 Found 478 hits with Last Name = 'gong' and Initial = 'l'
Found 478 hits with Last Name = 'gong' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM23165

(CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by radioligand competitive binding analy... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50169441

(CHEMBL3806205)Show SMILES CCCCCC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C22H36NO4P/c1-2-3-4-5-6-17-7-8-19-14-20(10-9-18(19)13-17)21-11-12-22(23,15-21)16-27-28(24,25)26/h9-10,14,17,21H,2-8,11-13,15-16,23H2,1H3,(H2,24,25,26)/t17-,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by radioligand competitive binding analy... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM258470
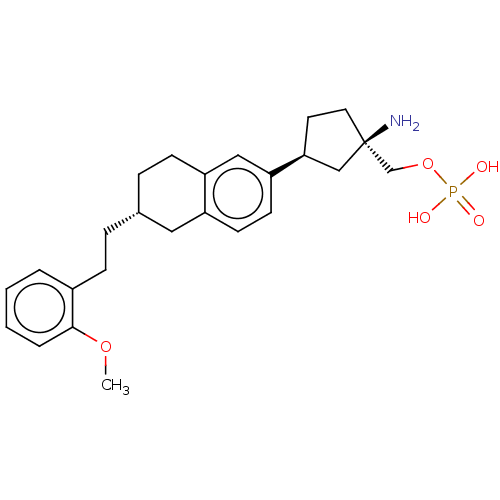
(US9522888, 697)Show SMILES COc1ccccc1CC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C25H34NO5P/c1-30-24-5-3-2-4-19(24)8-6-18-7-9-21-15-22(11-10-20(21)14-18)23-12-13-25(26,16-23)17-31-32(27,28)29/h2-5,10-11,15,18,23H,6-9,12-14,16-17,26H2,1H3,(H2,27,28,29)/t18-,23+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by radioligand competitive binding analy... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50312996

((R,S)-3-(5-chloro-1-methyl-1H-indol-3-yl)-4-(3-(2,...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cccc(NCC(O)CO)c2)c2cc(Cl)ccc12 |t:4| Show InChI InChI=1S/C22H20ClN3O4/c1-26-10-17(16-8-13(23)5-6-18(16)26)20-19(21(29)25-22(20)30)12-3-2-4-14(7-12)24-9-15(28)11-27/h2-8,10,15,24,27-28H,9,11H2,1H3,(H,25,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... |
Bioorg Med Chem Lett 20: 1693-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.038
BindingDB Entry DOI: 10.7270/Q2ZK5HNS |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313013
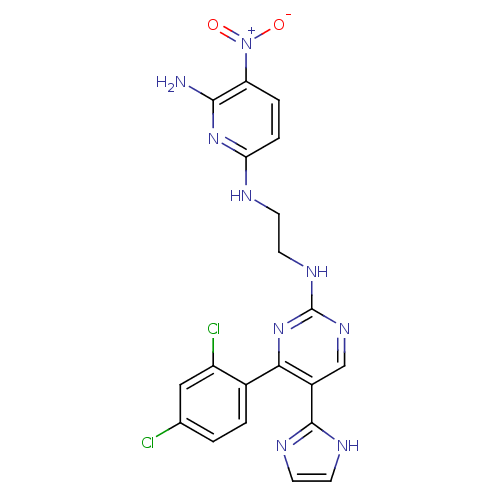
(CHEMBL1080901 | CT-98024 | N2-(2-(4-(2,4-dichlorop...)Show SMILES Nc1nc(NCCNc2ncc(-c3ncc[nH]3)c(n2)-c2ccc(Cl)cc2Cl)ccc1[N+]([O-])=O Show InChI InChI=1S/C20H17Cl2N9O2/c21-11-1-2-12(14(22)9-11)17-13(19-25-6-7-26-19)10-28-20(30-17)27-8-5-24-16-4-3-15(31(32)33)18(23)29-16/h1-4,6-7,9-10H,5,8H2,(H,25,26)(H3,23,24,29)(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... |
Bioorg Med Chem Lett 20: 1693-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.038
BindingDB Entry DOI: 10.7270/Q2ZK5HNS |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50451624

(CHEMBL331897)Show SMILES CC[N+]1(C\C2=C\CCCCCC2)CCC(CC1)NC(=O)C1c2cc(Cl)ccc2Oc2ccc(Cl)cc12 |t:4| Show InChI InChI=1S/C30H36Cl2N2O2/c1-2-34(20-21-8-6-4-3-5-7-9-21)16-14-24(15-17-34)33-30(35)29-25-18-22(31)10-12-27(25)36-28-13-11-23(32)19-26(28)29/h8,10-13,18-19,24,29H,2-7,9,14-17,20H2,1H3/p+1/b21-8+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity was determined towards C-C chemokine receptor type 3 using [125I]-labeled eotaxin as radioligand |
Bioorg Med Chem Lett 13: 3597-600 (2003)
BindingDB Entry DOI: 10.7270/Q228085J |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50451624

(CHEMBL331897)Show SMILES CC[N+]1(C\C2=C\CCCCCC2)CCC(CC1)NC(=O)C1c2cc(Cl)ccc2Oc2ccc(Cl)cc12 |t:4| Show InChI InChI=1S/C30H36Cl2N2O2/c1-2-34(20-21-8-6-4-3-5-7-9-21)16-14-24(15-17-34)33-30(35)29-25-18-22(31)10-12-27(25)36-28-13-11-23(32)19-26(28)29/h8,10-13,18-19,24,29H,2-7,9,14-17,20H2,1H3/p+1/b21-8+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity towards C-C chemokine receptor type 1 |
Bioorg Med Chem Lett 13: 3597-600 (2003)
BindingDB Entry DOI: 10.7270/Q228085J |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50312997
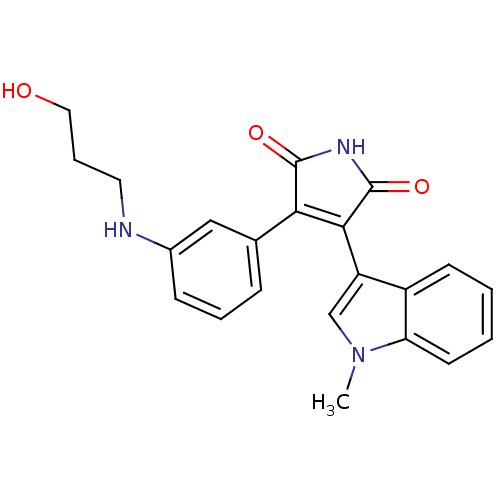
(3-(3-(3-hydroxypropylamino)phenyl)-4-(1-methyl-1H-...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cccc(NCCCO)c2)c2ccccc12 |t:4| Show InChI InChI=1S/C22H21N3O3/c1-25-13-17(16-8-2-3-9-18(16)25)20-19(21(27)24-22(20)28)14-6-4-7-15(12-14)23-10-5-11-26/h2-4,6-9,12-13,23,26H,5,10-11H2,1H3,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... |
Bioorg Med Chem Lett 20: 1693-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.038
BindingDB Entry DOI: 10.7270/Q2ZK5HNS |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM2647
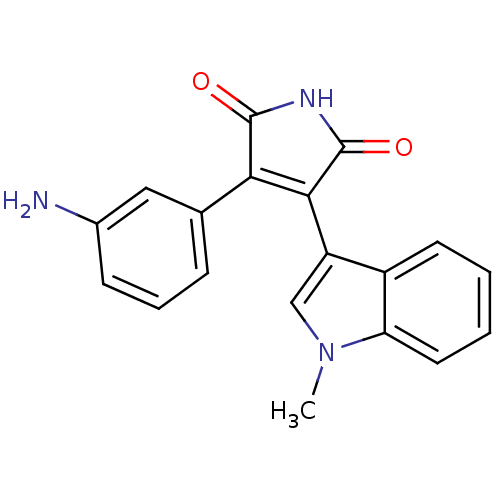
((Phenylindolyl)maleimide deriv. 69 | 3-(3-aminophe...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cccc(N)c2)c2ccccc12 |t:4| Show InChI InChI=1S/C19H15N3O2/c1-22-10-14(13-7-2-3-8-15(13)22)17-16(18(23)21-19(17)24)11-5-4-6-12(20)9-11/h2-10H,20H2,1H3,(H,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... |
Bioorg Med Chem Lett 20: 1693-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.038
BindingDB Entry DOI: 10.7270/Q2ZK5HNS |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50312998

