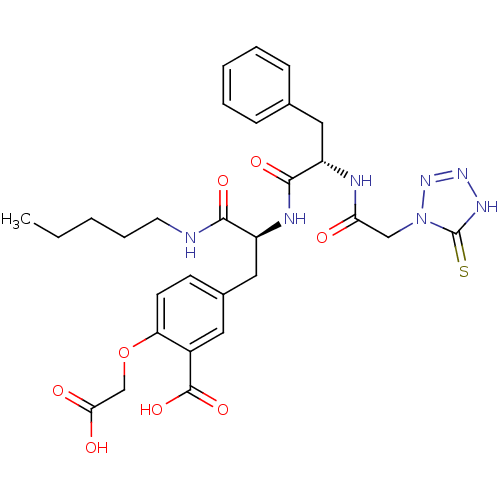
(2-Carboxymethoxy-5-[2-{(S)-2-[2-(5-mercapto-tetraz...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)c(c1)C(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)Cn1nn[nH]c1=S Show InChI InChI=1S/C29H35N7O8S/c1-2-3-7-12-30-26(40)21(15-19-10-11-23(44-17-25(38)39)20(13-19)28(42)43)32-27(41)22(14-18-8-5-4-6-9-18)31-24(37)16-36-29(45)33-34-35-36/h4-6,8-11,13,21-22H,2-3,7,12,14-17H2,1H3,(H,30,40)(H,31,37)(H,32,41)(H,38,39)(H,42,43)(H,33,35,45)/t21-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against human protein tyrosine phosphatase 1B (PTP1B) |
Bioorg Med Chem Lett 13: 971-5 (2003)
BindingDB Entry DOI: 10.7270/Q24M93WC |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50241372
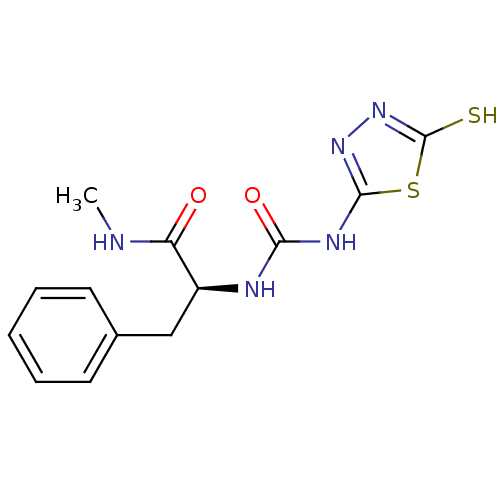
((S)-1-(1-(methylamino)-1-oxo-3-phenylpropan-2-yl)-...)Show InChI InChI=1S/C13H15N5O2S2/c1-14-10(19)9(7-8-5-3-2-4-6-8)15-11(20)16-12-17-18-13(21)22-12/h2-6,9H,7H2,1H3,(H,14,19)(H,18,21)(H2,15,16,17,20)/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-3 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50076372

