 Found 592 hits with Last Name = 'bordner' and Initial = 'j'
Found 592 hits with Last Name = 'bordner' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50113783
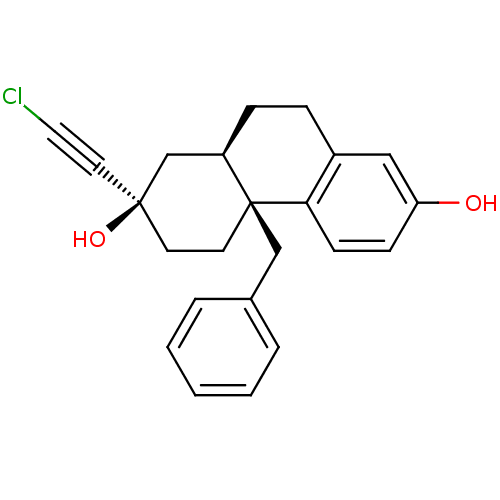
((2R,4aS,10aR)-4a-benzyl-2-(chloroethynyl)-1,2,3,4,...)Show SMILES Oc1ccc2c(CC[C@@H]3C[C@](O)(CC[C@@]23Cc2ccccc2)C#CCl)c1 Show InChI InChI=1S/C23H23ClO2/c24-13-12-22(26)10-11-23(15-17-4-2-1-3-5-17)19(16-22)7-6-18-14-20(25)8-9-21(18)23/h1-5,8-9,14,19,25-26H,6-7,10-11,15-16H2/t19-,22+,23+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of 10 nM [3H]dexamethasone from human Glucocorticoid receptor |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50113782

((10R,13S,17S)-17-hydroxy-13-methyl-10-(4-methylben...)Show SMILES CC#C[C@]1(O)CCC2C3CCC4=CC(=O)CC[C@]4(Cc4ccc(C)cc4)C3=CC[C@]12C |c:29,t:11| Show InChI InChI=1S/C29H34O2/c1-4-14-29(31)17-13-25-24-10-9-22-18-23(30)11-16-28(22,26(24)12-15-27(25,29)3)19-21-7-5-20(2)6-8-21/h5-8,12,18,24-25,31H,9-11,13,15-17,19H2,1-3H3/t24?,25?,27-,28+,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of 10 nM [3H]dexamethasone from human Glucocorticoid receptor |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50113780
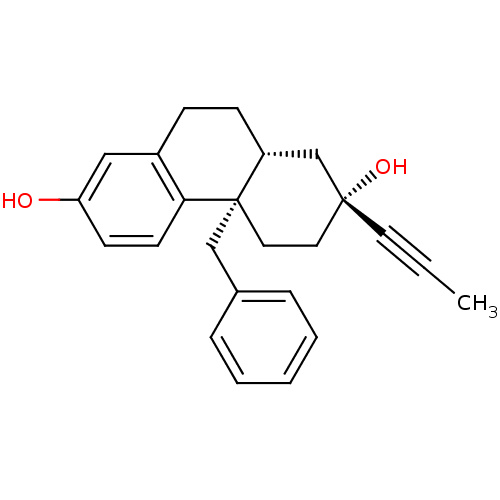
((2R,4aS,10aR)-4a-benzyl-2-(prop-1-ynyl)-1,2,3,4,4a...)Show SMILES CC#C[C@@]1(O)CC[C@]2(Cc3ccccc3)[C@H](CCc3cc(O)ccc23)C1 Show InChI InChI=1S/C24H26O2/c1-2-12-23(26)13-14-24(16-18-6-4-3-5-7-18)20(17-23)9-8-19-15-21(25)10-11-22(19)24/h3-7,10-11,15,20,25-26H,8-9,13-14,16-17H2,1H3/t20-,23-,24+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of 10 nM [3H]dexamethasone from human Glucocorticoid receptor |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to Alpha-1 adrenergic receptor by displacement of [3H]prazosin |
J Med Chem 31: 1036-9 (1988)
BindingDB Entry DOI: 10.7270/Q2639RZK |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627
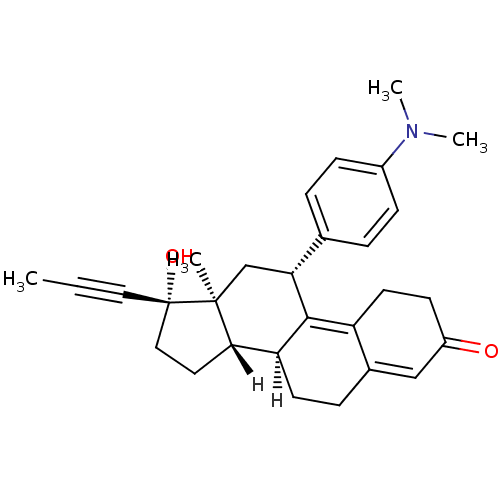
((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of 10 nM [3H]dexamethasone from human Glucocorticoid receptor |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Secretin receptor
(Homo sapiens (Human)) | BDBM50327887