((R,S)-3-(3-(2,3-dihydroxypropyl)phenyl)-4-(5-fluor...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cccc(CC(O)CO)c2)c2cc(F)ccc12 |t:4| Show InChI InChI=1S/C22H19FN2O4/c1-25-10-17(16-9-14(23)5-6-18(16)25)20-19(21(28)24-22(20)29)13-4-2-3-12(7-13)8-15(27)11-26/h2-7,9-10,15,26-27H,8,11H2,1H3,(H,24,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... |
Bioorg Med Chem Lett 20: 1693-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.038
BindingDB Entry DOI: 10.7270/Q2ZK5HNS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM102578
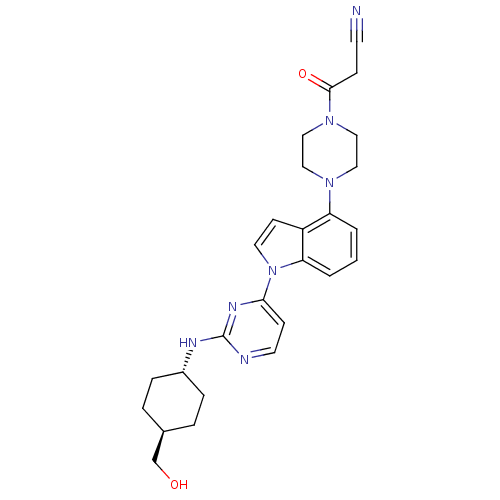
(US8536172, I-27)Show SMILES OC[C@H]1CC[C@@H](CC1)Nc1nccc(n1)-n1ccc2c(cccc12)N1CCN(CC1)C(=O)CC#N |r,wU:5.8,wD:2.1,(-5.68,-4.22,;-7.01,-3.45,;-7.01,-1.91,;-5.68,-1.14,;-5.68,.4,;-7.01,1.17,;-8.34,.4,;-8.34,-1.14,;-7.01,2.71,;-5.68,3.48,;-5.68,5.02,;-4.34,5.79,;-3.01,5.02,;-3.01,3.48,;-4.34,2.71,;-1.68,2.71,;-.21,3.18,;.69,1.94,;-.21,.69,;.11,-.82,;-1.04,-1.85,;-2.5,-1.37,;-2.82,.13,;-1.68,1.17,;1.6,-1.22,;2,-2.7,;3.48,-3.1,;4.57,-2.01,;4.17,-.53,;2.69,-.13,;6.06,-2.41,;7.15,-1.32,;6.46,-3.9,;7.95,-4.3,;8.34,-5.79,)| Show InChI InChI=1S/C26H31N7O2/c27-11-8-25(35)32-16-14-31(15-17-32)22-2-1-3-23-21(22)10-13-33(23)24-9-12-28-26(30-24)29-20-6-4-19(18-34)5-7-20/h1-3,9-10,12-13,19-20,34H,4-8,14-18H2,(H,28,29,30)/t19-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Roche Palo Alto LLC
US Patent
| Assay Description
JNK activity was measured by phosphorylation of GST-ATF2 (19-96) with [gamma-33P] ATP. |
US Patent US8536172 (2013)
BindingDB Entry DOI: 10.7270/Q23N222K |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50099482
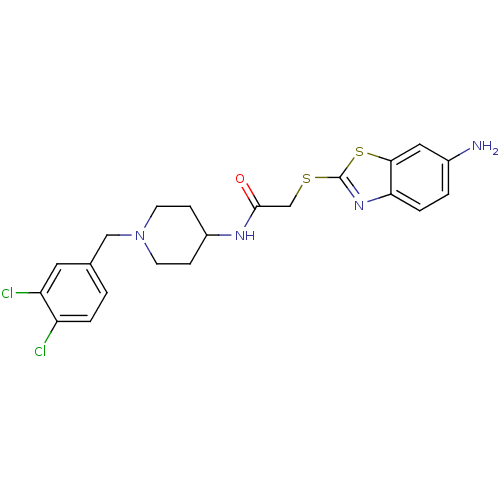
(2-(6-Amino-benzothiazol-2-ylsulfanyl)-N-[1-(3,4-di...)Show SMILES Nc1ccc2nc(SCC(=O)NC3CCN(Cc4ccc(Cl)c(Cl)c4)CC3)sc2c1 Show InChI InChI=1S/C21H22Cl2N4OS2/c22-16-3-1-13(9-17(16)23)11-27-7-5-15(6-8-27)25-20(28)12-29-21-26-18-4-2-14(24)10-19(18)30-21/h1-4,9-10,15H,5-8,11-12,24H2,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity was determined towards C-C chemokine receptor type 3 using [125I]-labeled eotaxin as radioligand |
Bioorg Med Chem Lett 13: 3597-600 (2003)
BindingDB Entry DOI: 10.7270/Q228085J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM102593
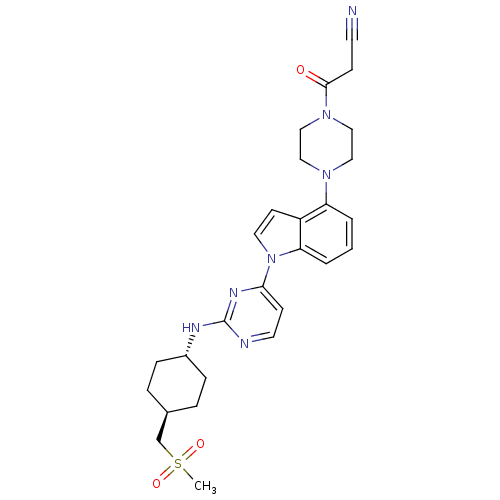
(US8536172, I-42)Show SMILES CS(=O)(=O)C[C@H]1CC[C@@H](CC1)Nc1nccc(n1)-n1ccc2c(cccc12)N1CCN(CC1)C(=O)CC#N |r,wU:8.11,wD:5.4,(-8.37,-5.78,;-8.37,-4.24,;-9.7,-3.46,;-9.7,-5.01,;-7.03,-3.47,;-7.03,-1.93,;-5.7,-1.16,;-5.7,.38,;-7.03,1.15,;-8.37,.38,;-8.37,-1.16,;-7.03,2.69,;-5.7,3.46,;-5.7,5,;-4.37,5.78,;-3.03,5,;-3.03,3.46,;-4.37,2.69,;-1.7,2.69,;-.23,3.17,;.67,1.92,;-.23,.68,;.09,-.83,;-1.06,-1.86,;-2.52,-1.38,;-2.84,.12,;-1.7,1.15,;1.57,-1.23,;1.97,-2.71,;3.46,-3.11,;4.55,-2.02,;4.15,-.54,;2.66,-.14,;6.04,-2.42,;6.43,-3.91,;7.12,-1.33,;8.61,-1.73,;9.7,-.64,)| Show InChI InChI=1S/C27H33N7O3S/c1-38(36,37)19-20-5-7-21(8-6-20)30-27-29-13-10-25(31-27)34-14-11-22-23(3-2-4-24(22)34)32-15-17-33(18-16-32)26(35)9-12-28/h2-4,10-11,13-14,20-21H,5-9,15-19H2,1H3,(H,29,30,31)/t20-,21- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Roche Palo Alto LLC
US Patent
| Assay Description
JNK activity was measured by phosphorylation of GST-ATF2 (19-96) with [gamma-33P] ATP. |
US Patent US8536172 (2013)
BindingDB Entry DOI: 10.7270/Q23N222K |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50133793
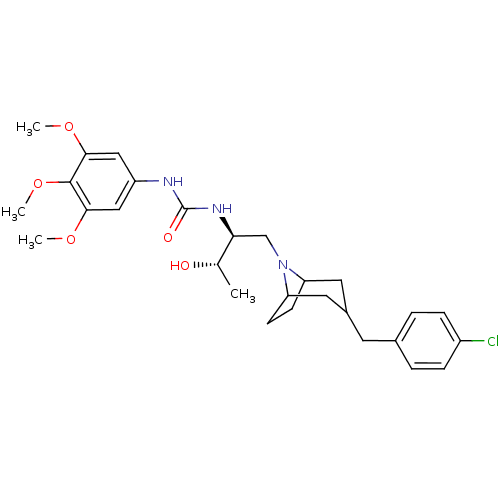
(1-{(S)-1-[3-(4-Chloro-benzyl)-8-aza-bicyclo[3.2.1]...)Show SMILES COc1cc(NC(=O)N[C@@H](CN2C3CCC2CC(Cc2ccc(Cl)cc2)C3)[C@H](C)O)cc(OC)c1OC |THB:18:17:11:13.14| Show InChI InChI=1S/C28H38ClN3O5/c1-17(33)24(31-28(34)30-21-14-25(35-2)27(37-4)26(15-21)36-3)16-32-22-9-10-23(32)13-19(12-22)11-18-5-7-20(29)8-6-18/h5-8,14-15,17,19,22-24,33H,9-13,16H2,1-4H3,(H2,30,31,34)/t17-,19?,22?,23?,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit C-C chemokine receptor type 3 receptor |
Bioorg Med Chem Lett 13: 3597-600 (2003)
BindingDB Entry DOI: 10.7270/Q228085J |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313010
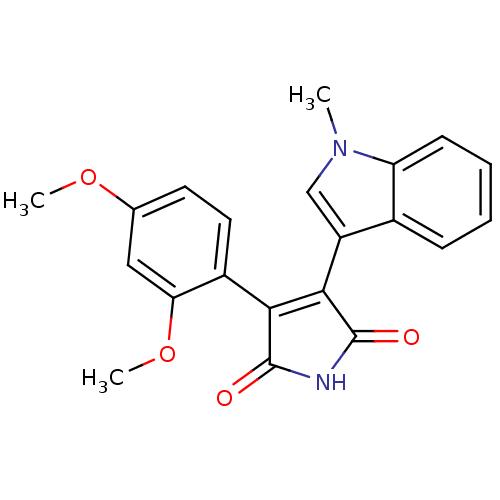
(3-(2,4-dimethoxyphenyl)-4-(1-methyl-1H-indol-3-yl)...)Show SMILES COc1ccc(C2=C(C(=O)NC2=O)c2cn(C)c3ccccc23)c(OC)c1 |t:6| Show InChI InChI=1S/C21H18N2O4/c1-23-11-15(13-6-4-5-7-16(13)23)19-18(20(24)22-21(19)25)14-9-8-12(26-2)10-17(14)27-3/h4-11H,1-3H3,(H,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... |
Bioorg Med Chem Lett 20: 1693-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.038
BindingDB Entry DOI: 10.7270/Q2ZK5HNS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM102557
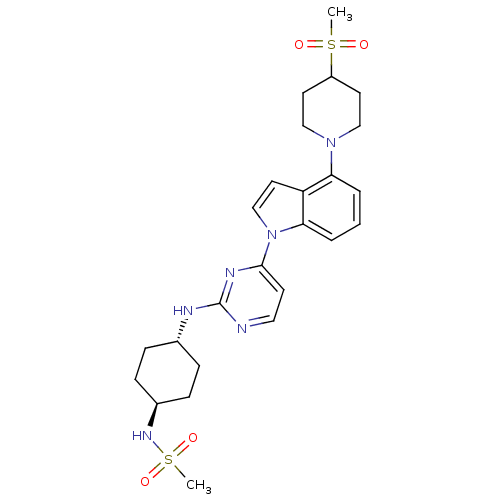
(US8536172, I-6)Show SMILES CS(=O)(=O)N[C@H]1CC[C@@H](CC1)Nc1nccc(n1)-n1ccc2c(cccc12)N1CCC(CC1)S(C)(=O)=O |r,wU:8.11,wD:5.4,(-6.41,-5.01,;-5.08,-4.24,;-5.08,-5.78,;-3.75,-3.47,;-6.41,-3.47,;-6.41,-1.93,;-5.08,-1.16,;-5.08,.38,;-6.41,1.15,;-7.75,.38,;-7.75,-1.16,;-6.41,2.69,;-5.08,3.46,;-5.08,5,;-3.75,5.78,;-2.41,5,;-2.41,3.46,;-3.75,2.69,;-1.08,2.69,;.39,3.17,;1.29,1.92,;.39,.68,;.71,-.83,;-.44,-1.86,;-1.9,-1.38,;-2.22,.12,;-1.08,1.15,;2.19,-1.23,;2.59,-2.71,;4.08,-3.11,;5.17,-2.02,;4.77,-.54,;3.28,-.14,;6.66,-2.42,;5.57,-3.51,;7.75,-1.33,;7.06,-3.91,)| Show InChI InChI=1S/C25H34N6O4S2/c1-36(32,33)20-11-15-30(16-12-20)22-4-3-5-23-21(22)13-17-31(23)24-10-14-26-25(28-24)27-18-6-8-19(9-7-18)29-37(2,34)35/h3-5,10,13-14,17-20,29H,6-9,11-12,15-16H2,1-2H3,(H,26,27,28)/t18-,19- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of human JNK1alpha1 using GST-tagged ATF2 as substrate preincubated for 10 mins prior to substrate addition measured after 30 mins by micr... |
Bioorg Med Chem Lett 23: 3565-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.029
BindingDB Entry DOI: 10.7270/Q2MG7SF1 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM102563
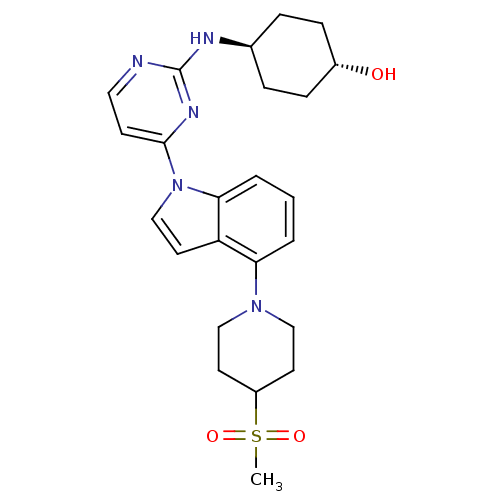
(US8536172, I-12)Show SMILES CS(=O)(=O)C1CCN(CC1)c1cccc2n(ccc12)-c1ccnc(N[C@H]2CC[C@H](O)CC2)n1 |r,wU:25.27,wD:28.31,(5.57,-4.44,;6.66,-3.35,;7.75,-2.27,;7.06,-4.84,;5.17,-2.96,;4.08,-4.04,;2.59,-3.65,;2.19,-2.16,;3.28,-1.07,;4.77,-1.47,;.71,-1.76,;-.44,-2.79,;-1.9,-2.31,;-2.22,-.81,;-1.08,.22,;-1.08,1.76,;.39,2.24,;1.29,.99,;.39,-.25,;-2.41,2.53,;-2.41,4.07,;-3.75,4.84,;-5.08,4.07,;-5.08,2.53,;-6.41,1.76,;-6.41,.22,;-5.08,-.55,;-5.08,-2.09,;-6.41,-2.86,;-6.41,-4.4,;-7.75,-2.09,;-7.75,-.55,;-3.75,1.76,)| Show InChI InChI=1S/C24H31N5O3S/c1-33(31,32)19-10-14-28(15-11-19)21-3-2-4-22-20(21)12-16-29(22)23-9-13-25-24(27-23)26-17-5-7-18(30)8-6-17/h2-4,9,12-13,16-19,30H,5-8,10-11,14-15H2,1H3,(H,25,26,27)/t17-,18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of human JNK1alpha1 using GST-tagged ATF2 as substrate preincubated for 10 mins prior to substrate addition measured after 30 mins by micr... |
Bioorg Med Chem Lett 23: 3565-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.029
BindingDB Entry DOI: 10.7270/Q2MG7SF1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM102565
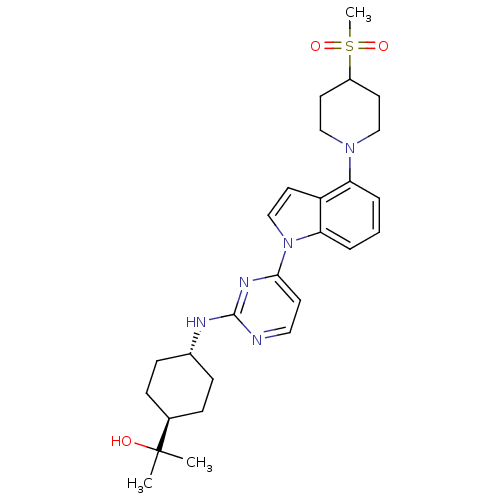
(US8536172, I-14)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)Nc1nccc(n1)-n1ccc2c(cccc12)N1CCC(CC1)S(C)(=O)=O |r,wU:7.10,wD:4.3,(-7.75,-5.01,;-6.41,-4.24,;-5.08,-3.47,;-5.08,-5.01,;-6.41,-2.7,;-5.08,-1.93,;-5.08,-.39,;-6.41,.38,;-7.75,-.39,;-7.75,-1.93,;-6.41,1.92,;-5.08,2.69,;-5.08,4.23,;-3.75,5.01,;-2.41,4.23,;-2.41,2.69,;-3.75,1.92,;-1.08,1.92,;.39,2.4,;1.29,1.15,;.39,-.09,;.71,-1.6,;-.44,-2.63,;-1.9,-2.15,;-2.22,-.65,;-1.08,.38,;2.19,-2,;2.59,-3.48,;4.08,-3.88,;5.17,-2.79,;4.77,-1.31,;3.28,-.91,;6.66,-3.19,;5.57,-4.28,;7.75,-2.1,;7.06,-4.68,)| Show InChI InChI=1S/C27H37N5O3S/c1-27(2,33)19-7-9-20(10-8-19)29-26-28-15-11-25(30-26)32-18-14-22-23(5-4-6-24(22)32)31-16-12-21(13-17-31)36(3,34)35/h4-6,11,14-15,18-21,33H,7-10,12-13,16-17H2,1-3H3,(H,28,29,30)/t19-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Roche Palo Alto LLC
US Patent
| Assay Description
JNK activity was measured by phosphorylation of GST-ATF2 (19-96) with [gamma-33P] ATP. |
US Patent US8536172 (2013)
BindingDB Entry DOI: 10.7270/Q23N222K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM102557