(1-[(S)-2-Oxo-1-pentafluorophenylmethyl-2-(4-pyridi...)Show SMILES Fc1c(F)c(F)c(C[C@H](NC(=O)Nc2nnc(S)s2)C(=O)N2CCN(CC2)c2ccccn2)c(F)c1F Show InChI InChI=1S/C21H18F5N7O2S2/c22-13-10(14(23)16(25)17(26)15(13)24)9-11(28-19(35)29-20-30-31-21(36)37-20)18(34)33-7-5-32(6-8-33)12-3-1-2-4-27-12/h1-4,11H,5-9H2,(H,31,36)(H2,28,29,30,35)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-1 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50076348
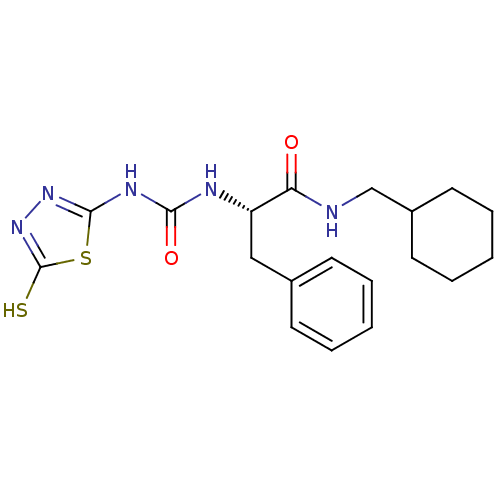
((S)-N-Cyclohexylmethyl-3-phenyl-2-[3-(5-thioxo-4,5...)Show SMILES Sc1nnc(NC(=O)N[C@@H](Cc2ccccc2)C(=O)NCC2CCCCC2)s1 Show InChI InChI=1S/C19H25N5O2S2/c25-16(20-12-14-9-5-2-6-10-14)15(11-13-7-3-1-4-8-13)21-17(26)22-18-23-24-19(27)28-18/h1,3-4,7-8,14-15H,2,5-6,9-12H2,(H,20,25)(H,24,27)(H2,21,22,23,26)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-3 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50076354
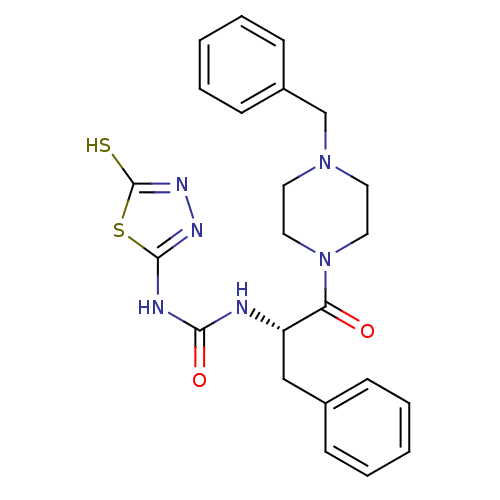
(1-[(S)-1-Benzyl-2-(4-benzyl-piperazin-1-yl)-2-oxo-...)Show SMILES Sc1nnc(NC(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCN(Cc3ccccc3)CC2)s1 Show InChI InChI=1S/C23H26N6O2S2/c30-20(29-13-11-28(12-14-29)16-18-9-5-2-6-10-18)19(15-17-7-3-1-4-8-17)24-21(31)25-22-26-27-23(32)33-22/h1-10,19H,11-16H2,(H,27,32)(H2,24,25,26,31)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-3 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50124513

(2-Carboxymethoxy-5-[(S)-2-pentylcarbamoyl-2-((S)-2...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)c(c1)C(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)Oc1ccccc1 Show InChI InChI=1S/C33H37N3O9/c1-2-3-10-17-34-30(39)26(20-23-15-16-28(44-21-29(37)38)25(18-23)32(41)42)35-31(40)27(19-22-11-6-4-7-12-22)36-33(43)45-24-13-8-5-9-14-24/h4-9,11-16,18,26-27H,2-3,10,17,19-21H2,1H3,(H,34,39)(H,35,40)(H,36,43)(H,37,38)(H,41,42)/t26-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against human protein tyrosine phosphatase 1B (PTP1B) |
Bioorg Med Chem Lett 13: 971-5 (2003)
BindingDB Entry DOI: 10.7270/Q24M93WC |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50076364
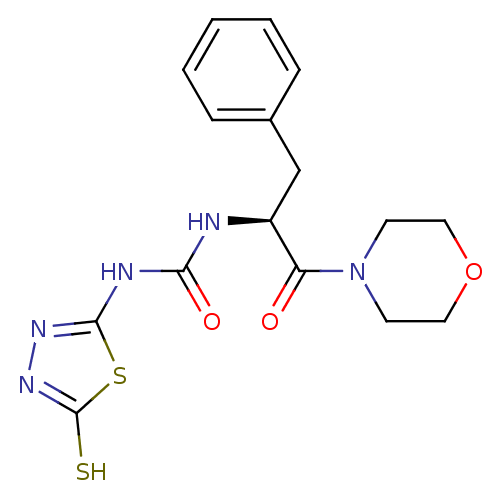
(1-((S)-1-Benzyl-2-morpholin-4-yl-2-oxo-ethyl)-3-(5...)Show SMILES Sc1nnc(NC(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCOCC2)s1 Show InChI InChI=1S/C16H19N5O3S2/c22-13(21-6-8-24-9-7-21)12(10-11-4-2-1-3-5-11)17-14(23)18-15-19-20-16(25)26-15/h1-5,12H,6-10H2,(H,20,25)(H2,17,18,19,23)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-3 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50076363
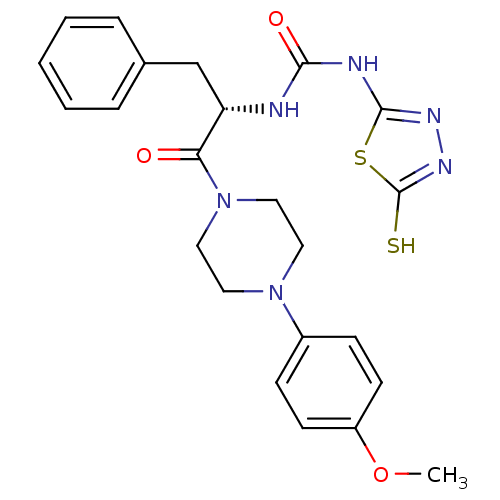
(1-{(S)-1-Benzyl-2-[4-(4-methoxy-phenyl)-piperazin-...)Show SMILES COc1ccc(cc1)N1CCN(CC1)C(=O)[C@H](Cc1ccccc1)NC(=O)Nc1nnc(S)s1 Show InChI InChI=1S/C23H26N6O3S2/c1-32-18-9-7-17(8-10-18)28-11-13-29(14-12-28)20(30)19(15-16-5-3-2-4-6-16)24-21(31)25-22-26-27-23(33)34-22/h2-10,19H,11-15H2,1H3,(H,27,33)(H2,24,25,26,31)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-3 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Rattus norvegicus (rat)) | BDBM13609