(CHEMBL1255581 | HSDGTFTSELSRLQDSARLQRLLQGLV-NH2)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CO)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)Cc1cnc[nH]1)[C@@H](C)O)[C@@H](C)O)C(=O)N[C@@H](C(C)C)C(N)=O |r,wU:81.83,129.133,65.72,156.164,48.53,29.33,112.119,12.17,146.148,4.4,95.100,183.186,wD:189.190,76.76,87.91,167.168,137.142,175.180,197.200,123.125,57.61,152.152,37.44,200.203,21.25,104.108,206.207,(111.34,-17.49,;111.34,-19.03,;112.68,-19.8,;110.01,-19.8,;110.01,-21.34,;108.68,-22.1,;107.35,-21.34,;107.35,-19.8,;106.02,-22.1,;104.68,-21.34,;103.34,-22.1,;103.34,-23.64,;102.01,-21.33,;102.01,-19.79,;103.34,-19.03,;103.34,-17.49,;102.02,-16.72,;104.67,-16.72,;100.68,-22.1,;99.34,-21.34,;99.34,-19.8,;98.01,-22.1,;98.01,-23.64,;99.34,-24.41,;99.34,-25.95,;100.67,-23.64,;96.67,-21.34,;95.33,-22.1,;95.33,-23.64,;94,-21.33,;94,-19.79,;95.33,-19.03,;95.33,-17.49,;96.66,-19.79,;92.67,-22.1,;91.34,-21.34,;91.34,-19.8,;90.01,-22.1,;90.01,-23.64,;91.34,-24.41,;91.34,-25.95,;92.67,-26.72,;92.66,-28.26,;91.34,-29.03,;94.01,-29.03,;88.67,-21.34,;87.33,-22.1,;87.33,-23.64,;86,-21.33,;86,-19.79,;87.33,-19.02,;87.33,-17.48,;86.02,-16.72,;88.66,-16.72,;84.68,-22.11,;83.34,-21.35,;83.34,-19.81,;82.01,-22.12,;82.01,-23.66,;83.34,-24.42,;83.34,-25.96,;84.67,-23.66,;80.68,-21.34,;79.33,-22.1,;79.33,-23.64,;78,-21.33,;78,-19.79,;79.33,-19.03,;79.33,-17.49,;80.67,-16.73,;80.66,-15.19,;79.35,-14.42,;82.01,-14.42,;76.68,-22.11,;75.34,-21.35,;75.34,-19.81,;74.01,-22.11,;74.01,-23.65,;72.68,-21.34,;71.34,-22.1,;71.34,-23.64,;70,-21.33,;70,-19.79,;71.36,-19.03,;68.67,-22.11,;67.33,-21.35,;67.33,-19.81,;66,-22.11,;66,-23.65,;67.33,-24.42,;68.68,-23.65,;67.33,-25.96,;64.67,-21.34,;63.33,-22.1,;63.33,-23.64,;62,-21.34,;62,-19.8,;63.33,-19.03,;63.33,-17.49,;62.02,-16.72,;64.66,-16.73,;60.67,-22.1,;59.33,-21.33,;59.33,-19.79,;58,-22.1,;58,-23.64,;59.33,-24.41,;59.33,-25.95,;60.66,-23.64,;56.67,-21.33,;55.33,-22.08,;55.33,-23.62,;54,-21.32,;54,-19.78,;55.33,-19.01,;55.33,-17.47,;56.68,-16.73,;56.65,-15.19,;55.33,-14.43,;58,-14.43,;52.67,-22.1,;51.34,-21.33,;51.34,-19.79,;50,-22.1,;50,-23.64,;51.37,-24.41,;48.67,-21.32,;47.32,-22.06,;47.32,-23.6,;45.99,-21.3,;45.99,-19.76,;47.32,-18.99,;47.32,-17.45,;48.65,-19.76,;44.67,-22.09,;43.33,-21.33,;43.33,-19.79,;42,-22.1,;42,-23.64,;43.33,-24.41,;43.33,-25.95,;42.05,-26.72,;44.66,-26.71,;40.67,-21.31,;39.32,-22.05,;39.32,-23.59,;37.99,-21.28,;37.99,-19.74,;39.38,-18.98,;36.66,-22.06,;35.33,-21.29,;35.33,-19.75,;34,-22.05,;32.66,-21.28,;31.32,-22.03,;31.32,-23.57,;29.99,-21.26,;29.99,-19.72,;31.32,-18.95,;32.63,-19.72,;33.97,-18.95,;33.97,-17.42,;32.63,-16.65,;31.32,-17.42,;28.67,-22.06,;27.32,-21.3,;27.32,-19.76,;26,-22.07,;24.68,-21.27,;23.33,-22.01,;23.33,-23.55,;21.99,-21.24,;20.66,-22.02,;19.33,-21.26,;19.33,-19.72,;18,-22.02,;18,-23.56,;19.33,-24.33,;20.71,-23.57,;19.33,-25.87,;16.68,-21.22,;15.32,-21.95,;15.32,-23.49,;13.99,-21.18,;13.99,-19.64,;15.37,-18.89,;12.66,-21.95,;11.33,-21.18,;11.33,-19.63,;9.99,-21.95,;8.64,-21.17,;9.99,-23.48,;9.07,-24.71,;9.51,-26.17,;8.25,-27.06,;7.01,-26.13,;7.53,-24.68,;26,-23.61,;24.67,-24.38,;27.39,-24.39,;34,-23.59,;32.66,-24.36,;35.35,-24.37,;111.34,-22.1,;111.34,-23.64,;112.68,-21.34,;114.01,-22.11,;114.01,-23.65,;115.34,-24.41,;112.68,-24.41,;115.34,-21.34,;116.67,-22.11,;115.34,-19.8,)| Show InChI InChI=1S/C129H216N42O42/c1-58(2)40-78(119(206)170-99(64(13)14)102(134)189)149-94(181)51-145-105(192)74(29-33-91(131)178)153-113(200)81(43-61(7)8)161-116(203)82(44-62(9)10)159-108(195)72(27-22-38-143-128(137)138)151-110(197)75(30-34-92(132)179)154-114(201)79(41-59(3)4)157-107(194)71(26-21-37-142-127(135)136)150-103(190)65(15)148-121(208)87(53-172)166-118(205)86(49-98(187)188)163-111(198)76(31-35-93(133)180)155-115(202)80(42-60(5)6)158-109(196)73(28-23-39-144-129(139)140)152-122(209)89(55-174)167-117(204)83(45-63(11)12)160-112(199)77(32-36-96(183)184)156-123(210)90(56-175)168-126(213)101(67(17)177)171-120(207)84(46-68-24-19-18-20-25-68)164-125(212)100(66(16)176)169-95(182)52-146-106(193)85(48-97(185)186)162-124(211)88(54-173)165-104(191)70(130)47-69-50-141-57-147-69/h18-20,24-25,50,57-67,70-90,99-101,172-177H,21-23,26-49,51-56,130H2,1-17H3,(H2,131,178)(H2,132,179)(H2,133,180)(H2,134,189)(H,141,147)(H,145,192)(H,146,193)(H,148,208)(H,149,181)(H,150,190)(H,151,197)(H,152,209)(H,153,200)(H,154,201)(H,155,202)(H,156,210)(H,157,194)(H,158,196)(H,159,195)(H,160,199)(H,161,203)(H,162,211)(H,163,198)(H,164,212)(H,165,191)(H,166,205)(H,167,204)(H,168,213)(H,169,182)(H,170,206)(H,171,207)(H,183,184)(H,185,186)(H,187,188)(H4,135,136,142)(H4,137,138,143)(H4,139,140,144)/t65-,66+,67+,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,99-,100-,101-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I]-secretin-27 from secretin receptor expressed in CHO cells by gamma-spectrometer analysis |
Bioorg Med Chem Lett 20: 6040-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.062
BindingDB Entry DOI: 10.7270/Q23778ZG |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM96939

((4-amino-6,7-dimethoxy-quinazolin-2-yl)-dimethyl-a...)Show InChI InChI=1S/C12H16N4O2/c1-16(2)12-14-8-6-10(18-4)9(17-3)5-7(8)11(13)15-12/h5-6H,1-4H3,(H2,13,14,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to Alpha-1 adrenergic receptor by displacement of [3H]prazosin |
J Med Chem 31: 1036-9 (1988)
BindingDB Entry DOI: 10.7270/Q2639RZK |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human Androgen receptor |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50113777

