 Found 1082 hits with Last Name = 'chen' and Initial = 'jm'
Found 1082 hits with Last Name = 'chen' and Initial = 'jm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human norepinephrine transporter |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP12 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Stromelysin-2
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP10 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-15
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP15 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP7 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-20
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP20 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-24
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP24 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-25
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP25 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-26
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP26 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human TACE |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP1 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP3 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP8 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP9 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP14 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-16
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP16 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50118985
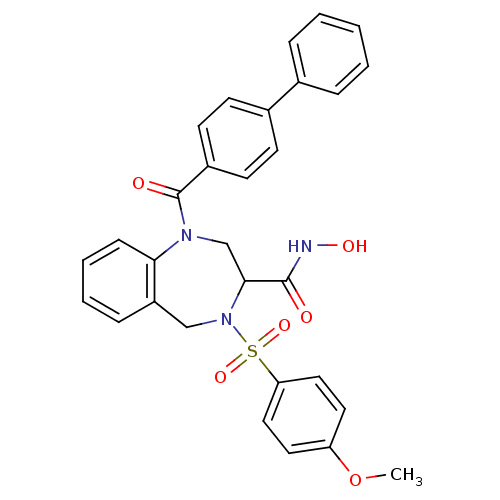
(1-(Biphenyl-4-carbonyl)-4-(4-methoxy-benzenesulfon...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccccc2N(CC1C(=O)NO)C(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C30H27N3O6S/c1-39-25-15-17-26(18-16-25)40(37,38)33-19-24-9-5-6-10-27(24)32(20-28(33)29(34)31-36)30(35)23-13-11-22(12-14-23)21-7-3-2-4-8-21/h2-18,28,36H,19-20H2,1H3,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration of the compound required in vitro to inhibit Matrix metalloproteinase-13 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50118983