(2-{4-[(2S)-2-[(2S)-2-(3-formamidopropanoic acid)-3...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OC(C(O)=O)C(O)=O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CCC(O)=O |r| Show InChI InChI=1S/C30H37N3O10/c1-2-3-7-16-31-27(37)22(18-20-10-12-21(13-11-20)43-26(29(39)40)30(41)42)33-28(38)23(17-19-8-5-4-6-9-19)32-24(34)14-15-25(35)36/h4-6,8-13,22-23,26H,2-3,7,14-18H2,1H3,(H,31,37)(H,32,34)(H,33,38)(H,35,36)(H,39,40)(H,41,42)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20E+3 | -33.5 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Pharmacia Corporation
| Assay Description
Hydrolysis of substrate p-nitrophenyl phosphate was monitored on a 96-well microplate reader. Concentrations of PTP1B inhibitors that resulted in 50%... |
Biochemistry 40: 5642-54 (2001)
Article DOI: 10.1021/bi002865v
BindingDB Entry DOI: 10.7270/Q2D50K6S |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50076358

((S)-N-Isopropyl-3-phenyl-2-[3-(5-thioxo-4,5-dihydr...)Show SMILES CC(C)NC(=O)[C@H](Cc1ccccc1)NC(=O)Nc1nnc(S)s1 Show InChI InChI=1S/C15H19N5O2S2/c1-9(2)16-12(21)11(8-10-6-4-3-5-7-10)17-13(22)18-14-19-20-15(23)24-14/h3-7,9,11H,8H2,1-2H3,(H,16,21)(H,20,23)(H2,17,18,19,22)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-3 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50124515
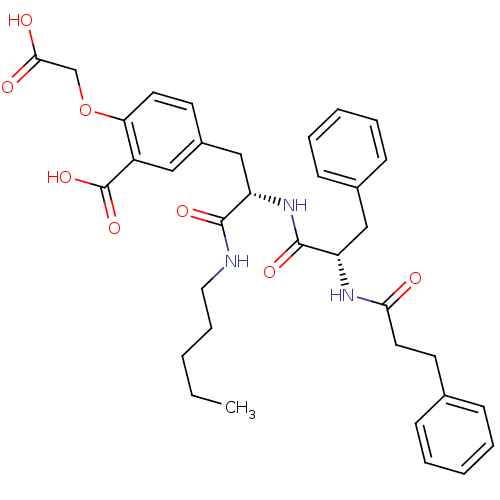
(2-Carboxymethoxy-5-{2-pentylcarbamoyl-2-[(S)-(S)-3...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)c(c1)C(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)CCc1ccccc1 Show InChI InChI=1S/C35H41N3O8/c1-2-3-10-19-36-33(42)28(22-26-15-17-30(46-23-32(40)41)27(20-26)35(44)45)38-34(43)29(21-25-13-8-5-9-14-25)37-31(39)18-16-24-11-6-4-7-12-24/h4-9,11-15,17,20,28-29H,2-3,10,16,18-19,21-23H2,1H3,(H,36,42)(H,37,39)(H,38,43)(H,40,41)(H,44,45)/t28-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against human protein tyrosine phosphatase 1B (PTP1B) |
Bioorg Med Chem Lett 13: 971-5 (2003)
BindingDB Entry DOI: 10.7270/Q24M93WC |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50076342