((4aS,10aR)-4a-benzyl-7-hydroxy-3,4,4a,9,10,10a-hex...)Show SMILES Oc1ccc2c(CC[C@@H]3CC(=O)CC[C@@]23Cc2ccccc2)c1 Show InChI InChI=1S/C21H22O2/c22-18-8-9-20-16(12-18)6-7-17-13-19(23)10-11-21(17,20)14-15-4-2-1-3-5-15/h1-5,8-9,12,17,22H,6-7,10-11,13-14H2/t17-,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of 10 nM [3H]dexamethasone from human Glucocorticoid receptor |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | |
Secretin receptor
(Homo sapiens (Human)) | BDBM50327888
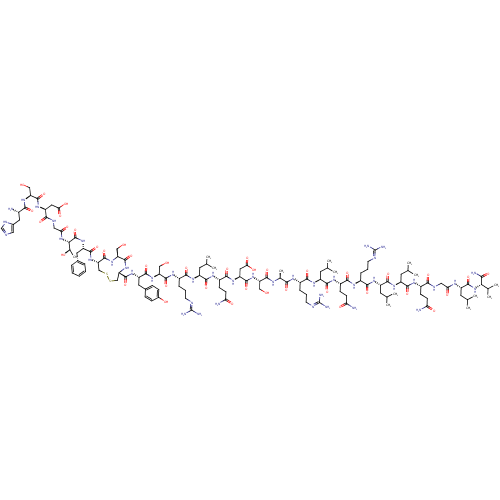
(CHEMBL1256314)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)Cc2cnc[nH]2)[C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N1)C(=O)N[C@@H](C(C)C)C(N)=O |r,wU:197.200,177.181,81.83,150.159,65.72,48.53,112.119,29.33,12.17,95.100,4.4,129.138,wD:183.185,146.147,141.141,87.91,76.76,37.44,169.175,191.195,123.125,57.61,161.163,21.25,104.108,206.209,(102.97,-19.16,;102.97,-20.7,;104.3,-21.48,;101.64,-21.48,;101.64,-23.02,;100.31,-23.79,;98.97,-23.02,;98.97,-21.48,;97.64,-23.79,;96.3,-23.02,;94.97,-23.79,;94.97,-25.33,;93.63,-23.02,;93.63,-21.48,;94.97,-20.71,;94.97,-19.17,;93.64,-18.4,;96.3,-18.4,;92.3,-23.79,;90.96,-23.02,;90.96,-21.48,;89.63,-23.79,;89.63,-25.33,;90.96,-26.1,;90.96,-27.64,;92.29,-25.33,;88.29,-23.02,;86.96,-23.79,;86.96,-25.33,;85.62,-23.02,;85.62,-21.48,;86.96,-20.71,;86.96,-19.17,;88.29,-21.48,;84.28,-23.78,;82.95,-23.01,;82.95,-21.47,;81.61,-23.78,;81.61,-25.32,;82.95,-26.09,;82.95,-27.63,;84.28,-28.4,;84.29,-29.94,;82.94,-30.71,;85.62,-30.7,;80.29,-23.01,;78.95,-23.78,;78.95,-25.32,;77.62,-23.01,;77.62,-21.47,;78.95,-20.7,;78.95,-19.16,;77.6,-18.38,;80.29,-18.39,;76.27,-23.77,;74.94,-23,;74.94,-21.46,;73.6,-23.77,;73.6,-25.31,;74.94,-26.08,;74.94,-27.62,;76.27,-25.31,;72.27,-23,;70.94,-23.78,;70.94,-25.32,;69.61,-23.02,;69.61,-21.48,;70.94,-20.7,;70.94,-19.16,;72.27,-18.38,;72.28,-16.84,;70.95,-16.04,;73.61,-16.05,;68.26,-23.77,;66.92,-23.01,;66.92,-21.47,;65.59,-23.78,;65.59,-25.32,;64.26,-23,;62.94,-23.79,;62.94,-25.33,;61.6,-23.02,;61.6,-21.48,;62.92,-20.68,;60.25,-23.77,;58.91,-23.01,;58.91,-21.47,;57.58,-23.78,;57.58,-25.32,;58.91,-26.09,;60.23,-25.29,;58.91,-27.63,;56.24,-23.01,;54.91,-23.78,;54.91,-25.32,;53.57,-23.01,;53.57,-21.47,;54.91,-20.7,;54.91,-19.16,;53.58,-18.39,;56.24,-18.39,;52.24,-23.78,;50.9,-23.01,;50.9,-21.47,;49.57,-23.77,;49.57,-25.31,;50.9,-26.09,;50.9,-27.63,;52.23,-25.31,;48.23,-23.01,;46.89,-23.77,;46.89,-25.31,;45.56,-23,;45.56,-21.46,;46.89,-20.69,;46.89,-19.15,;48.22,-18.38,;48.22,-16.84,;46.89,-16.05,;49.56,-16.06,;44.22,-23.77,;42.89,-23,;42.89,-21.46,;41.55,-23.77,;41.55,-25.31,;42.89,-26.07,;40.22,-22.99,;38.89,-23.76,;38.89,-25.3,;37.56,-22.99,;37.56,-21.45,;38.89,-20.68,;40.22,-21.46,;41.55,-20.68,;41.55,-19.14,;42.89,-18.35,;40.22,-18.37,;38.89,-19.14,;36.22,-23.76,;34.89,-23,;34.89,-21.46,;33.56,-23.76,;33.56,-25.3,;34.89,-26.07,;26.89,-26.07,;25.56,-25.3,;25.56,-23.76,;24.22,-23,;22.89,-23.76,;22.89,-25.3,;21.55,-23,;21.55,-21.46,;22.89,-20.68,;24.21,-21.45,;25.55,-20.68,;25.55,-19.14,;24.21,-18.37,;22.89,-19.14,;20.21,-23.76,;18.87,-23,;18.87,-21.46,;17.54,-23.76,;16.2,-23,;14.86,-23.77,;14.86,-25.31,;13.53,-23,;12.18,-23.76,;10.84,-23,;10.84,-21.46,;9.52,-23.77,;9.52,-25.31,;10.84,-26.08,;12.17,-25.31,;10.84,-27.62,;8.18,-23,;6.85,-23.77,;6.85,-25.31,;5.51,-23,;5.51,-21.46,;6.86,-20.66,;4.17,-23.76,;2.83,-23,;2.83,-21.45,;1.5,-23.76,;.15,-22.99,;1.49,-25.29,;.58,-26.52,;1.01,-27.99,;-.24,-28.87,;-1.48,-27.94,;-.96,-26.49,;17.54,-25.3,;16.2,-26.08,;18.86,-26.05,;26.89,-23,;26.89,-21.46,;28.22,-23.76,;29.56,-23,;29.56,-21.46,;30.9,-20.68,;30.89,-23.76,;30.89,-25.3,;32.22,-23,;102.97,-23.78,;102.97,-25.32,;104.31,-23.02,;105.65,-23.78,;105.65,-25.32,;106.98,-26.1,;104.31,-26.1,;106.98,-23.02,;108.32,-23.79,;106.98,-21.48,)| Show InChI InChI=1S/C129H208N42O40S2/c1-59(2)39-78(119(204)171-100(64(11)12)102(134)187)149-96(181)50-145-105(190)75(30-33-93(131)178)153-112(197)81(42-62(7)8)159-115(200)82(43-63(9)10)158-108(193)73(24-19-37-143-128(137)138)151-110(195)76(31-34-94(132)179)154-113(198)79(40-60(3)4)156-107(192)72(23-18-36-142-127(135)136)150-103(188)65(13)148-120(205)87(52-172)165-118(203)86(48-99(185)186)162-111(196)77(32-35-95(133)180)155-114(199)80(41-61(5)6)157-109(194)74(25-20-38-144-129(139)140)152-121(206)89(54-174)166-116(201)83(45-68-26-28-70(177)29-27-68)160-124(209)91-56-212-213-57-92(125(210)167-90(55-175)123(208)169-91)168-117(202)84(44-67-21-16-15-17-22-67)163-126(211)101(66(14)176)170-97(182)51-146-106(191)85(47-98(183)184)161-122(207)88(53-173)164-104(189)71(130)46-69-49-141-58-147-69/h15-17,21-22,26-29,49,58-66,71-92,100-101,172-177H,18-20,23-25,30-48,50-57,130H2,1-14H3,(H2,131,178)(H2,132,179)(H2,133,180)(H2,134,187)(H,141,147)(H,145,190)(H,146,191)(H,148,205)(H,149,181)(H,150,188)(H,151,195)(H,152,206)(H,153,197)(H,154,198)(H,155,199)(H,156,192)(H,157,194)(H,158,193)(H,159,200)(H,160,209)(H,161,207)(H,162,196)(H,163,211)(H,164,189)(H,165,203)(H,166,201)(H,167,210)(H,168,202)(H,169,208)(H,170,182)(H,171,204)(H,183,184)(H,185,186)(H4,135,136,142)(H4,137,138,143)(H4,139,140,144)/t65-,66+,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,100-,101-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I]-secretin-27 from secretin receptor expressed in CHO cells by gamma-spectrometer analysis |
Bioorg Med Chem Lett 20: 6040-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.062
BindingDB Entry DOI: 10.7270/Q23778ZG |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50227377