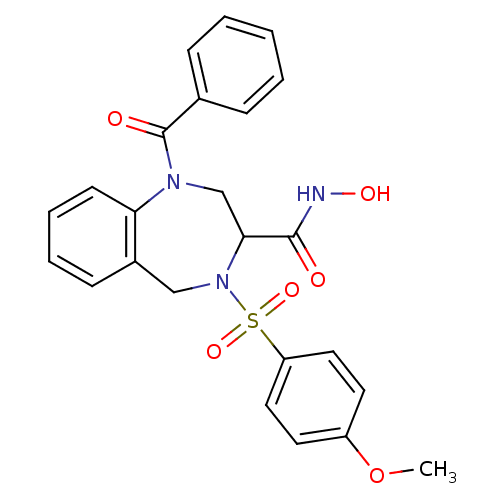
(1-Benzoyl-4-(4-methoxy-benzenesulfonyl)-2,3,4,5-te...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccccc2N(CC1C(=O)NO)C(=O)c1ccccc1 Show InChI InChI=1S/C24H23N3O6S/c1-33-19-11-13-20(14-12-19)34(31,32)27-15-18-9-5-6-10-21(18)26(16-22(27)23(28)25-30)24(29)17-7-3-2-4-8-17/h2-14,22,30H,15-16H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration of the compound required in vitro to inhibit Matrix metalloproteinase-13 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50106133
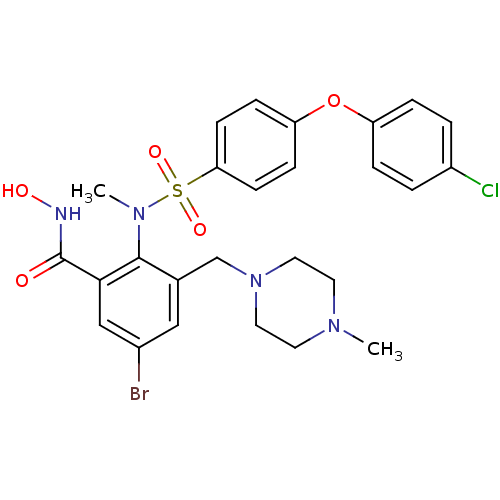
(5-Bromo-2-{[4-(4-chloro-phenoxy)-benzenesulfonyl]-...)Show SMILES CN(c1c(CN2CCN(C)CC2)cc(Br)cc1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C26H28BrClN4O5S/c1-30-11-13-32(14-12-30)17-18-15-19(27)16-24(26(33)29-34)25(18)31(2)38(35,36)23-9-7-22(8-10-23)37-21-5-3-20(28)4-6-21/h3-10,15-16,34H,11-14,17H2,1-2H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of Matrix metalloprotease-9. |
Bioorg Med Chem Lett 11: 2975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2125T64 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395921
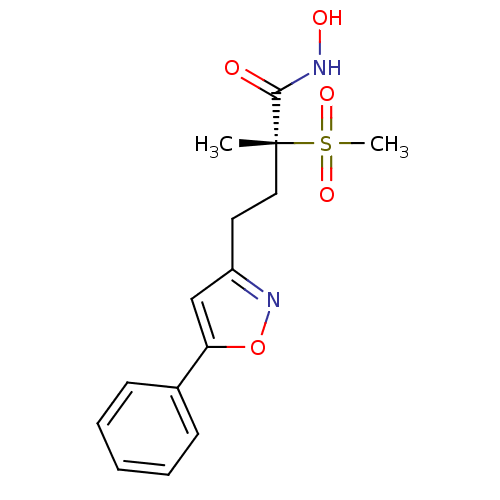
(CHEMBL2164511)Show SMILES C[C@@](CCc1cc(on1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C15H18N2O5S/c1-15(14(18)16-19,23(2,20)21)9-8-12-10-13(22-17-12)11-6-4-3-5-7-11/h3-7,10,19H,8-9H2,1-2H3,(H,16,18)/t15-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.511 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485077
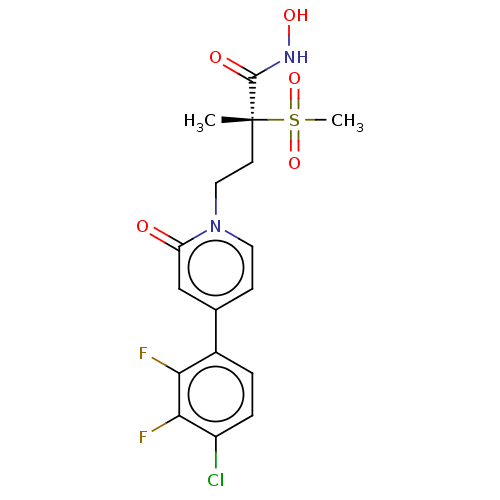
(CHEMBL2023517)Show SMILES C[C@@](CCn1ccc(cc1=O)-c1ccc(Cl)c(F)c1F)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C17H17ClF2N2O5S/c1-17(16(24)21-25,28(2,26)27)6-8-22-7-5-10(9-13(22)23)11-3-4-12(18)15(20)14(11)19/h3-5,7,9,25H,6,8H2,1-2H3,(H,21,24)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485061

(CHEMBL2023524)Show SMILES C[C@@](CCn1ccc(cc1=O)-c1ccc(cc1)-n1nccn1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C19H21N5O5S/c1-19(18(26)22-27,30(2,28)29)8-12-23-11-7-15(13-17(23)25)14-3-5-16(6-4-14)24-20-9-10-21-24/h3-7,9-11,13,27H,8,12H2,1-2H3,(H,22,26)/t19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50118983