(1-[(S)-1-Benzyl-2-oxo-2-(4-pyridin-2-yl-piperazin-...)Show SMILES Sc1nnc(NC(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCN(CC2)c2ccccn2)s1 Show InChI InChI=1S/C21H23N7O2S2/c29-18(28-12-10-27(11-13-28)17-8-4-5-9-22-17)16(14-15-6-2-1-3-7-15)23-19(30)24-20-25-26-21(31)32-20/h1-9,16H,10-14H2,(H,26,31)(H2,23,24,25,30)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-1 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50076367

(1-[(S)-1-(4-Fluoro-benzyl)-2-oxo-2-(4-pyridin-2-yl...)Show SMILES Fc1ccc(C[C@H](NC(=O)Nc2nnc(S)s2)C(=O)N2CCN(CC2)c2ccccn2)cc1 Show InChI InChI=1S/C21H22FN7O2S2/c22-15-6-4-14(5-7-15)13-16(24-19(31)25-20-26-27-21(32)33-20)18(30)29-11-9-28(10-12-29)17-3-1-2-8-23-17/h1-8,16H,9-13H2,(H,27,32)(H2,24,25,26,31)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-1 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50112101

(5-[2-(2-tert-Butoxycarbonylamino-3-phenyl-propiony...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)c(c1)C(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C31H41N3O9/c1-5-6-10-15-32-27(37)23(18-21-13-14-25(42-19-26(35)36)22(16-21)29(39)40)33-28(38)24(17-20-11-8-7-9-12-20)34-30(41)43-31(2,3)4/h7-9,11-14,16,23-24H,5-6,10,15,17-19H2,1-4H3,(H,32,37)(H,33,38)(H,34,41)(H,35,36)(H,39,40)/t23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against human protein tyrosine phosphatase 1B (PTP1B) |
Bioorg Med Chem Lett 13: 971-5 (2003)
BindingDB Entry DOI: 10.7270/Q24M93WC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50241378
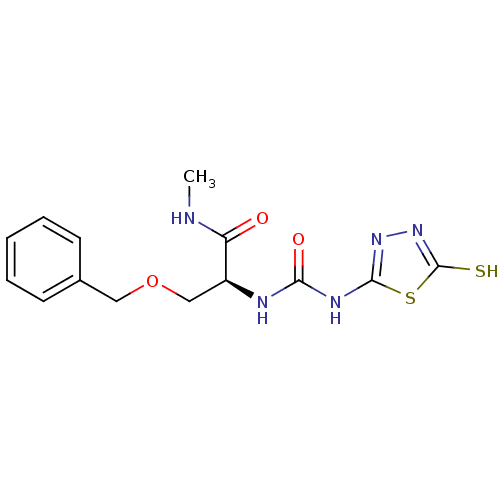
((S)-1-(3-(benzyloxy)-1-(methylamino)-1-oxopropan-2...)Show InChI InChI=1S/C14H17N5O3S2/c1-15-11(20)10(8-22-7-9-5-3-2-4-6-9)16-12(21)17-13-18-19-14(23)24-13/h2-6,10H,7-8H2,1H3,(H,15,20)(H,19,23)(H2,16,17,18,21)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-3 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50076370
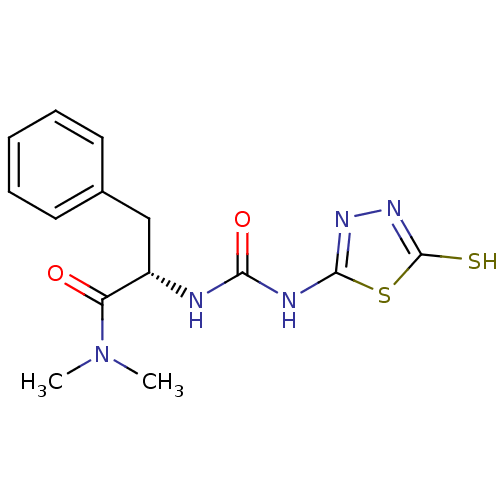
((S)-N,N-Dimethyl-3-phenyl-2-[3-(5-thioxo-4,5-dihyd...)Show SMILES CN(C)C(=O)[C@H](Cc1ccccc1)NC(=O)Nc1nnc(S)s1 Show InChI InChI=1S/C14H17N5O2S2/c1-19(2)11(20)10(8-9-6-4-3-5-7-9)15-12(21)16-13-17-18-14(22)23-13/h3-7,10H,8H2,1-2H3,(H,18,22)(H2,15,16,17,21)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-3 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Rattus norvegicus (rat)) | BDBM13610