(CHEMBL414822)Show InChI InChI=1S/C13H17N3O2/c1-16(2)13-6-9(14)8-5-11(17-3)12(18-4)7-10(8)15-13/h5-7H,1-4H3,(H2,14,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to Alpha-1 adrenergic receptor by displacement of [3H]prazosin |
J Med Chem 31: 1036-9 (1988)
BindingDB Entry DOI: 10.7270/Q2639RZK |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human Progesterone receptor |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50113778
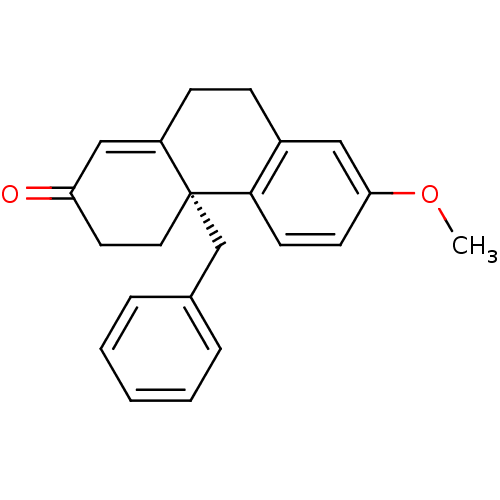
((S)-4a-Benzyl-7-methoxy-4,4a,9,10-tetrahydro-3H-ph...)Show SMILES COc1ccc2c(CCC3=CC(=O)CC[C@]23Cc2ccccc2)c1 |t:9| Show InChI InChI=1S/C22H22O2/c1-24-20-9-10-21-17(13-20)7-8-18-14-19(23)11-12-22(18,21)15-16-5-3-2-4-6-16/h2-6,9-10,13-14H,7-8,11-12,15H2,1H3/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human Androgen receptor |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50113782

((10R,13S,17S)-17-hydroxy-13-methyl-10-(4-methylben...)Show SMILES CC#C[C@]1(O)CCC2C3CCC4=CC(=O)CC[C@]4(Cc4ccc(C)cc4)C3=CC[C@]12C |c:29,t:11| Show InChI InChI=1S/C29H34O2/c1-4-14-29(31)17-13-25-24-10-9-22-18-23(30)11-16-28(22,26(24)12-15-27(25,29)3)19-21-7-5-20(2)6-8-21/h5-8,12,18,24-25,31H,9-11,13,15-17,19H2,1-3H3/t24?,25?,27-,28+,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human Androgen receptor |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50113783

((2R,4aS,10aR)-4a-benzyl-2-(chloroethynyl)-1,2,3,4,...)Show SMILES Oc1ccc2c(CC[C@@H]3C[C@](O)(CC[C@@]23Cc2ccccc2)C#CCl)c1 Show InChI InChI=1S/C23H23ClO2/c24-13-12-22(26)10-11-23(15-17-4-2-1-3-5-17)19(16-22)7-6-18-14-20(25)8-9-21(18)23/h1-5,8-9,14,19,25-26H,6-7,10-11,15-16H2/t19-,22+,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human Androgen receptor |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50113781
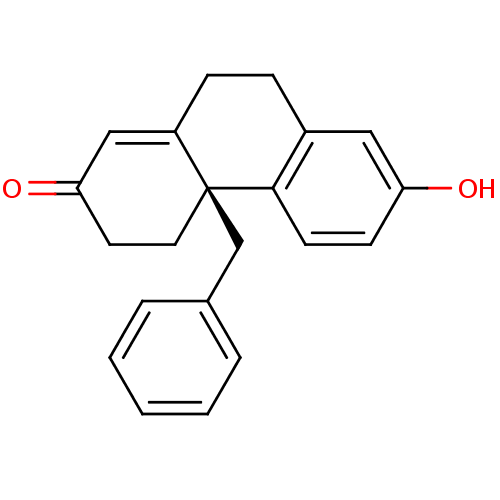
((R)-4a-Benzyl-7-hydroxy-4,4a,9,10-tetrahydro-3H-ph...)Show SMILES Oc1ccc2c(CCC3=CC(=O)CC[C@@]23Cc2ccccc2)c1 |t:8| Show InChI InChI=1S/C21H20O2/c22-18-8-9-20-16(12-18)6-7-17-13-19(23)10-11-21(17,20)14-15-4-2-1-3-5-15/h1-5,8-9,12-13,22H,6-7,10-11,14H2/t21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of 10 nM [3H]dexamethasone from human Glucocorticoid receptor |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50227382

(CHEMBL8588)Show SMILES COc1cc2cc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C20H22N4O4/c1-26-16-10-13-11-18(22-19(21)14(13)12-17(16)27-2)23-5-7-24(8-6-23)20(25)15-4-3-9-28-15/h3-4,9-12H,5-8H2,1-2H3,(H2,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to Alpha-1 adrenergic receptor by displacement of [3H]prazosin |
J Med Chem 31: 1036-9 (1988)
BindingDB Entry DOI: 10.7270/Q2639RZK |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50113781

((R)-4a-Benzyl-7-hydroxy-4,4a,9,10-tetrahydro-3H-ph...)Show SMILES Oc1ccc2c(CCC3=CC(=O)CC[C@@]23Cc2ccccc2)c1 |t:8| Show InChI InChI=1S/C21H20O2/c22-18-8-9-20-16(12-18)6-7-17-13-19(23)10-11-21(17,20)14-15-4-2-1-3-5-15/h1-5,8-9,12-13,22H,6-7,10-11,14H2/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human Androgen receptor |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50113780