(US8536172, I-6)Show SMILES CS(=O)(=O)N[C@H]1CC[C@@H](CC1)Nc1nccc(n1)-n1ccc2c(cccc12)N1CCC(CC1)S(C)(=O)=O |r,wU:8.11,wD:5.4,(-6.41,-5.01,;-5.08,-4.24,;-5.08,-5.78,;-3.75,-3.47,;-6.41,-3.47,;-6.41,-1.93,;-5.08,-1.16,;-5.08,.38,;-6.41,1.15,;-7.75,.38,;-7.75,-1.16,;-6.41,2.69,;-5.08,3.46,;-5.08,5,;-3.75,5.78,;-2.41,5,;-2.41,3.46,;-3.75,2.69,;-1.08,2.69,;.39,3.17,;1.29,1.92,;.39,.68,;.71,-.83,;-.44,-1.86,;-1.9,-1.38,;-2.22,.12,;-1.08,1.15,;2.19,-1.23,;2.59,-2.71,;4.08,-3.11,;5.17,-2.02,;4.77,-.54,;3.28,-.14,;6.66,-2.42,;5.57,-3.51,;7.75,-1.33,;7.06,-3.91,)| Show InChI InChI=1S/C25H34N6O4S2/c1-36(32,33)20-11-15-30(16-12-20)22-4-3-5-23-21(22)13-17-31(23)24-10-14-26-25(28-24)27-18-6-8-19(9-7-18)29-37(2,34)35/h3-5,10,13-14,17-20,29H,6-9,11-12,15-16H2,1-2H3,(H,26,27,28)/t18-,19- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Roche Palo Alto LLC
US Patent
| Assay Description
JNK activity was measured by phosphorylation of GST-ATF2 (19-96) with [gamma-33P] ATP. |
US Patent US8536172 (2013)
BindingDB Entry DOI: 10.7270/Q23N222K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM102563