(2-(carboxymethoxy)-5-[(2S)-2-(3-formamidopropanoic...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)c(c1)C(O)=O)NC(=O)CCC(O)=O |r| Show InChI InChI=1S/C21H28N2O9/c1-2-3-4-9-22-20(29)15(23-17(24)7-8-18(25)26)11-13-5-6-16(32-12-19(27)28)14(10-13)21(30)31/h5-6,10,15H,2-4,7-9,11-12H2,1H3,(H,22,29)(H,23,24)(H,25,26)(H,27,28)(H,30,31)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+3 | -31.4 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Pharmacia Corporation
| Assay Description
Hydrolysis of substrate p-nitrophenyl phosphate was monitored on a 96-well microplate reader. Concentrations of PTP1B inhibitors that resulted in 50%... |
Biochemistry 40: 5642-54 (2001)
Article DOI: 10.1021/bi002865v
BindingDB Entry DOI: 10.7270/Q2D50K6S |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50076341

((S)-N-Methyl-3-pentafluorophenyl-2-[3-(5-thioxo-4,...)Show SMILES CNC(=O)[C@H](Cc1c(F)c(F)c(F)c(F)c1F)NC(=O)Nc1nnc(S)s1 Show InChI InChI=1S/C13H10F5N5O2S2/c1-19-10(24)4(20-11(25)21-12-22-23-13(26)27-12)2-3-5(14)7(16)9(18)8(17)6(3)15/h4H,2H2,1H3,(H,19,24)(H,23,26)(H2,20,21,22,25)/t4-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-1 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50076333

((S)-3-Phenyl-2-[3-(5-thioxo-4,5-dihydro-[1,3,4]thi...)Show InChI InChI=1S/C12H12N4O3S2/c17-9(18)8(6-7-4-2-1-3-5-7)13-10(19)14-11-15-16-12(20)21-11/h1-5,8H,6H2,(H,16,20)(H,17,18)(H2,13,14,15,19)/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-3 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50076343
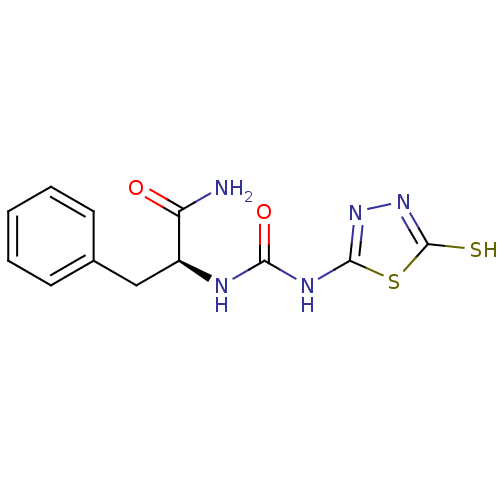
((S)-3-Phenyl-2-[3-(5-thioxo-4,5-dihydro-[1,3,4]thi...)Show InChI InChI=1S/C12H13N5O2S2/c13-9(18)8(6-7-4-2-1-3-5-7)14-10(19)15-11-16-17-12(20)21-11/h1-5,8H,6H2,(H2,13,18)(H,17,20)(H2,14,15,16,19)/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-3 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Rattus norvegicus (rat)) | BDBM13613