((2R,4aS,10aR)-4a-benzyl-2-(prop-1-ynyl)-1,2,3,4,4a...)Show SMILES CC#C[C@@]1(O)CC[C@]2(Cc3ccccc3)[C@H](CCc3cc(O)ccc23)C1 Show InChI InChI=1S/C24H26O2/c1-2-12-23(26)13-14-24(16-18-6-4-3-5-7-18)20(17-23)9-8-19-15-21(25)10-11-22(19)24/h3-7,10-11,15,20,25-26H,8-9,13-14,16-17H2,1H3/t20-,23-,24+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human Androgen receptor |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50113776

((R)-4a-Benzyl-7-methoxy-4,4a,9,10-tetrahydro-3H-ph...)Show SMILES COc1ccc2c(CCC3=CC(=O)CC[C@@]23Cc2ccccc2)c1 |t:9| Show InChI InChI=1S/C22H22O2/c1-24-20-9-10-21-17(13-20)7-8-18-14-19(23)11-12-22(18,21)15-16-5-3-2-4-6-16/h2-6,9-10,13-14H,7-8,11-12,15H2,1H3/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human Androgen receptor |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50113777

((4aS,10aR)-4a-benzyl-7-hydroxy-3,4,4a,9,10,10a-hex...)Show SMILES Oc1ccc2c(CC[C@@H]3CC(=O)CC[C@@]23Cc2ccccc2)c1 Show InChI InChI=1S/C21H22O2/c22-18-8-9-20-16(12-18)6-7-17-13-19(23)10-11-21(17,20)14-15-4-2-1-3-5-15/h1-5,8-9,12,17,22H,6-7,10-11,13-14H2/t17-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human Androgen receptor |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 4.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to alpha-2 adrenergic receptor by displacement of [3H]clonidine |
J Med Chem 31: 1036-9 (1988)
BindingDB Entry DOI: 10.7270/Q2639RZK |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50113778

((S)-4a-Benzyl-7-methoxy-4,4a,9,10-tetrahydro-3H-ph...)Show SMILES COc1ccc2c(CCC3=CC(=O)CC[C@]23Cc2ccccc2)c1 |t:9| Show InChI InChI=1S/C22H22O2/c1-24-20-9-10-21-17(13-20)7-8-18-14-19(23)11-12-22(18,21)15-16-5-3-2-4-6-16/h2-6,9-10,13-14H,7-8,11-12,15H2,1H3/t22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human ER beta |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50113777

((4aS,10aR)-4a-benzyl-7-hydroxy-3,4,4a,9,10,10a-hex...)Show SMILES Oc1ccc2c(CC[C@@H]3CC(=O)CC[C@@]23Cc2ccccc2)c1 Show InChI InChI=1S/C21H22O2/c22-18-8-9-20-16(12-18)6-7-17-13-19(23)10-11-21(17,20)14-15-4-2-1-3-5-15/h1-5,8-9,12,17,22H,6-7,10-11,13-14H2/t17-,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human Progesterone receptor |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50113778

((S)-4a-Benzyl-7-methoxy-4,4a,9,10-tetrahydro-3H-ph...)Show SMILES COc1ccc2c(CCC3=CC(=O)CC[C@]23Cc2ccccc2)c1 |t:9| Show InChI InChI=1S/C22H22O2/c1-24-20-9-10-21-17(13-20)7-8-18-14-19(23)11-12-22(18,21)15-16-5-3-2-4-6-16/h2-6,9-10,13-14H,7-8,11-12,15H2,1H3/t22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of 10 nM [3H]dexamethasone from human Glucocorticoid receptor |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50113776

((R)-4a-Benzyl-7-methoxy-4,4a,9,10-tetrahydro-3H-ph...)Show SMILES COc1ccc2c(CCC3=CC(=O)CC[C@@]23Cc2ccccc2)c1 |t:9| Show InChI InChI=1S/C22H22O2/c1-24-20-9-10-21-17(13-20)7-8-18-14-19(23)11-12-22(18,21)15-16-5-3-2-4-6-16/h2-6,9-10,13-14H,7-8,11-12,15H2,1H3/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human ER beta |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50113780

((2R,4aS,10aR)-4a-benzyl-2-(prop-1-ynyl)-1,2,3,4,4a...)Show SMILES CC#C[C@@]1(O)CC[C@]2(Cc3ccccc3)[C@H](CCc3cc(O)ccc23)C1 Show InChI InChI=1S/C24H26O2/c1-2-12-23(26)13-14-24(16-18-6-4-3-5-7-18)20(17-23)9-8-19-15-21(25)10-11-22(19)24/h3-7,10-11,15,20,25-26H,8-9,13-14,16-17H2,1H3/t20-,23-,24+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human Progesterone receptor |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50113776

((R)-4a-Benzyl-7-methoxy-4,4a,9,10-tetrahydro-3H-ph...)Show SMILES COc1ccc2c(CCC3=CC(=O)CC[C@@]23Cc2ccccc2)c1 |t:9| Show InChI InChI=1S/C22H22O2/c1-24-20-9-10-21-17(13-20)7-8-18-14-19(23)11-12-22(18,21)15-16-5-3-2-4-6-16/h2-6,9-10,13-14H,7-8,11-12,15H2,1H3/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human Progesterone receptor |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50113781

((R)-4a-Benzyl-7-hydroxy-4,4a,9,10-tetrahydro-3H-ph...)Show SMILES Oc1ccc2c(CCC3=CC(=O)CC[C@@]23Cc2ccccc2)c1 |t:8| Show InChI InChI=1S/C21H20O2/c22-18-8-9-20-16(12-18)6-7-17-13-19(23)10-11-21(17,20)14-15-4-2-1-3-5-15/h1-5,8-9,12-13,22H,6-7,10-11,14H2/t21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human Progesterone receptor |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50113778

((S)-4a-Benzyl-7-methoxy-4,4a,9,10-tetrahydro-3H-ph...)Show SMILES COc1ccc2c(CCC3=CC(=O)CC[C@]23Cc2ccccc2)c1 |t:9| Show InChI InChI=1S/C22H22O2/c1-24-20-9-10-21-17(13-20)7-8-18-14-19(23)11-12-22(18,21)15-16-5-3-2-4-6-16/h2-6,9-10,13-14H,7-8,11-12,15H2,1H3/t22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human Progesterone receptor |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50113782

((10R,13S,17S)-17-hydroxy-13-methyl-10-(4-methylben...)Show SMILES CC#C[C@]1(O)CCC2C3CCC4=CC(=O)CC[C@]4(Cc4ccc(C)cc4)C3=CC[C@]12C |c:29,t:11| Show InChI InChI=1S/C29H34O2/c1-4-14-29(31)17-13-25-24-10-9-22-18-23(30)11-16-28(22,26(24)12-15-27(25,29)3)19-21-7-5-20(2)6-8-21/h5-8,12,18,24-25,31H,9-11,13,15-17,19H2,1-3H3/t24?,25?,27-,28+,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human ER beta |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50113783