(US8536172, I-12)Show SMILES CS(=O)(=O)C1CCN(CC1)c1cccc2n(ccc12)-c1ccnc(N[C@H]2CC[C@H](O)CC2)n1 |r,wU:25.27,wD:28.31,(5.57,-4.44,;6.66,-3.35,;7.75,-2.27,;7.06,-4.84,;5.17,-2.96,;4.08,-4.04,;2.59,-3.65,;2.19,-2.16,;3.28,-1.07,;4.77,-1.47,;.71,-1.76,;-.44,-2.79,;-1.9,-2.31,;-2.22,-.81,;-1.08,.22,;-1.08,1.76,;.39,2.24,;1.29,.99,;.39,-.25,;-2.41,2.53,;-2.41,4.07,;-3.75,4.84,;-5.08,4.07,;-5.08,2.53,;-6.41,1.76,;-6.41,.22,;-5.08,-.55,;-5.08,-2.09,;-6.41,-2.86,;-6.41,-4.4,;-7.75,-2.09,;-7.75,-.55,;-3.75,1.76,)| Show InChI InChI=1S/C24H31N5O3S/c1-33(31,32)19-10-14-28(15-11-19)21-3-2-4-22-20(21)12-16-29(22)23-9-13-25-24(27-23)26-17-5-7-18(30)8-6-17/h2-4,9,12-13,16-19,30H,5-8,10-11,14-15H2,1H3,(H,25,26,27)/t17-,18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Roche Palo Alto LLC
US Patent
| Assay Description
JNK activity was measured by phosphorylation of GST-ATF2 (19-96) with [gamma-33P] ATP. |
US Patent US8536172 (2013)
BindingDB Entry DOI: 10.7270/Q23N222K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313000