(2-{4-[(2S)-2-({[(1S)-1-carboxy-2-phenylethyl]carba...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OC(C(O)=O)C(O)=O)cc1)NC(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C27H33N3O9/c1-2-3-7-14-28-23(31)20(15-18-10-12-19(13-11-18)39-22(25(34)35)26(36)37)29-27(38)30-21(24(32)33)16-17-8-5-4-6-9-17/h4-6,8-13,20-22H,2-3,7,14-16H2,1H3,(H,28,31)(H,32,33)(H,34,35)(H,36,37)(H2,29,30,38)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| MMDB
PDB
Article
PubMed
| 3.40E+3 | -30.9 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Pharmacia Corporation
| Assay Description
Hydrolysis of substrate p-nitrophenyl phosphate was monitored on a 96-well microplate reader. Concentrations of PTP1B inhibitors that resulted in 50%... |
Biochemistry 40: 5642-54 (2001)
Article DOI: 10.1021/bi002865v
BindingDB Entry DOI: 10.7270/Q2D50K6S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50241376
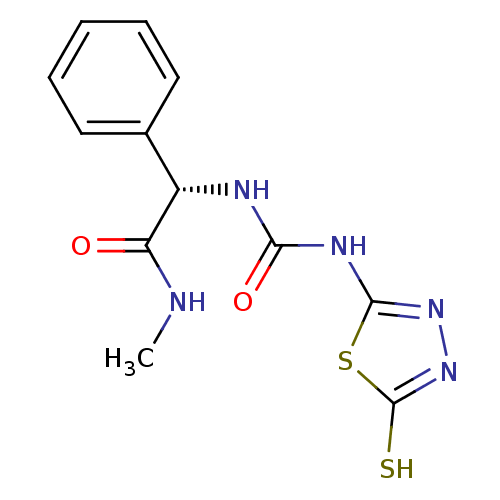
((S)-1-(2-(methylamino)-2-oxo-1-phenylethyl)-3-(5-t...)Show InChI InChI=1S/C12H13N5O2S2/c1-13-9(18)8(7-5-3-2-4-6-7)14-10(19)15-11-16-17-12(20)21-11/h2-6,8H,1H3,(H,13,18)(H,17,20)(H2,14,15,16,19)/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-3 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50241374

((S)-1-(3-cyclohexyl-1-(methylamino)-1-oxopropan-2-...)Show InChI InChI=1S/C13H21N5O2S2/c1-14-10(19)9(7-8-5-3-2-4-6-8)15-11(20)16-12-17-18-13(21)22-12/h8-9H,2-7H2,1H3,(H,14,19)(H,18,21)(H2,15,16,17,20)/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-3 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Rattus norvegicus (rat)) | BDBM13606
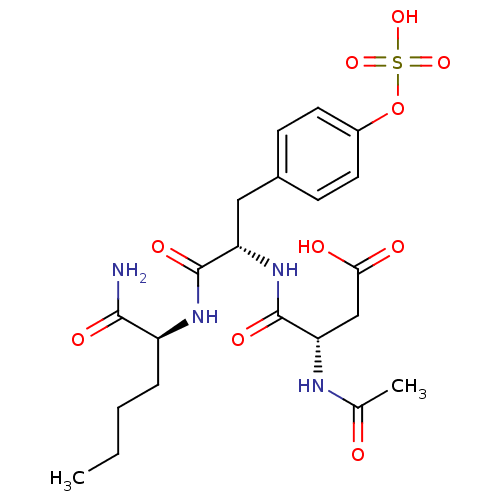
((3S)-3-{[(1S)-1-{[(1S)-1-carbamoylpentyl]carbamoyl...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(N)=O |r| Show InChI InChI=1S/C21H30N4O10S/c1-3-4-5-15(19(22)29)24-20(30)16(25-21(31)17(11-18(27)28)23-12(2)26)10-13-6-8-14(9-7-13)35-36(32,33)34/h6-9,15-17H,3-5,10-11H2,1-2H3,(H2,22,29)(H,23,26)(H,24,30)(H,25,31)(H,27,28)(H,32,33,34)/t15-,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.00E+3 | -30.0 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Pharmacia Corporation
| Assay Description
Hydrolysis of substrate p-nitrophenyl phosphate was monitored on a 96-well microplate reader. Concentrations of PTP1B inhibitors that resulted in 50%... |
Biochemistry 40: 5642-54 (2001)
Article DOI: 10.1021/bi002865v
BindingDB Entry DOI: 10.7270/Q2D50K6S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13606