((2R,4aS,10aR)-4a-benzyl-2-(chloroethynyl)-1,2,3,4,...)Show SMILES Oc1ccc2c(CC[C@@H]3C[C@](O)(CC[C@@]23Cc2ccccc2)C#CCl)c1 Show InChI InChI=1S/C23H23ClO2/c24-13-12-22(26)10-11-23(15-17-4-2-1-3-5-17)19(16-22)7-6-18-14-20(25)8-9-21(18)23/h1-5,8-9,14,19,25-26H,6-7,10-11,15-16H2/t19-,22+,23+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human Progesterone receptor |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50113782

((10R,13S,17S)-17-hydroxy-13-methyl-10-(4-methylben...)Show SMILES CC#C[C@]1(O)CCC2C3CCC4=CC(=O)CC[C@]4(Cc4ccc(C)cc4)C3=CC[C@]12C |c:29,t:11| Show InChI InChI=1S/C29H34O2/c1-4-14-29(31)17-13-25-24-10-9-22-18-23(30)11-16-28(22,26(24)12-15-27(25,29)3)19-21-7-5-20(2)6-8-21/h5-8,12,18,24-25,31H,9-11,13,15-17,19H2,1-3H3/t24?,25?,27-,28+,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human Estrogen receptor alpha |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50113779
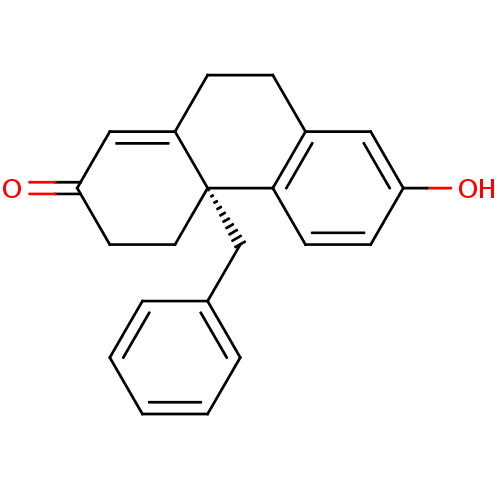
((S)-4a-Benzyl-7-hydroxy-4,4a,9,10-tetrahydro-3H-ph...)Show SMILES Oc1ccc2c(CCC3=CC(=O)CC[C@]23Cc2ccccc2)c1 |t:8| Show InChI InChI=1S/C21H20O2/c22-18-8-9-20-16(12-18)6-7-17-13-19(23)10-11-21(17,20)14-15-4-2-1-3-5-15/h1-5,8-9,12-13,22H,6-7,10-11,14H2/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human Androgen receptor |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50113778

((S)-4a-Benzyl-7-methoxy-4,4a,9,10-tetrahydro-3H-ph...)Show SMILES COc1ccc2c(CCC3=CC(=O)CC[C@]23Cc2ccccc2)c1 |t:9| Show InChI InChI=1S/C22H22O2/c1-24-20-9-10-21-17(13-20)7-8-18-14-19(23)11-12-22(18,21)15-16-5-3-2-4-6-16/h2-6,9-10,13-14H,7-8,11-12,15H2,1H3/t22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human Estrogen receptor alpha |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50113779

((S)-4a-Benzyl-7-hydroxy-4,4a,9,10-tetrahydro-3H-ph...)Show SMILES Oc1ccc2c(CCC3=CC(=O)CC[C@]23Cc2ccccc2)c1 |t:8| Show InChI InChI=1S/C21H20O2/c22-18-8-9-20-16(12-18)6-7-17-13-19(23)10-11-21(17,20)14-15-4-2-1-3-5-15/h1-5,8-9,12-13,22H,6-7,10-11,14H2/t21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of 10 nM [3H]dexamethasone from human Glucocorticoid receptor |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50113779

((S)-4a-Benzyl-7-hydroxy-4,4a,9,10-tetrahydro-3H-ph...)Show SMILES Oc1ccc2c(CCC3=CC(=O)CC[C@]23Cc2ccccc2)c1 |t:8| Show InChI InChI=1S/C21H20O2/c22-18-8-9-20-16(12-18)6-7-17-13-19(23)10-11-21(17,20)14-15-4-2-1-3-5-15/h1-5,8-9,12-13,22H,6-7,10-11,14H2/t21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human Progesterone receptor |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50113776

((R)-4a-Benzyl-7-methoxy-4,4a,9,10-tetrahydro-3H-ph...)Show SMILES COc1ccc2c(CCC3=CC(=O)CC[C@@]23Cc2ccccc2)c1 |t:9| Show InChI InChI=1S/C22H22O2/c1-24-20-9-10-21-17(13-20)7-8-18-14-19(23)11-12-22(18,21)15-16-5-3-2-4-6-16/h2-6,9-10,13-14H,7-8,11-12,15H2,1H3/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of 10 nM [3H]dexamethasone from human Glucocorticoid receptor |
J Med Chem 45: 2417-24 (2002)
BindingDB Entry DOI: 10.7270/Q2HT2Q1F |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50081070
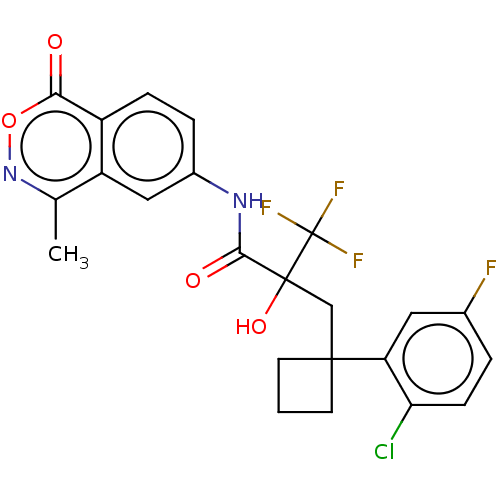
(CHEMBL3421892)Show SMILES Cc1noc(=O)c2ccc(NC(=O)C(O)(CC3(CCC3)c3cc(F)ccc3Cl)C(F)(F)F)cc12 Show InChI InChI=1S/C23H19ClF4N2O4/c1-12-16-10-14(4-5-15(16)19(31)34-30-12)29-20(32)22(33,23(26,27)28)11-21(7-2-8-21)17-9-13(25)3-6-18(17)24/h3-6,9-10,33H,2,7-8,11H2,1H3,(H,29,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human PR |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50081073
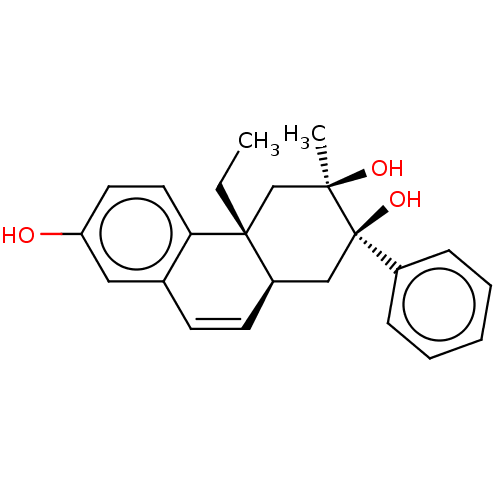
(CHEMBL3421889)Show SMILES [H][C@@]12C[C@@](O)(c3ccccc3)[C@](C)(O)C[C@@]1(CC)c1ccc(O)cc1C=C2 |r,c:28| Show InChI InChI=1S/C23H26O3/c1-3-22-15-21(2,25)23(26,17-7-5-4-6-8-17)14-18(22)10-9-16-13-19(24)11-12-20(16)22/h4-13,18,24-26H,3,14-15H2,1-2H3/t18-,21-,22-,23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human GR |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50081070