((R)-3-(3-(2,3-dihydroxypropyl)phenyl)-4-(1-methyl-...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cccc(C[C@@H](O)CO)c2)c2ccccc12 |r,t:4| Show InChI InChI=1S/C22H20N2O4/c1-24-11-17(16-7-2-3-8-18(16)24)20-19(21(27)23-22(20)28)14-6-4-5-13(9-14)10-15(26)12-25/h2-9,11,15,25-26H,10,12H2,1H3,(H,23,27,28)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... |
Bioorg Med Chem Lett 20: 1693-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.038
BindingDB Entry DOI: 10.7270/Q2ZK5HNS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM102602
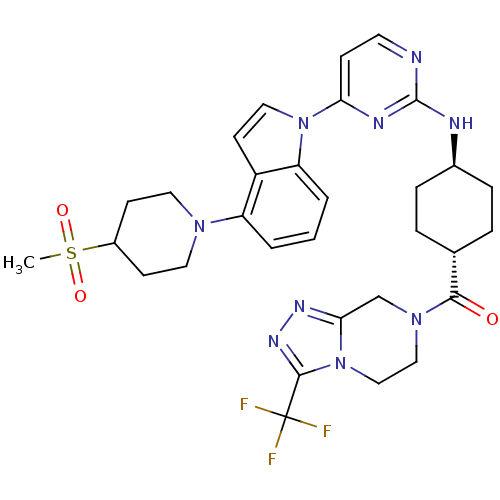
(US8536172, I-51)Show SMILES CS(=O)(=O)C1CCN(CC1)c1cccc2n(ccc12)-c1ccnc(N[C@H]2CC[C@@H](CC2)C(=O)N2CCn3c(C2)nnc3C(F)(F)F)n1 |r,wU:25.27,wD:28.34,(8.29,12.12,;7.2,11.03,;6.11,12.12,;8.29,9.94,;6.11,9.94,;6.51,8.46,;5.42,7.37,;3.93,7.77,;3.53,9.25,;4.62,10.34,;2.84,6.68,;3.47,5.27,;2.56,4.03,;1.03,4.19,;.4,5.59,;-1.06,6.07,;-1.06,7.61,;.4,8.08,;1.31,6.84,;-2.15,4.98,;-3.64,5.38,;-4.73,4.29,;-4.33,2.8,;-2.84,2.4,;-2.44,.92,;-3.53,-.17,;-3.13,-1.66,;-4.22,-2.75,;-5.71,-2.35,;-6.11,-.86,;-5.02,.23,;-6.8,-3.44,;-8.29,-3.04,;-6.4,-4.93,;-7.65,-5.83,;-7.48,-7.36,;-6.08,-7.99,;-4.83,-7.09,;-4.99,-5.55,;-3.59,-7.99,;-4.06,-9.46,;-5.6,-9.46,;-6.37,-10.79,;-5.6,-12.12,;-7.91,-10.79,;-7.14,-9.46,;-1.75,3.49,)| Show InChI InChI=1S/C31H36F3N9O3S/c1-47(45,46)22-10-14-40(15-11-22)24-3-2-4-25-23(24)12-16-42(25)26-9-13-35-30(37-26)36-21-7-5-20(6-8-21)28(44)41-17-18-43-27(19-41)38-39-29(43)31(32,33)34/h2-4,9,12-13,16,20-22H,5-8,10-11,14-15,17-19H2,1H3,(H,35,36,37)/t20-,21- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Roche Palo Alto LLC
US Patent
| Assay Description
JNK activity was measured by phosphorylation of GST-ATF2 (19-96) with [gamma-33P] ATP. |
US Patent US8536172 (2013)
BindingDB Entry DOI: 10.7270/Q23N222K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM102566
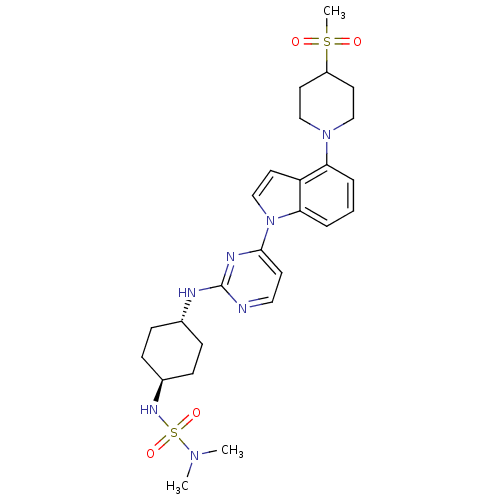
(US8536172, I-15)Show SMILES CN(C)S(=O)(=O)N[C@H]1CC[C@@H](CC1)Nc1nccc(n1)-n1ccc2c(cccc12)N1CCC(CC1)S(C)(=O)=O |r,wU:10.13,wD:7.6,(-2.21,-4.72,;-3.75,-4.72,;-2.98,-6.06,;-5.08,-3.95,;-5.08,-5.49,;-3.75,-3.18,;-6.41,-3.18,;-6.41,-1.64,;-5.08,-.87,;-5.08,.67,;-6.41,1.44,;-7.75,.67,;-7.75,-.87,;-6.41,2.98,;-5.08,3.75,;-5.08,5.29,;-3.75,6.06,;-2.41,5.29,;-2.41,3.75,;-3.75,2.98,;-1.08,2.98,;.39,3.45,;1.29,2.21,;.39,.96,;.71,-.55,;-.44,-1.58,;-1.9,-1.1,;-2.22,.41,;-1.08,1.44,;2.19,-.94,;2.59,-2.43,;4.08,-2.83,;5.17,-1.74,;4.77,-.25,;3.28,.15,;6.66,-2.14,;5.57,-3.23,;7.75,-1.05,;7.06,-3.63,)| Show InChI InChI=1S/C26H37N7O4S2/c1-31(2)39(36,37)30-20-9-7-19(8-10-20)28-26-27-15-11-25(29-26)33-18-14-22-23(5-4-6-24(22)33)32-16-12-21(13-17-32)38(3,34)35/h4-6,11,14-15,18-21,30H,7-10,12-13,16-17H2,1-3H3,(H,27,28,29)/t19-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Roche Palo Alto LLC
US Patent
| Assay Description
JNK activity was measured by phosphorylation of GST-ATF2 (19-96) with [gamma-33P] ATP. |
US Patent US8536172 (2013)
BindingDB Entry DOI: 10.7270/Q23N222K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM102573
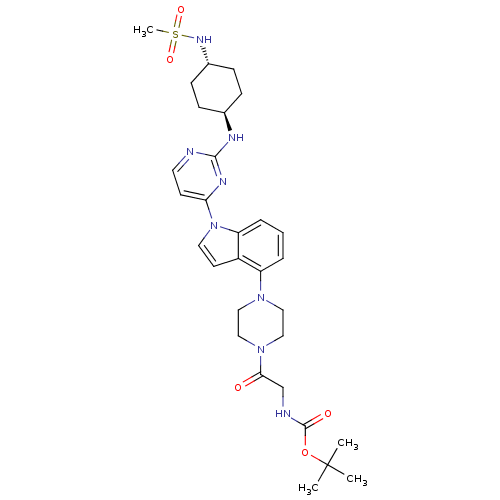
(US8536172, I-22)Show SMILES CC(C)(C)OC(=O)NCC(=O)N1CCN(CC1)c1cccc2n(ccc12)-c1ccnc(N[C@H]2CC[C@@H](CC2)NS(C)(=O)=O)n1 |r,wU:32.34,wD:35.41,(10.7,-6.58,;9.21,-6.18,;8.12,-7.27,;9.61,-4.7,;8.81,-4.7,;7.32,-4.3,;6.24,-5.39,;6.93,-2.81,;5.44,-2.41,;5.04,-.92,;6.13,.17,;3.55,-.53,;2.46,-1.61,;.98,-1.22,;.58,.27,;1.67,1.36,;3.15,.96,;-.91,.67,;-2.06,-.36,;-3.52,.12,;-3.84,1.62,;-2.7,2.65,;-2.7,4.19,;-1.23,4.67,;-.33,3.42,;-1.23,2.18,;-4.03,4.96,;-4.03,6.5,;-5.36,7.27,;-6.7,6.5,;-6.7,4.96,;-8.03,4.19,;-8.03,2.65,;-6.7,1.88,;-6.7,.34,;-8.03,-.43,;-9.36,.34,;-9.36,1.88,;-8.03,-1.97,;-9.36,-2.74,;-10.7,-1.97,;-9.36,-4.28,;-8.03,-3.51,;-5.36,4.19,)| Show InChI InChI=1S/C30H42N8O5S/c1-30(2,3)43-29(40)32-20-27(39)37-18-16-36(17-19-37)24-6-5-7-25-23(24)13-15-38(25)26-12-14-31-28(34-26)33-21-8-10-22(11-9-21)35-44(4,41)42/h5-7,12-15,21-22,35H,8-11,16-20H2,1-4H3,(H,32,40)(H,31,33,34)/t21-,22- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Roche Palo Alto LLC
US Patent
| Assay Description
JNK activity was measured by phosphorylation of GST-ATF2 (19-96) with [gamma-33P] ATP. |
US Patent US8536172 (2013)
BindingDB Entry DOI: 10.7270/Q23N222K |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM2651
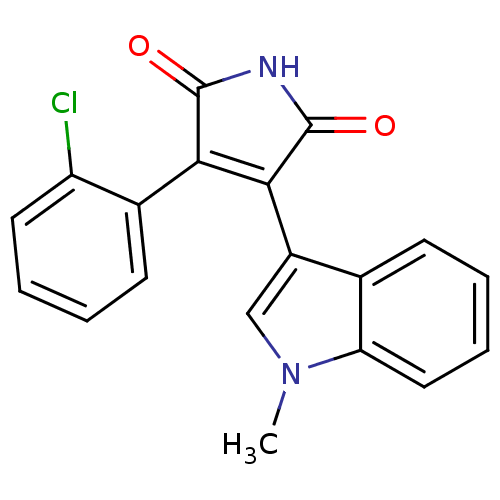
((Phenylindolyl)maleimide deriv. 73 | 3-(2-chloroph...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2ccccc2Cl)c2ccccc12 |t:4| Show InChI InChI=1S/C19H13ClN2O2/c1-22-10-13(11-6-3-5-9-15(11)22)17-16(18(23)21-19(17)24)12-7-2-4-8-14(12)20/h2-10H,1H3,(H,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... |
Bioorg Med Chem Lett 20: 1693-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.038
BindingDB Entry DOI: 10.7270/Q2ZK5HNS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM102577
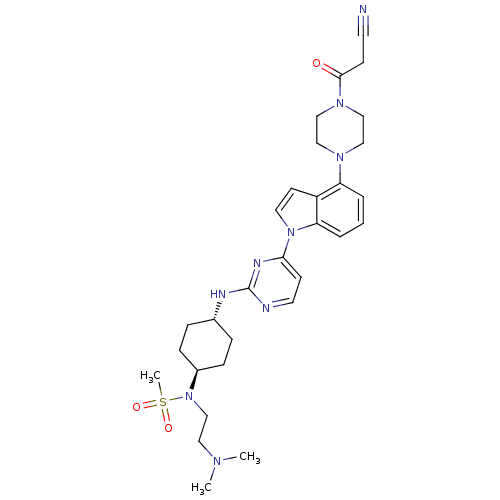
(US8536172, I-26)Show SMILES CN(C)CCN([C@H]1CC[C@@H](CC1)Nc1nccc(n1)-n1ccc2c(cccc12)N1CCN(CC1)C(=O)CC#N)S(C)(=O)=O |r,wU:9.12,wD:6.5,(-3.01,-6.51,;-3.41,-5.02,;-2.32,-3.93,;-4.9,-4.62,;-5.3,-3.13,;-6.78,-2.73,;-6.78,-1.19,;-5.45,-.42,;-5.45,1.12,;-6.78,1.89,;-8.12,1.12,;-8.12,-.42,;-6.78,3.43,;-5.45,4.2,;-5.45,5.74,;-4.12,6.51,;-2.78,5.74,;-2.78,4.2,;-4.12,3.43,;-1.45,3.43,;.01,3.9,;.92,2.66,;.01,1.41,;.33,-.1,;-.81,-1.13,;-2.27,-.65,;-2.59,.86,;-1.45,1.89,;1.82,-.49,;2.22,-1.98,;3.71,-2.38,;4.8,-1.29,;4.4,.2,;2.91,.59,;6.28,-1.69,;7.37,-.6,;6.68,-3.18,;8.17,-3.58,;9.66,-3.98,;-8.12,-3.5,;-8.12,-5.04,;-9.66,-3.5,;-8.12,-1.96,)| Show InChI InChI=1S/C30H41N9O3S/c1-35(2)17-22-39(43(3,41)42)24-9-7-23(8-10-24)33-30-32-15-12-28(34-30)38-16-13-25-26(5-4-6-27(25)38)36-18-20-37(21-19-36)29(40)11-14-31/h4-6,12-13,15-16,23-24H,7-11,17-22H2,1-3H3,(H,32,33,34)/t23-,24- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Roche Palo Alto LLC
US Patent
| Assay Description
JNK activity was measured by phosphorylation of GST-ATF2 (19-96) with [gamma-33P] ATP. |
US Patent US8536172 (2013)
BindingDB Entry DOI: 10.7270/Q23N222K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM102576
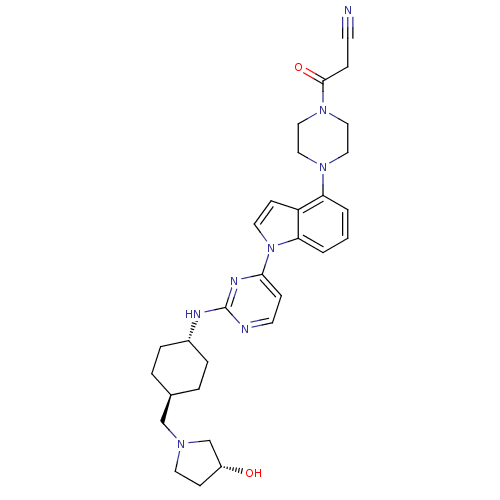
(US8536172, I-25)Show SMILES O[C@@H]1CCN(C[C@H]2CC[C@@H](CC2)Nc2nccc(n2)-n2ccc3c(cccc23)N2CCN(CC2)C(=O)CC#N)C1 |r,wU:9.12,wD:6.5,1.0,(-13.08,1.6,;-12.31,2.94,;-12.78,4.4,;-11.54,5.31,;-10.29,4.4,;-8.96,5.17,;-7.63,4.4,;-7.63,2.86,;-6.29,2.09,;-4.96,2.86,;-4.96,4.4,;-6.29,5.17,;-3.62,2.09,;-2.29,2.86,;-2.29,4.4,;-.96,5.17,;.38,4.4,;.38,2.86,;-.96,2.09,;1.71,2.09,;3.17,2.57,;4.08,1.32,;3.17,.08,;3.49,-1.43,;2.35,-2.46,;.89,-1.98,;.57,-.48,;1.71,.55,;4.98,-1.83,;5.38,-3.32,;6.87,-3.71,;7.96,-2.62,;7.56,-1.14,;6.07,-.74,;9.44,-3.02,;10.53,-1.93,;9.84,-4.51,;11.33,-4.91,;12.82,-5.31,;-10.77,2.94,)| Show InChI InChI=1S/C30H38N8O2/c31-12-8-29(40)37-18-16-36(17-19-37)26-2-1-3-27-25(26)11-15-38(27)28-9-13-32-30(34-28)33-23-6-4-22(5-7-23)20-35-14-10-24(39)21-35/h1-3,9,11,13,15,22-24,39H,4-8,10,14,16-21H2,(H,32,33,34)/t22-,23-,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Roche Palo Alto LLC
US Patent
| Assay Description
JNK activity was measured by phosphorylation of GST-ATF2 (19-96) with [gamma-33P] ATP. |
US Patent US8536172 (2013)
BindingDB Entry DOI: 10.7270/Q23N222K |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50133792
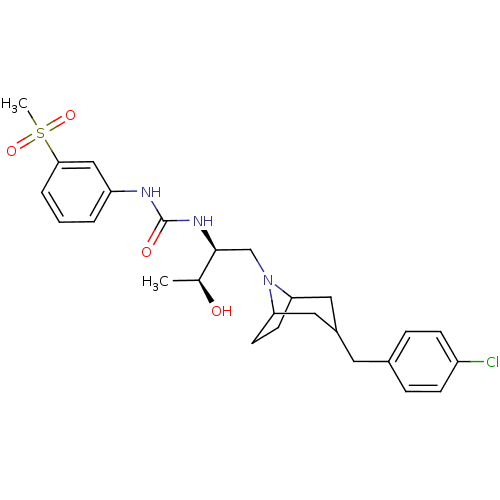
(1-{(S)-1-[3-(4-Chloro-benzyl)-8-aza-bicyclo[3.2.1]...)Show SMILES C[C@H](O)[C@H](CN1C2CCC1CC(Cc1ccc(Cl)cc1)C2)NC(=O)Nc1cccc(c1)S(C)(=O)=O |THB:12:11:5:7.8| Show InChI InChI=1S/C26H34ClN3O4S/c1-17(31)25(29-26(32)28-21-4-3-5-24(15-21)35(2,33)34)16-30-22-10-11-23(30)14-19(13-22)12-18-6-8-20(27)9-7-18/h3-9,15,17,19,22-23,25,31H,10-14,16H2,1-2H3,(H2,28,29,32)/t17-,19?,22?,23?,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit C-C chemokine receptor type 3 receptor |
Bioorg Med Chem Lett 13: 3597-600 (2003)
BindingDB Entry DOI: 10.7270/Q228085J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM102606
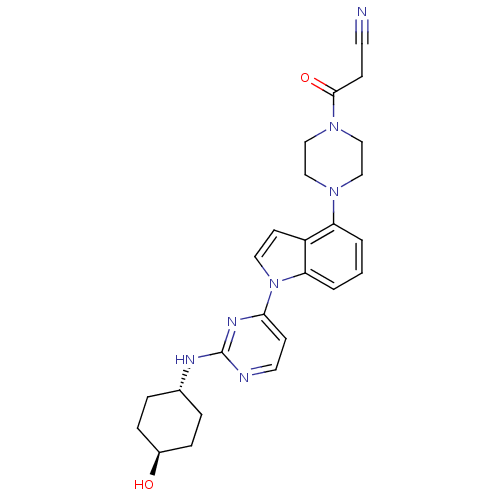
(US8536172, I-55)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nccc(n1)-n1ccc2c(cccc12)N1CCN(CC1)C(=O)CC#N |r,wU:4.7,wD:1.0,(-7.01,-3.45,;-7.01,-1.91,;-5.68,-1.14,;-5.68,.4,;-7.01,1.17,;-8.34,.4,;-8.34,-1.14,;-7.01,2.71,;-5.68,3.48,;-5.68,5.02,;-4.34,5.79,;-3.01,5.02,;-3.01,3.48,;-4.34,2.71,;-1.68,2.71,;-.21,3.18,;.69,1.94,;-.21,.69,;.11,-.82,;-1.04,-1.85,;-2.5,-1.37,;-2.82,.13,;-1.68,1.17,;1.6,-1.22,;2,-2.7,;3.48,-3.1,;4.57,-2.01,;4.17,-.53,;2.69,-.13,;6.06,-2.41,;7.15,-1.32,;6.46,-3.9,;7.95,-4.3,;8.34,-5.79,)| Show InChI InChI=1S/C25H29N7O2/c26-11-8-24(34)31-16-14-30(15-17-31)21-2-1-3-22-20(21)10-13-32(22)23-9-12-27-25(29-23)28-18-4-6-19(33)7-5-18/h1-3,9-10,12-13,18-19,33H,4-8,14-17H2,(H,27,28,29)/t18-,19- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Roche Palo Alto LLC
US Patent
| Assay Description
JNK activity was measured by phosphorylation of GST-ATF2 (19-96) with [gamma-33P] ATP. |
US Patent US8536172 (2013)
BindingDB Entry DOI: 10.7270/Q23N222K |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50133796
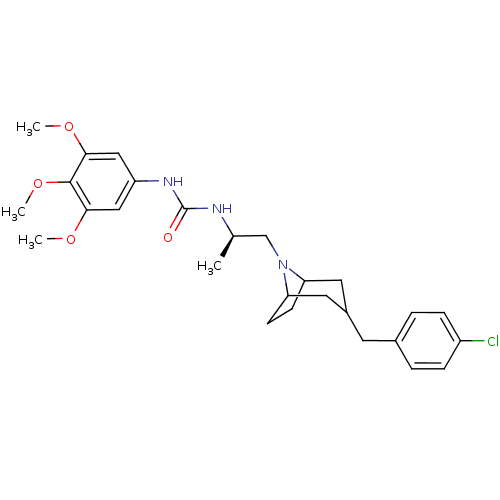
(1-{(R)-2-[3-(4-Chloro-benzyl)-8-aza-bicyclo[3.2.1]...)Show SMILES COc1cc(NC(=O)N[C@H](C)CN2C3CCC2CC(Cc2ccc(Cl)cc2)C3)cc(OC)c1OC |THB:19:18:12:14.15| Show InChI InChI=1S/C27H36ClN3O4/c1-17(29-27(32)30-21-14-24(33-2)26(35-4)25(15-21)34-3)16-31-22-9-10-23(31)13-19(12-22)11-18-5-7-20(28)8-6-18/h5-8,14-15,17,19,22-23H,9-13,16H2,1-4H3,(H2,29,30,32)/t17-,19?,22?,23?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit C-C chemokine receptor type 3 receptor |
Bioorg Med Chem Lett 13: 3597-600 (2003)
BindingDB Entry DOI: 10.7270/Q228085J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM102589
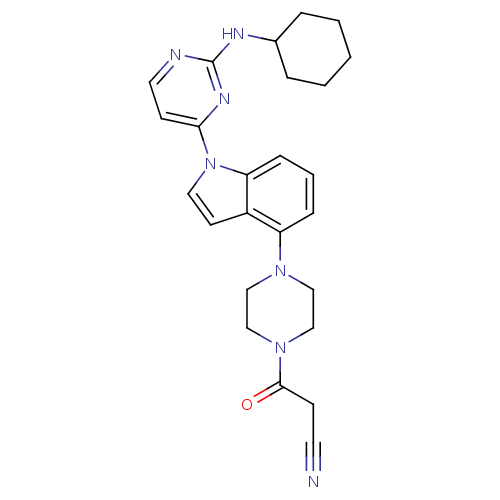
(US8536172, I-38)Show SMILES O=C(CC#N)N1CCN(CC1)c1cccc2n(ccc12)-c1ccnc(NC2CCCCC2)n1 Show InChI InChI=1S/C25H29N7O/c26-12-9-24(33)31-17-15-30(16-18-31)21-7-4-8-22-20(21)11-14-32(22)23-10-13-27-25(29-23)28-19-5-2-1-3-6-19/h4,7-8,10-11,13-14,19H,1-3,5-6,9,15-18H2,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Roche Palo Alto LLC
US Patent
| Assay Description
JNK activity was measured by phosphorylation of GST-ATF2 (19-96) with [gamma-33P] ATP. |
US Patent US8536172 (2013)
BindingDB Entry DOI: 10.7270/Q23N222K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM102560