(1-Benzoyl-4-(4-methoxy-benzenesulfonyl)-2,3,4,5-te...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccccc2N(CC1C(=O)NO)C(=O)c1ccccc1 Show InChI InChI=1S/C24H23N3O6S/c1-33-19-11-13-20(14-12-19)34(31,32)27-15-18-9-5-6-10-21(18)26(16-22(27)23(28)25-30)24(29)17-7-3-2-4-8-17/h2-14,22,30H,15-16H2,1H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration required in vitro to inhibit Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395911
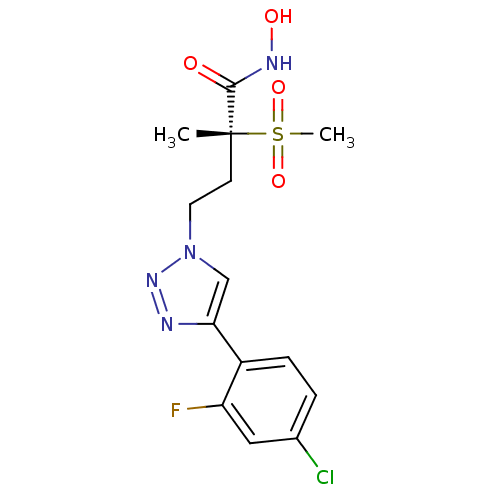
(CHEMBL2164521)Show SMILES C[C@@](CCn1cc(nn1)-c1ccc(Cl)cc1F)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C14H16ClFN4O4S/c1-14(13(21)18-22,25(2,23)24)5-6-20-8-12(17-19-20)10-4-3-9(15)7-11(10)16/h3-4,7-8,22H,5-6H2,1-2H3,(H,18,21)/t14-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.657 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50118985

(1-(Biphenyl-4-carbonyl)-4-(4-methoxy-benzenesulfon...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccccc2N(CC1C(=O)NO)C(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C30H27N3O6S/c1-39-25-15-17-26(18-16-25)40(37,38)33-19-24-9-5-6-10-27(24)32(20-28(33)29(34)31-36)30(35)23-13-11-22(12-14-23)21-7-3-2-4-8-21/h2-18,28,36H,19-20H2,1H3,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration required in vitro to inhibit Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50106133

(5-Bromo-2-{[4-(4-chloro-phenoxy)-benzenesulfonyl]-...)Show SMILES CN(c1c(CN2CCN(C)CC2)cc(Br)cc1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C26H28BrClN4O5S/c1-30-11-13-32(14-12-30)17-18-15-19(27)16-24(26(33)29-34)25(18)31(2)38(35,36)23-9-7-22(8-10-23)37-21-5-3-20(28)4-6-21/h3-10,15-16,34H,11-14,17H2,1-2H3,(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-13 |
Bioorg Med Chem Lett 11: 2975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2125T64 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50106136
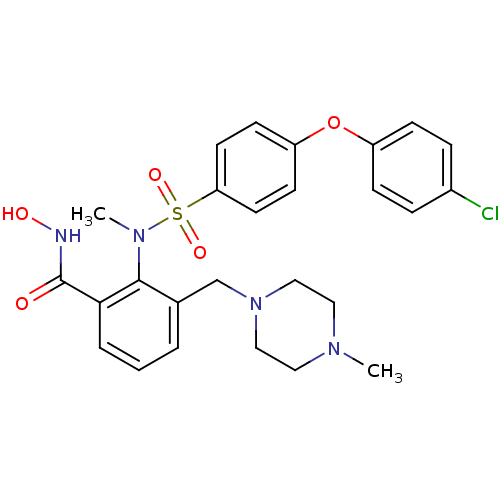