(CHEMBL3421892)Show SMILES Cc1noc(=O)c2ccc(NC(=O)C(O)(CC3(CCC3)c3cc(F)ccc3Cl)C(F)(F)F)cc12 Show InChI InChI=1S/C23H19ClF4N2O4/c1-12-16-10-14(4-5-15(16)19(31)34-30-12)29-20(32)22(33,23(26,27)28)11-21(7-2-8-21)17-9-13(25)3-6-18(17)24/h3-6,9-10,33H,2,7-8,11H2,1H3,(H,29,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human GR |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000040
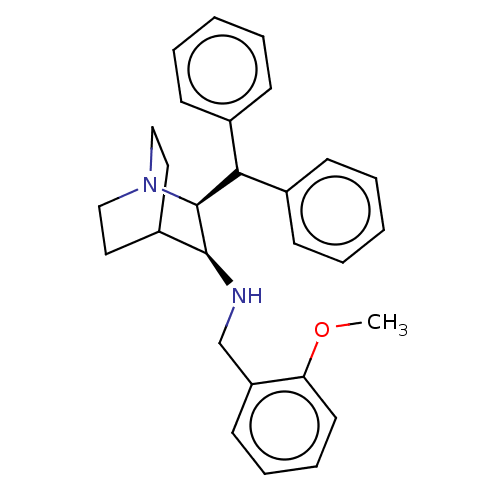
(((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1ccccc1CN[C@H]1C2CCN(CC2)[C@H]1C(c1ccccc1)c1ccccc1 |wD:10.10,17.20,(11.79,-2.71,;10.31,-3.13,;9.22,-2.04,;10.55,-1.27,;10.57,.27,;9.22,1.04,;7.89,.27,;7.89,-1.26,;6.56,-2.01,;6.56,-3.55,;5.21,-4.32,;3.92,-3.55,;3.16,-4.88,;4.65,-5.3,;3.88,-6.63,;2.57,-5.86,;2.57,-4.32,;5.21,-5.86,;6.56,-6.63,;6.54,-8.17,;5.21,-8.94,;5.21,-10.48,;6.54,-11.25,;7.89,-10.48,;7.87,-8.94,;7.89,-5.86,;9.22,-6.63,;10.55,-5.88,;10.55,-4.34,;9.22,-3.55,;7.89,-4.34,)| Show InChI InChI=1S/C28H32N2O/c1-31-25-15-9-8-14-24(25)20-29-27-23-16-18-30(19-17-23)28(27)26(21-10-4-2-5-11-21)22-12-6-3-7-13-22/h2-15,23,26-29H,16-20H2,1H3/t27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for its Tachykinin receptor 1 affinity by the displacement of [125I]-Bolton-Hunter substance p from human IM-9 cells |
Bioorg Med Chem Lett 4: 1153-1156 (1994)
Article DOI: 10.1016/S0960-894X(01)80246-8
BindingDB Entry DOI: 10.7270/Q2VQ32N3 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50081069

(CHEMBL3421871)Show SMILES [H][C@]12CCc3cc(O)ccc3[C@]1(CC)C[C@@](C)(O)[C@@](O)(C2)c1ccccc1 |r| Show InChI InChI=1S/C23H28O3/c1-3-22-15-21(2,25)23(26,17-7-5-4-6-8-17)14-18(22)10-9-16-13-19(24)11-12-20(16)22/h4-8,11-13,18,24-26H,3,9-10,14-15H2,1-2H3/t18-,21-,22-,23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human GR |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50037869
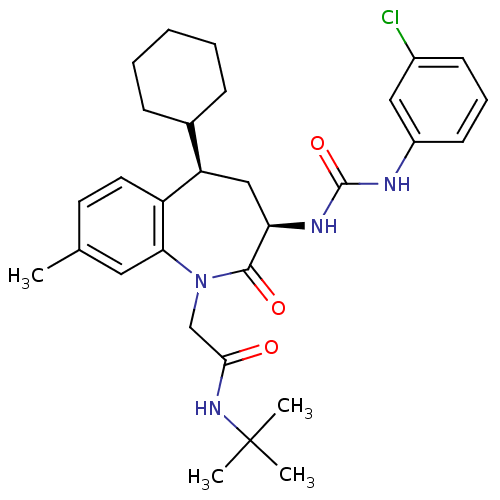
(CHEMBL334100 | N-tert-Butyl-2-{(3R,5R)-3-[3-(3-chl...)Show SMILES Cc1ccc2[C@H](C[C@@H](NC(=O)Nc3cccc(Cl)c3)C(=O)N(CC(=O)NC(C)(C)C)c2c1)C1CCCCC1 Show InChI InChI=1S/C30H39ClN4O3/c1-19-13-14-23-24(20-9-6-5-7-10-20)17-25(33-29(38)32-22-12-8-11-21(31)16-22)28(37)35(26(23)15-19)18-27(36)34-30(2,3)4/h8,11-16,20,24-25H,5-7,9-10,17-18H2,1-4H3,(H,34,36)(H2,32,33,38)/t24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro affinity to the cholecystokinin type B receptor in guinea pig cortex assayed using [125I]-BH-CCK-8 as radioligand |
J Med Chem 37: 3789-811 (1994)
BindingDB Entry DOI: 10.7270/Q2KK99TN |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50081072
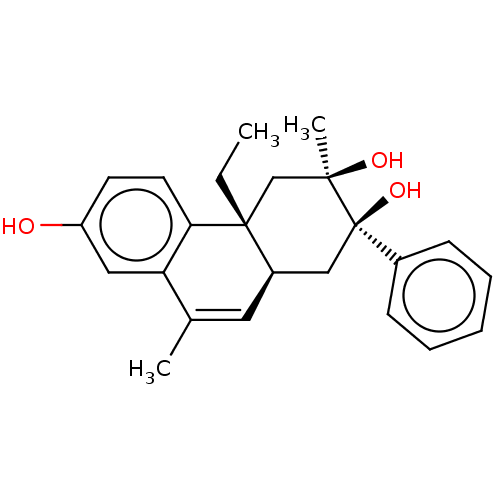
(CHEMBL3421890)Show SMILES [H][C@@]12C[C@@](O)(c3ccccc3)[C@](C)(O)C[C@@]1(CC)c1ccc(O)cc1C(C)=C2 |r,c:29| Show InChI InChI=1S/C24H28O3/c1-4-23-15-22(3,26)24(27,17-8-6-5-7-9-17)14-18(23)12-16(2)20-13-19(25)10-11-21(20)23/h5-13,18,25-27H,4,14-15H2,1-3H3/t18-,22-,23-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transrepression activity at glucocorticoid receptor in human SW1353 cells assessed as repression of IL-1-induced MMP-13 production after 24 hrs by EL... |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50081072