(US8536172, I-9)Show SMILES CS(=O)(=O)N[C@H]1CC[C@@H](CC1)Nc1nccc(n1)-n1ccc2c(cccc12)N1CCN(CC1)C(=O)CC#N |r,wU:8.11,wD:5.4,(-9.56,-3.47,;-8.22,-4.24,;-8.22,-5.78,;-6.89,-5.01,;-6.89,-3.47,;-6.89,-1.93,;-5.55,-1.16,;-5.55,.38,;-6.89,1.15,;-8.22,.38,;-8.22,-1.16,;-6.89,2.69,;-5.55,3.46,;-5.55,5,;-4.22,5.78,;-2.89,5,;-2.89,3.46,;-4.22,2.69,;-1.55,2.69,;-.09,3.17,;.82,1.92,;-.09,.68,;.23,-.83,;-.91,-1.86,;-2.38,-1.38,;-2.7,.12,;-1.55,1.15,;1.72,-1.23,;2.12,-2.71,;3.61,-3.11,;4.69,-2.02,;4.3,-.54,;2.81,-.14,;6.18,-2.42,;7.27,-1.33,;6.58,-3.91,;8.07,-4.31,;9.56,-4.71,)| Show InChI InChI=1S/C26H32N8O3S/c1-38(36,37)31-20-7-5-19(6-8-20)29-26-28-13-10-24(30-26)34-14-11-21-22(3-2-4-23(21)34)32-15-17-33(18-16-32)25(35)9-12-27/h2-4,10-11,13-14,19-20,31H,5-9,15-18H2,1H3,(H,28,29,30)/t19-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of human JNK1alpha1 using GST-tagged ATF2 as substrate preincubated for 10 mins prior to substrate addition measured after 30 mins by micr... |
Bioorg Med Chem Lett 23: 3565-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.029
BindingDB Entry DOI: 10.7270/Q2MG7SF1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50100021

((S)-2-[(Naphthalene-1-carbonyl)-amino]-3-(4-nitro-...)Show SMILES COC(=O)[C@H](Cc1ccc(cc1)[N+]([O-])=O)NC(=O)c1cccc2ccccc12 Show InChI InChI=1S/C21H18N2O5/c1-28-21(25)19(13-14-9-11-16(12-10-14)23(26)27)22-20(24)18-8-4-6-15-5-2-3-7-17(15)18/h2-12,19H,13H2,1H3,(H,22,24)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity was determined towards C-C chemokine receptor type 3 using [125I]-labeled eotaxin as radioligand |
Bioorg Med Chem Lett 13: 3597-600 (2003)
BindingDB Entry DOI: 10.7270/Q228085J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM102560