(2-{[4-(4-Chloro-phenoxy)-benzenesulfonyl]-methyl-a...)Show SMILES CN(c1c(CN2CCN(C)CC2)cccc1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C26H29ClN4O5S/c1-29-14-16-31(17-15-29)18-19-4-3-5-24(26(32)28-33)25(19)30(2)37(34,35)23-12-10-22(11-13-23)36-21-8-6-20(27)7-9-21/h3-13,33H,14-18H2,1-2H3,(H,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of Matrix metalloprotease-13. |
Bioorg Med Chem Lett 11: 2975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2125T64 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395920
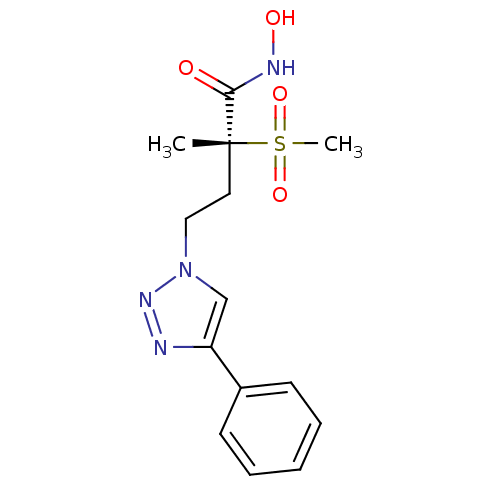
(CHEMBL2164512)Show SMILES C[C@@](CCn1cc(nn1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C14H18N4O4S/c1-14(13(19)16-20,23(2,21)22)8-9-18-10-12(15-17-18)11-6-4-3-5-7-11/h3-7,10,20H,8-9H2,1-2H3,(H,16,19)/t14-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50395910
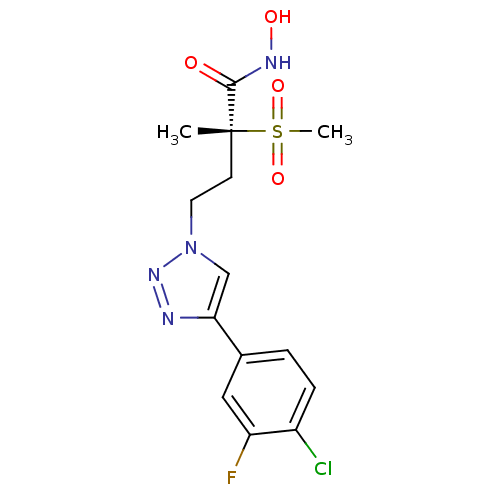
(CHEMBL2164522)Show SMILES C[C@@](CCn1cc(nn1)-c1ccc(Cl)c(F)c1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C14H16ClFN4O4S/c1-14(13(21)18-22,25(2,23)24)5-6-20-8-12(17-19-20)9-3-4-10(15)11(16)7-9/h3-4,7-8,22H,5-6H2,1-2H3,(H,18,21)/t14-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.912 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 6832-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.058
BindingDB Entry DOI: 10.7270/Q2GF0VMV |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2 [G2019S]
(Homo sapiens (Human)) | BDBM50508758
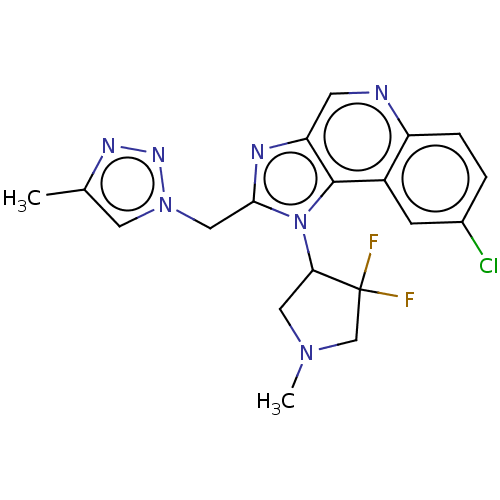
(CHEMBL4460909 | US11312713, Example 82)Show SMILES CN1CC(n2c(Cn3cc(C)nn3)nc3cnc4ccc(Cl)cc4c23)C(F)(F)C1 Show InChI InChI=1S/C19H18ClF2N7/c1-11-7-28(26-25-11)9-17-24-15-6-23-14-4-3-12(20)5-13(14)18(15)29(17)16-8-27(2)10-19(16,21)22/h3-7,16H,8-10H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.938 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
LRRK2 kinase activity was measured using Lantha Screen technology from Invitrogen. GST-tagged truncated LRRK2 from Invitrogen (Cat #PV4874) was incub... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FN19C7 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50126612