((3S)-3-{[(1S)-1-{[(1S)-1-carbamoylpentyl]carbamoyl...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(N)=O |r| Show InChI InChI=1S/C21H30N4O10S/c1-3-4-5-15(19(22)29)24-20(30)16(25-21(31)17(11-18(27)28)23-12(2)26)10-13-6-8-14(9-7-13)35-36(32,33)34/h6-9,15-17H,3-5,10-11H2,1-2H3,(H2,22,29)(H,23,26)(H,24,30)(H,25,31)(H,27,28)(H,32,33,34)/t15-,16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against human protein tyrosine phosphatase 1B (PTP1B) |
Bioorg Med Chem Lett 13: 971-5 (2003)
BindingDB Entry DOI: 10.7270/Q24M93WC |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50241373
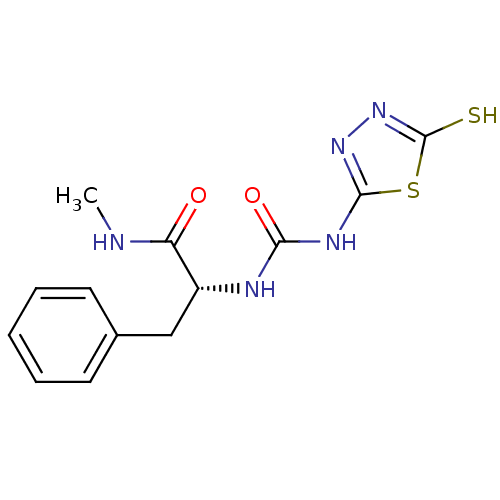
((R)-1-(1-(methylamino)-1-oxo-3-phenylpropan-2-yl)-...)Show InChI InChI=1S/C13H15N5O2S2/c1-14-10(19)9(7-8-5-3-2-4-6-8)15-11(20)16-12-17-18-13(21)22-12/h2-6,9H,7H2,1H3,(H,14,19)(H,18,21)(H2,15,16,17,20)/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-3 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50076363

(1-{(S)-1-Benzyl-2-[4-(4-methoxy-phenyl)-piperazin-...)Show SMILES COc1ccc(cc1)N1CCN(CC1)C(=O)[C@H](Cc1ccccc1)NC(=O)Nc1nnc(S)s1 Show InChI InChI=1S/C23H26N6O3S2/c1-32-18-9-7-17(8-10-18)28-11-13-29(14-12-28)20(30)19(15-16-5-3-2-4-6-16)24-21(31)25-22-26-27-23(33)34-22/h2-10,19H,11-15H2,1H3,(H,27,33)(H2,24,25,26,31)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-1 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50076334

((S)-N-(3,4-Dimethoxy-benzyl)-3-phenyl-2-[3-(5-thio...)Show SMILES COc1ccc(CNC(=O)[C@H](Cc2ccccc2)NC(=O)Nc2nnc(S)s2)cc1OC Show InChI InChI=1S/C21H23N5O4S2/c1-29-16-9-8-14(11-17(16)30-2)12-22-18(27)15(10-13-6-4-3-5-7-13)23-19(28)24-20-25-26-21(31)32-20/h3-9,11,15H,10,12H2,1-2H3,(H,22,27)(H,26,31)(H2,23,24,25,28)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn
Curated by ChEMBL
| Assay Description
Binding affinity for matrix metalloproteinase-1 |
J Med Chem 42: 1525-36 (1999)
Article DOI: 10.1021/jm9803222
BindingDB Entry DOI: 10.7270/Q2C828G4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data