(US8536172, I-9)Show SMILES CS(=O)(=O)N[C@H]1CC[C@@H](CC1)Nc1nccc(n1)-n1ccc2c(cccc12)N1CCN(CC1)C(=O)CC#N |r,wU:8.11,wD:5.4,(-9.56,-3.47,;-8.22,-4.24,;-8.22,-5.78,;-6.89,-5.01,;-6.89,-3.47,;-6.89,-1.93,;-5.55,-1.16,;-5.55,.38,;-6.89,1.15,;-8.22,.38,;-8.22,-1.16,;-6.89,2.69,;-5.55,3.46,;-5.55,5,;-4.22,5.78,;-2.89,5,;-2.89,3.46,;-4.22,2.69,;-1.55,2.69,;-.09,3.17,;.82,1.92,;-.09,.68,;.23,-.83,;-.91,-1.86,;-2.38,-1.38,;-2.7,.12,;-1.55,1.15,;1.72,-1.23,;2.12,-2.71,;3.61,-3.11,;4.69,-2.02,;4.3,-.54,;2.81,-.14,;6.18,-2.42,;7.27,-1.33,;6.58,-3.91,;8.07,-4.31,;9.56,-4.71,)| Show InChI InChI=1S/C26H32N8O3S/c1-38(36,37)31-20-7-5-19(6-8-20)29-26-28-13-10-24(30-26)34-14-11-21-22(3-2-4-23(21)34)32-15-17-33(18-16-32)25(35)9-12-27/h2-4,10-11,13-14,19-20,31H,5-9,15-18H2,1H3,(H,28,29,30)/t19-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Roche Palo Alto LLC
US Patent
| Assay Description
JNK activity was measured by phosphorylation of GST-ATF2 (19-96) with [gamma-33P] ATP. |
US Patent US8536172 (2013)
BindingDB Entry DOI: 10.7270/Q23N222K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM102617
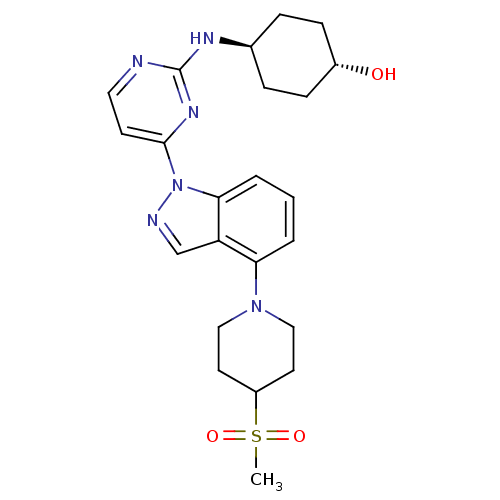
(US8536172, I-66)Show SMILES CS(=O)(=O)C1CCN(CC1)c1cccc2n(ncc12)-c1ccnc(N[C@H]2CC[C@H](O)CC2)n1 |r,wU:25.27,wD:28.31,(10.53,3.6,;9.19,2.83,;8.42,4.17,;9.96,1.5,;7.86,2.06,;7.86,.52,;6.52,-.25,;5.19,.52,;5.19,2.06,;6.52,2.83,;3.86,-.25,;3.86,-1.79,;2.52,-2.56,;1.19,-1.79,;1.19,-.25,;.04,.78,;.67,2.19,;2.2,2.03,;2.52,.52,;-1.5,.78,;-2.27,2.12,;-3.81,2.12,;-4.58,.78,;-3.81,-.55,;-4.58,-1.88,;-6.06,-2.28,;-6.46,-3.77,;-7.95,-4.17,;-9.04,-3.08,;-10.53,-3.48,;-8.64,-1.59,;-7.15,-1.19,;-2.27,-.55,)| Show InChI InChI=1S/C23H30N6O3S/c1-33(31,32)18-10-13-28(14-11-18)20-3-2-4-21-19(20)15-25-29(21)22-9-12-24-23(27-22)26-16-5-7-17(30)8-6-16/h2-4,9,12,15-18,30H,5-8,10-11,13-14H2,1H3,(H,24,26,27)/t16-,17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Roche Palo Alto LLC
US Patent
| Assay Description
JNK activity was measured by phosphorylation of GST-ATF2 (19-96) with [gamma-33P] ATP. |
US Patent US8536172 (2013)
BindingDB Entry DOI: 10.7270/Q23N222K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM102617

(US8536172, I-66)Show SMILES CS(=O)(=O)C1CCN(CC1)c1cccc2n(ncc12)-c1ccnc(N[C@H]2CC[C@H](O)CC2)n1 |r,wU:25.27,wD:28.31,(10.53,3.6,;9.19,2.83,;8.42,4.17,;9.96,1.5,;7.86,2.06,;7.86,.52,;6.52,-.25,;5.19,.52,;5.19,2.06,;6.52,2.83,;3.86,-.25,;3.86,-1.79,;2.52,-2.56,;1.19,-1.79,;1.19,-.25,;.04,.78,;.67,2.19,;2.2,2.03,;2.52,.52,;-1.5,.78,;-2.27,2.12,;-3.81,2.12,;-4.58,.78,;-3.81,-.55,;-4.58,-1.88,;-6.06,-2.28,;-6.46,-3.77,;-7.95,-4.17,;-9.04,-3.08,;-10.53,-3.48,;-8.64,-1.59,;-7.15,-1.19,;-2.27,-.55,)| Show InChI InChI=1S/C23H30N6O3S/c1-33(31,32)18-10-13-28(14-11-18)20-3-2-4-21-19(20)15-25-29(21)22-9-12-24-23(27-22)26-16-5-7-17(30)8-6-16/h2-4,9,12,15-18,30H,5-8,10-11,13-14H2,1H3,(H,24,26,27)/t16-,17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of human JNK1alpha1 using GST-tagged ATF2 as substrate preincubated for 10 mins prior to substrate addition measured after 30 mins by micr... |
Bioorg Med Chem Lett 23: 3565-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.029
BindingDB Entry DOI: 10.7270/Q2MG7SF1 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-15
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against MMP15 |
J Med Chem 49: 456-8 (2006)
Article DOI: 10.1021/jm051101g
BindingDB Entry DOI: 10.7270/Q2WQ03C3 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against MMP1 |
J Med Chem 49: 456-8 (2006)
Article DOI: 10.1021/jm051101g
BindingDB Entry DOI: 10.7270/Q2WQ03C3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM102570

(US8536172, I-19)Show SMILES CCNC(=O)[C@H]1CC[C@@H](CC1)Nc1nccc(n1)-n1ccc2c(cccc12)N1CCC(CC1)S(C)(=O)=O |r,wU:8.11,wD:5.4,(-2.41,-5.01,;-3.75,-4.24,;-5.08,-5.01,;-6.41,-4.24,;-7.75,-5.01,;-6.41,-2.7,;-5.08,-1.93,;-5.08,-.39,;-6.41,.38,;-7.75,-.39,;-7.75,-1.93,;-6.41,1.92,;-5.08,2.69,;-5.08,4.23,;-3.75,5.01,;-2.41,4.23,;-2.41,2.69,;-3.75,1.92,;-1.08,1.92,;.39,2.4,;1.29,1.15,;.39,-.09,;.71,-1.6,;-.44,-2.63,;-1.9,-2.15,;-2.22,-.65,;-1.08,.38,;2.19,-2,;2.59,-3.48,;4.08,-3.88,;5.17,-2.79,;4.77,-1.31,;3.28,-.91,;6.66,-3.19,;5.57,-4.28,;7.75,-2.1,;7.06,-4.68,)| Show InChI InChI=1S/C27H36N6O3S/c1-3-28-26(34)19-7-9-20(10-8-19)30-27-29-15-11-25(31-27)33-18-14-22-23(5-4-6-24(22)33)32-16-12-21(13-17-32)37(2,35)36/h4-6,11,14-15,18-21H,3,7-10,12-13,16-17H2,1-2H3,(H,28,34)(H,29,30,31)/t19-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Roche Palo Alto LLC
US Patent
| Assay Description
JNK activity was measured by phosphorylation of GST-ATF2 (19-96) with [gamma-33P] ATP. |
US Patent US8536172 (2013)
BindingDB Entry DOI: 10.7270/Q23N222K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM102602