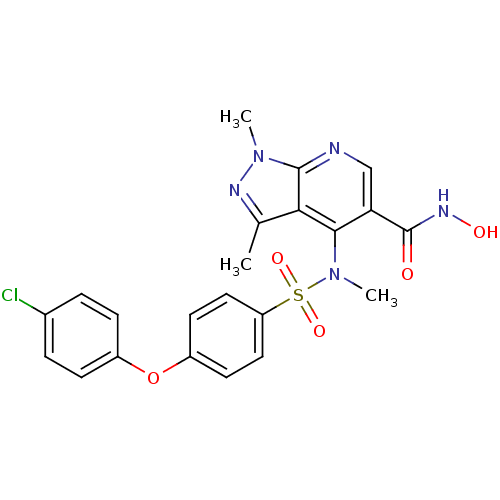
(4-{[4-(4-Chloro-phenoxy)-benzenesulfonyl]-methyl-a...)Show SMILES CN(c1c(cnc2n(C)nc(C)c12)C(=O)NO)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C22H20ClN5O5S/c1-13-19-20(18(22(29)26-30)12-24-21(19)27(2)25-13)28(3)34(31,32)17-10-8-16(9-11-17)33-15-6-4-14(23)5-7-15/h4-12,30H,1-3H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 9 (MMP-9). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50118975
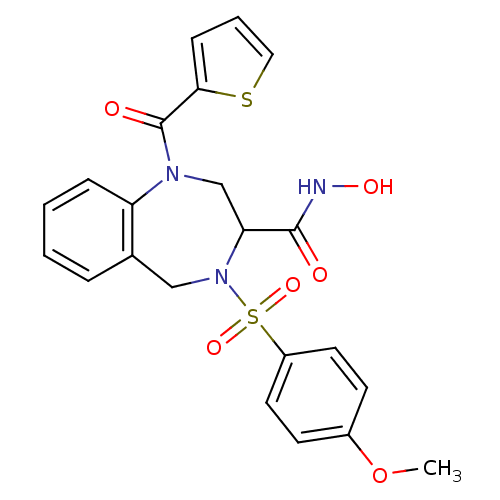
(4-(4-Methoxy-benzenesulfonyl)-1-(thiophene-2-carbo...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccccc2N(CC1C(=O)NO)C(=O)c1cccs1 Show InChI InChI=1S/C22H21N3O6S2/c1-31-16-8-10-17(11-9-16)33(29,30)25-13-15-5-2-3-6-18(15)24(14-19(25)21(26)23-28)22(27)20-7-4-12-32-20/h2-12,19,28H,13-14H2,1H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration required in vitro to inhibit Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50485056
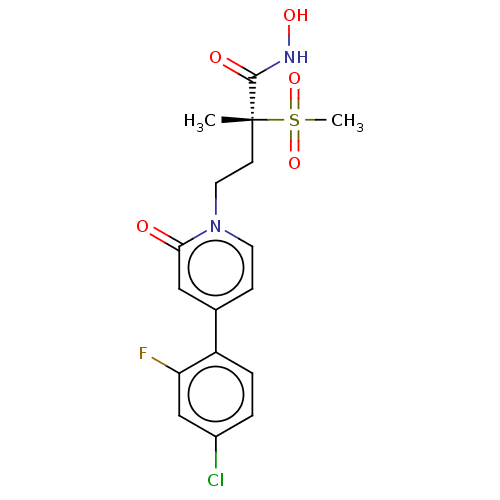
(CHEMBL2023515)Show SMILES C[C@@](CCn1ccc(cc1=O)-c1ccc(Cl)cc1F)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C17H18ClFN2O5S/c1-17(16(23)20-24,27(2,25)26)6-8-21-7-5-11(9-15(21)22)13-4-3-12(18)10-14(13)19/h3-5,7,9-10,24H,6,8H2,1-2H3,(H,20,23)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC expressed in Escherichia coli using UDP-3-O-N-acetylglucosamine substrate measured after 30 mins by mass sp... |
J Med Chem 55: 1662-70 (2012)
Article DOI: 10.1021/jm2014875
BindingDB Entry DOI: 10.7270/Q2W38050 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50118975

(4-(4-Methoxy-benzenesulfonyl)-1-(thiophene-2-carbo...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccccc2N(CC1C(=O)NO)C(=O)c1cccs1 Show InChI InChI=1S/C22H21N3O6S2/c1-31-16-8-10-17(11-9-16)33(29,30)25-13-15-5-2-3-6-18(15)24(14-19(25)21(26)23-28)22(27)20-7-4-12-32-20/h2-12,19,28H,13-14H2,1H3,(H,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration of the compound required in vitro to inhibit Matrix metalloproteinase-13 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50126626