(CHEMBL3421890)Show SMILES [H][C@@]12C[C@@](O)(c3ccccc3)[C@](C)(O)C[C@@]1(CC)c1ccc(O)cc1C(C)=C2 |r,c:29| Show InChI InChI=1S/C24H28O3/c1-4-23-15-22(3,26)24(27,17-8-6-5-7-9-17)14-18(23)12-16(2)20-13-19(25)10-11-21(20)23/h5-13,18,25-27H,4,14-15H2,1-3H3/t18-,22-,23-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human GR |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000040

(((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1ccccc1CN[C@H]1C2CCN(CC2)[C@H]1C(c1ccccc1)c1ccccc1 |wD:10.10,17.20,(11.79,-2.71,;10.31,-3.13,;9.22,-2.04,;10.55,-1.27,;10.57,.27,;9.22,1.04,;7.89,.27,;7.89,-1.26,;6.56,-2.01,;6.56,-3.55,;5.21,-4.32,;3.92,-3.55,;3.16,-4.88,;4.65,-5.3,;3.88,-6.63,;2.57,-5.86,;2.57,-4.32,;5.21,-5.86,;6.56,-6.63,;6.54,-8.17,;5.21,-8.94,;5.21,-10.48,;6.54,-11.25,;7.89,-10.48,;7.87,-8.94,;7.89,-5.86,;9.22,-6.63,;10.55,-5.88,;10.55,-4.34,;9.22,-3.55,;7.89,-4.34,)| Show InChI InChI=1S/C28H32N2O/c1-31-25-15-9-8-14-24(25)20-29-27-23-16-18-30(19-17-23)28(27)26(21-10-4-2-5-11-21)22-12-6-3-7-13-22/h2-15,23,26-29H,16-20H2,1H3/t27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards Tachykinin receptor 1 in human IM-9 cells using [3H]-substance P as ligand |
J Med Chem 35: 2591-600 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1JJF |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50081101
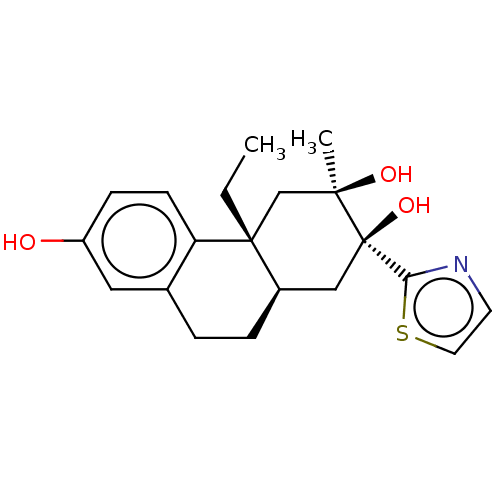
(CHEMBL3421879)Show SMILES [H][C@]12CCc3cc(O)ccc3[C@]1(CC)C[C@@](C)(O)[C@@](O)(C2)c1nccs1 |r| Show InChI InChI=1S/C20H25NO3S/c1-3-19-12-18(2,23)20(24,17-21-8-9-25-17)11-14(19)5-4-13-10-15(22)6-7-16(13)19/h6-10,14,22-24H,3-5,11-12H2,1-2H3/t14-,18-,19-,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human GR |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM14775
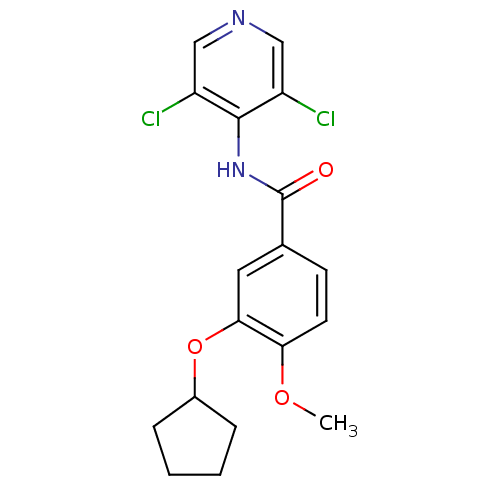
(3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...)Show InChI InChI=1S/C18H18Cl2N2O3/c1-24-15-7-6-11(8-16(15)25-12-4-2-3-5-12)18(23)22-17-13(19)9-21-10-14(17)20/h6-10,12H,2-5H2,1H3,(H,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for its inhibitory activity on unpurified recombinant Phosphodiesterase 4D |
J Med Chem 41: 266-70 (1998)
Article DOI: 10.1021/jm970685m
BindingDB Entry DOI: 10.7270/Q2KD1X11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50037849
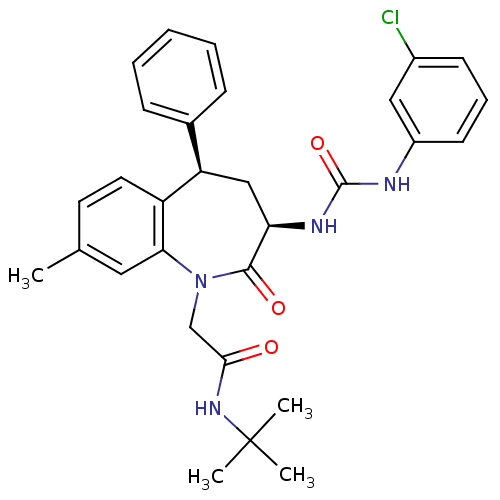
(CHEMBL291458 | N-tert-Butyl-2-{(3R,5R)-3-[3-(3-chl...)Show SMILES Cc1ccc2[C@H](C[C@@H](NC(=O)Nc3cccc(Cl)c3)C(=O)N(CC(=O)NC(C)(C)C)c2c1)c1ccccc1 Show InChI InChI=1S/C30H33ClN4O3/c1-19-13-14-23-24(20-9-6-5-7-10-20)17-25(33-29(38)32-22-12-8-11-21(31)16-22)28(37)35(26(23)15-19)18-27(36)34-30(2,3)4/h5-16,24-25H,17-18H2,1-4H3,(H,34,36)(H2,32,33,38)/t24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro affinity to the cholecystokinin type B receptor in guinea pig cortex assayed using [125I]-BH-CCK-8 as radioligand |
J Med Chem 37: 3789-811 (1994)
BindingDB Entry DOI: 10.7270/Q2KK99TN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data