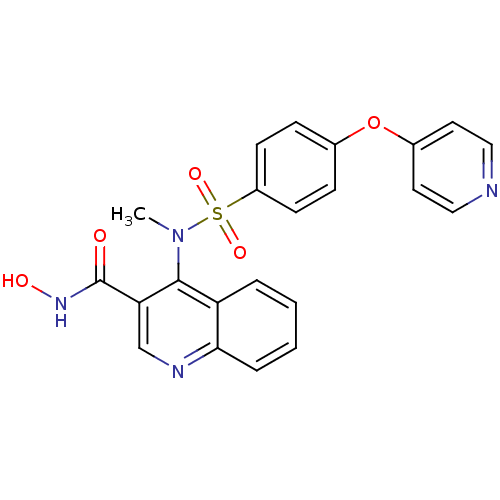
(4-{Methyl-[4-(pyridin-4-yloxy)-benzenesulfonyl]-am...)Show SMILES CN(c1c(cnc2ccccc12)C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C22H18N4O5S/c1-26(21-18-4-2-3-5-20(18)24-14-19(21)22(27)25-28)32(29,30)17-8-6-15(7-9-17)31-16-10-12-23-13-11-16/h2-14,28H,1H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 9 (MMP-9). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50106130
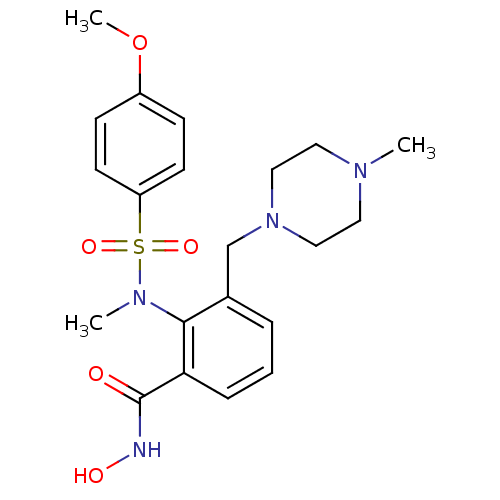
(CHEMBL100512 | N-Hydroxy-2-[(4-methoxy-benzenesulf...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(C)c1c(CN2CCN(C)CC2)cccc1C(=O)NO Show InChI InChI=1S/C21H28N4O5S/c1-23-11-13-25(14-12-23)15-16-5-4-6-19(21(26)22-27)20(16)24(2)31(28,29)18-9-7-17(30-3)8-10-18/h4-10,27H,11-15H2,1-3H3,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of Matrix metalloprotease-9. |
Bioorg Med Chem Lett 11: 2975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2125T64 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50106136

(2-{[4-(4-Chloro-phenoxy)-benzenesulfonyl]-methyl-a...)Show SMILES CN(c1c(CN2CCN(C)CC2)cccc1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C26H29ClN4O5S/c1-29-14-16-31(17-15-29)18-19-4-3-5-24(26(32)28-33)25(19)30(2)37(34,35)23-12-10-22(11-13-23)36-21-8-6-20(27)7-9-21/h3-13,33H,14-18H2,1-2H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of Matrix metalloprotease-9. |
Bioorg Med Chem Lett 11: 2975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2125T64 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50181002

((S)-N-hydroxy-2,2-dimethyl-4-(4-(prop-2-ynyloxy)ph...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(OCC#C)cc1 Show InChI InChI=1S/C16H20N2O5S2/c1-4-10-23-12-5-7-13(8-6-12)25(21,22)18-9-11-24-16(2,3)14(18)15(19)17-20/h1,5-8,14,20H,9-11H2,2-3H3,(H,17,19)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 16: 1605-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.020
BindingDB Entry DOI: 10.7270/Q2GF0T3Q |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50181006
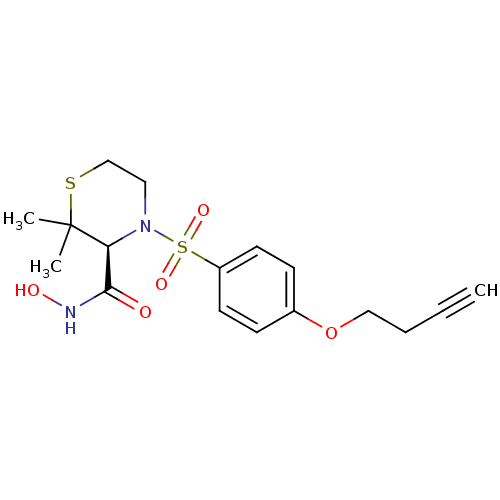
((S)-4-(4-(but-3-ynyloxy)phenylsulfonyl)-N-hydroxy-...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(OCCC#C)cc1 Show InChI InChI=1S/C17H22N2O5S2/c1-4-5-11-24-13-6-8-14(9-7-13)26(22,23)19-10-12-25-17(2,3)15(19)16(20)18-21/h1,6-9,15,21H,5,10-12H2,2-3H3,(H,18,20)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 16: 1605-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.020
BindingDB Entry DOI: 10.7270/Q2GF0T3Q |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50181002

((S)-N-hydroxy-2,2-dimethyl-4-(4-(prop-2-ynyloxy)ph...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(OCC#C)cc1 Show InChI InChI=1S/C16H20N2O5S2/c1-4-10-23-12-5-7-13(8-6-12)25(21,22)18-9-11-24-16(2,3)14(18)15(19)17-20/h1,5-8,14,20H,9-11H2,2-3H3,(H,17,19)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of MMP1 |
Bioorg Med Chem Lett 16: 1605-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.020
BindingDB Entry DOI: 10.7270/Q2GF0T3Q |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126623
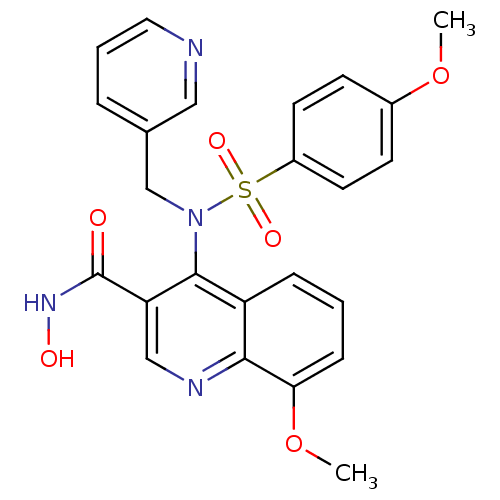
(8-Methoxy-4-[(4-methoxy-benzenesulfonyl)-pyridin-3...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(cnc2c(OC)cccc12)C(=O)NO Show InChI InChI=1S/C24H22N4O6S/c1-33-17-8-10-18(11-9-17)35(31,32)28(15-16-5-4-12-25-13-16)23-19-6-3-7-21(34-2)22(19)26-14-20(23)24(29)27-30/h3-14,30H,15H2,1-2H3,(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126626

(4-{Methyl-[4-(pyridin-4-yloxy)-benzenesulfonyl]-am...)Show SMILES CN(c1c(cnc2ccccc12)C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C22H18N4O5S/c1-26(21-18-4-2-3-5-20(18)24-14-19(21)22(27)25-28)32(29,30)17-8-6-15(7-9-17)31-16-10-12-23-13-11-16/h2-14,28H,1H3,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126622
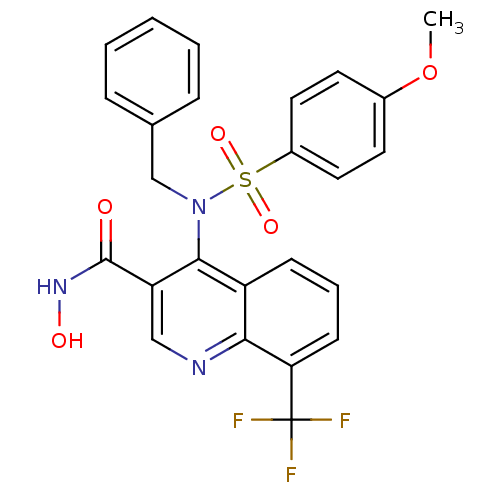
(4-(N-benzyl-4-methoxyphenylsulfonamido)-N-hydroxy-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccccc1)c1c(cnc2c(cccc12)C(F)(F)F)C(=O)NO Show InChI InChI=1S/C25H20F3N3O5S/c1-36-17-10-12-18(13-11-17)37(34,35)31(15-16-6-3-2-4-7-16)23-19-8-5-9-21(25(26,27)28)22(19)29-14-20(23)24(32)30-33/h2-14,33H,15H2,1H3,(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126612

(4-{[4-(4-Chloro-phenoxy)-benzenesulfonyl]-methyl-a...)Show SMILES CN(c1c(cnc2n(C)nc(C)c12)C(=O)NO)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C22H20ClN5O5S/c1-13-19-20(18(22(29)26-30)12-24-21(19)27(2)25-13)28(3)34(31,32)17-10-8-16(9-11-17)33-15-6-4-14(23)5-7-15/h4-12,30H,1-3H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126600
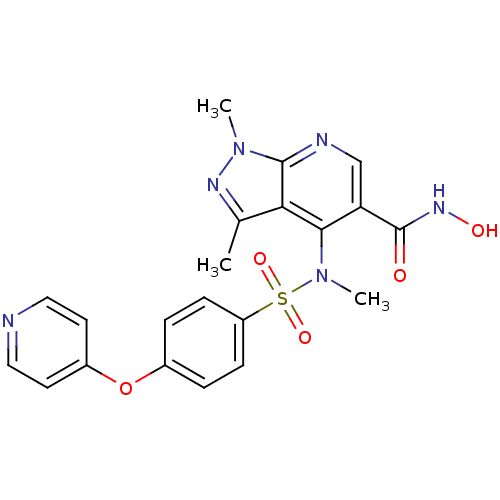
(1,3-Dimethyl-4-{methyl-[4-(pyridin-4-yloxy)-benzen...)Show SMILES CN(c1c(cnc2n(C)nc(C)c12)C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C21H20N6O5S/c1-13-18-19(17(21(28)25-29)12-23-20(18)26(2)24-13)27(3)33(30,31)16-6-4-14(5-7-16)32-15-8-10-22-11-9-15/h4-12,29H,1-3H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126624
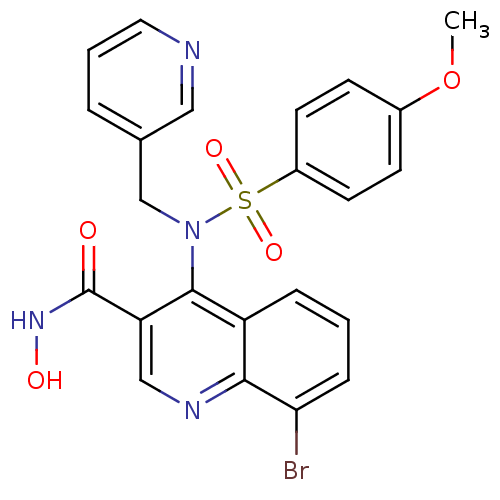
(8-Bromo-4-[(4-methoxy-benzenesulfonyl)-pyridin-3-y...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(cnc2c(Br)cccc12)C(=O)NO Show InChI InChI=1S/C23H19BrN4O5S/c1-33-16-7-9-17(10-8-16)34(31,32)28(14-15-4-3-11-25-12-15)22-18-5-2-6-20(24)21(18)26-13-19(22)23(29)27-30/h2-13,30H,14H2,1H3,(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126620
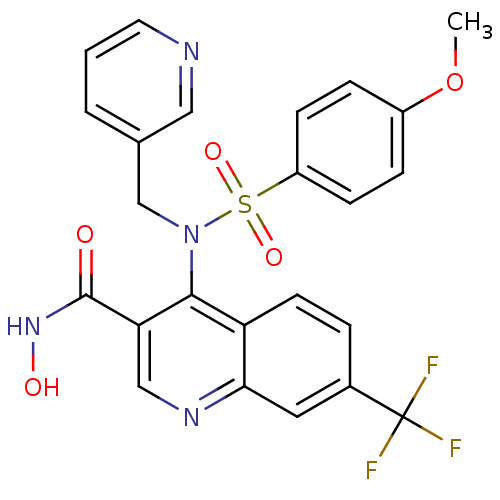
(4-[(4-Methoxy-benzenesulfonyl)-pyridin-3-ylmethyl-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(cnc2cc(ccc12)C(F)(F)F)C(=O)NO Show InChI InChI=1S/C24H19F3N4O5S/c1-36-17-5-7-18(8-6-17)37(34,35)31(14-15-3-2-10-28-12-15)22-19-9-4-16(24(25,26)27)11-21(19)29-13-20(22)23(32)30-33/h2-13,33H,14H2,1H3,(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50118974
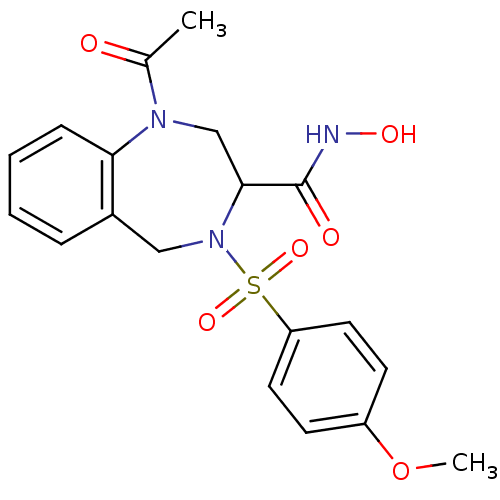
(1-Acetyl-4-(4-methoxy-benzenesulfonyl)-2,3,4,5-tet...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccccc2N(CC1C(=O)NO)C(C)=O Show InChI InChI=1S/C19H21N3O6S/c1-13(23)21-12-18(19(24)20-25)22(11-14-5-3-4-6-17(14)21)29(26,27)16-9-7-15(28-2)8-10-16/h3-10,18,25H,11-12H2,1-2H3,(H,20,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration of the compound required in vitro to inhibit Matrix metalloproteinase-13 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data