(US8536172, I-51)Show SMILES CS(=O)(=O)C1CCN(CC1)c1cccc2n(ccc12)-c1ccnc(N[C@H]2CC[C@@H](CC2)C(=O)N2CCn3c(C2)nnc3C(F)(F)F)n1 |r,wU:25.27,wD:28.34,(8.29,12.12,;7.2,11.03,;6.11,12.12,;8.29,9.94,;6.11,9.94,;6.51,8.46,;5.42,7.37,;3.93,7.77,;3.53,9.25,;4.62,10.34,;2.84,6.68,;3.47,5.27,;2.56,4.03,;1.03,4.19,;.4,5.59,;-1.06,6.07,;-1.06,7.61,;.4,8.08,;1.31,6.84,;-2.15,4.98,;-3.64,5.38,;-4.73,4.29,;-4.33,2.8,;-2.84,2.4,;-2.44,.92,;-3.53,-.17,;-3.13,-1.66,;-4.22,-2.75,;-5.71,-2.35,;-6.11,-.86,;-5.02,.23,;-6.8,-3.44,;-8.29,-3.04,;-6.4,-4.93,;-7.65,-5.83,;-7.48,-7.36,;-6.08,-7.99,;-4.83,-7.09,;-4.99,-5.55,;-3.59,-7.99,;-4.06,-9.46,;-5.6,-9.46,;-6.37,-10.79,;-5.6,-12.12,;-7.91,-10.79,;-7.14,-9.46,;-1.75,3.49,)| Show InChI InChI=1S/C31H36F3N9O3S/c1-47(45,46)22-10-14-40(15-11-22)24-3-2-4-25-23(24)12-16-42(25)26-9-13-35-30(37-26)36-21-7-5-20(6-8-21)28(44)41-17-18-43-27(19-41)38-39-29(43)31(32,33)34/h2-4,9,12-13,16,20-22H,5-8,10-11,14-15,17-19H2,1H3,(H,35,36,37)/t20-,21- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Roche Palo Alto LLC
US Patent
| Assay Description
JNK activity was measured by phosphorylation of GST-ATF2 (19-96) with [gamma-33P] ATP. |
US Patent US8536172 (2013)
BindingDB Entry DOI: 10.7270/Q23N222K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50492029
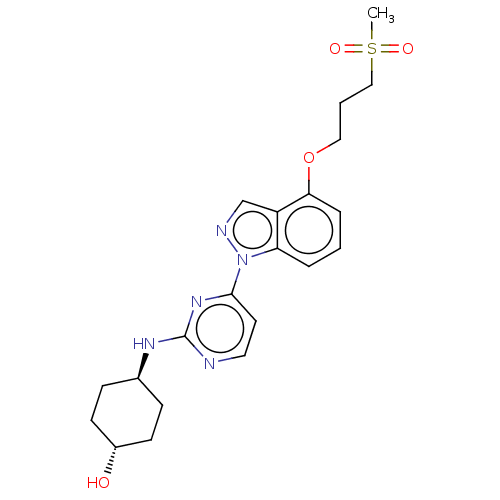
(CHEMBL2390974)Show SMILES CS(=O)(=O)CCCOc1cccc2n(ncc12)-c1ccnc(N[C@H]2CC[C@H](O)CC2)n1 |r,wU:23.24,wD:26.28,(24.35,-15.74,;23.55,-17.06,;23.51,-18.6,;24.86,-17.86,;22.01,-17.02,;21.21,-18.34,;19.67,-18.3,;18.87,-19.62,;17.33,-19.59,;16.54,-20.9,;14.99,-20.86,;14.26,-19.51,;15.06,-18.2,;14.61,-16.74,;15.88,-15.86,;17.11,-16.79,;16.6,-18.24,;13.16,-16.23,;13.16,-14.69,;11.82,-13.92,;10.49,-14.69,;10.5,-16.22,;9.16,-16.99,;9.16,-18.53,;10.49,-19.31,;10.49,-20.85,;9.16,-21.61,;9.16,-23.15,;7.83,-20.85,;7.83,-19.31,;11.83,-17,)| Show InChI InChI=1S/C21H27N5O4S/c1-31(28,29)13-3-12-30-19-5-2-4-18-17(19)14-23-26(18)20-10-11-22-21(25-20)24-15-6-8-16(27)9-7-15/h2,4-5,10-11,14-16,27H,3,6-9,12-13H2,1H3,(H,22,24,25)/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of human JNK1alpha1 using GST-tagged ATF2 as substrate preincubated for 10 mins prior to substrate addition measured after 30 mins by micr... |
Bioorg Med Chem Lett 23: 3565-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.029
BindingDB Entry DOI: 10.7270/Q2MG7SF1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313003

(3-(5-chloro-1-methyl-1H-indol-3-yl)-4-(3-methoxyph...)Show SMILES COc1cccc(c1)C1=C(C(=O)NC1=O)c1cn(C)c2ccc(Cl)cc12 |t:9| Show InChI InChI=1S/C20H15ClN2O3/c1-23-10-15(14-9-12(21)6-7-16(14)23)18-17(19(24)22-20(18)25)11-4-3-5-13(8-11)26-2/h3-10H,1-2H3,(H,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... |
Bioorg Med Chem Lett 20: 1693-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.038
BindingDB Entry DOI: 10.7270/Q2ZK5HNS |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313009
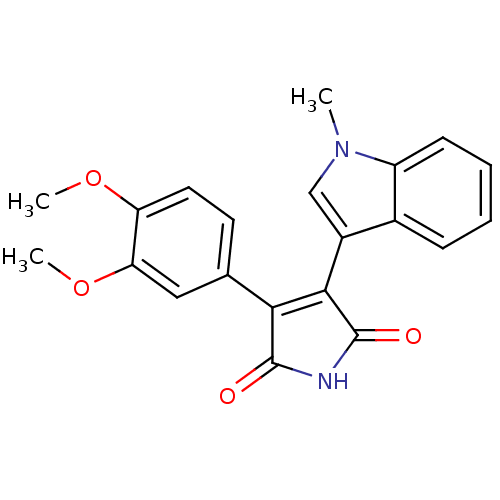
(3-(3,4-dimethoxyphenyl)-4-(1-methyl-1H-indol-3-yl)...)Show SMILES COc1ccc(cc1OC)C1=C(C(=O)NC1=O)c1cn(C)c2ccccc12 |t:11| Show InChI InChI=1S/C21H18N2O4/c1-23-11-14(13-6-4-5-7-15(13)23)19-18(20(24)22-21(19)25)12-8-9-16(26-2)17(10-12)27-3/h4-11H,1-3H3,(H,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... |
Bioorg Med Chem Lett 20: 1693-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.038
BindingDB Entry DOI: 10.7270/Q2ZK5HNS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM102564
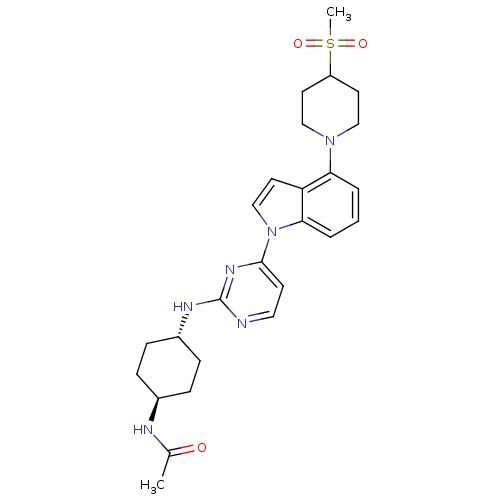
(US8536172, I-13)Show SMILES CC(=O)N[C@H]1CC[C@@H](CC1)Nc1nccc(n1)-n1ccc2c(cccc12)N1CCC(CC1)S(C)(=O)=O |r,wU:7.10,wD:4.3,(-3.75,-3.47,;-5.08,-4.24,;-5.08,-5.78,;-6.41,-3.47,;-6.41,-1.93,;-5.08,-1.16,;-5.08,.38,;-6.41,1.15,;-7.75,.38,;-7.75,-1.16,;-6.41,2.69,;-5.08,3.46,;-5.08,5,;-3.75,5.78,;-2.41,5,;-2.41,3.46,;-3.75,2.69,;-1.08,2.69,;.39,3.17,;1.29,1.92,;.39,.68,;.71,-.83,;-.44,-1.86,;-1.9,-1.38,;-2.22,.12,;-1.08,1.15,;2.19,-1.23,;2.59,-2.71,;4.08,-3.11,;5.17,-2.02,;4.77,-.54,;3.28,-.14,;6.66,-2.42,;5.57,-3.51,;7.75,-1.33,;7.06,-3.91,)| Show InChI InChI=1S/C26H34N6O3S/c1-18(33)28-19-6-8-20(9-7-19)29-26-27-14-10-25(30-26)32-17-13-22-23(4-3-5-24(22)32)31-15-11-21(12-16-31)36(2,34)35/h3-5,10,13-14,17,19-21H,6-9,11-12,15-16H2,1-2H3,(H,28,33)(H,27,29,30)/t19-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Roche Palo Alto LLC
US Patent
| Assay Description
JNK activity was measured by phosphorylation of GST-ATF2 (19-96) with [gamma-33P] ATP. |
US Patent US8536172 (2013)
BindingDB Entry DOI: 10.7270/Q23N222K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM102571
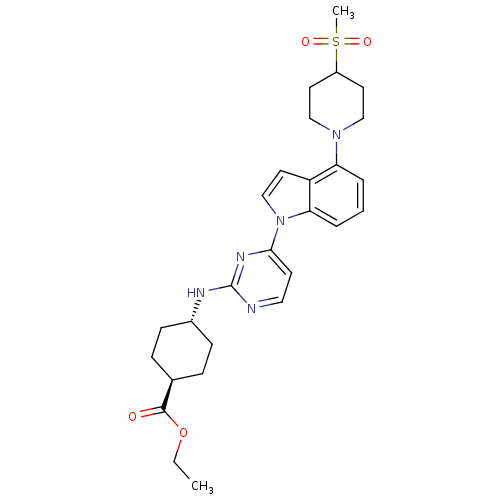
(US8536172, I-20)Show SMILES CCOC(=O)[C@H]1CC[C@@H](CC1)Nc1nccc(n1)-n1ccc2c(cccc12)N1CCC(CC1)S(C)(=O)=O |r,wU:8.11,wD:5.4,(-2.41,-5.01,;-3.75,-4.24,;-5.08,-5.01,;-6.41,-4.24,;-7.75,-5.01,;-6.41,-2.7,;-5.08,-1.93,;-5.08,-.39,;-6.41,.38,;-7.75,-.39,;-7.75,-1.93,;-6.41,1.92,;-5.08,2.69,;-5.08,4.23,;-3.75,5.01,;-2.41,4.23,;-2.41,2.69,;-3.75,1.92,;-1.08,1.92,;.39,2.4,;1.29,1.15,;.39,-.09,;.71,-1.6,;-.44,-2.63,;-1.9,-2.15,;-2.22,-.65,;-1.08,.38,;2.19,-2,;2.59,-3.48,;4.08,-3.88,;5.17,-2.79,;4.77,-1.31,;3.28,-.91,;6.66,-3.19,;5.57,-4.28,;7.75,-2.1,;7.06,-4.68,)| Show InChI InChI=1S/C27H35N5O4S/c1-3-36-26(33)19-7-9-20(10-8-19)29-27-28-15-11-25(30-27)32-18-14-22-23(5-4-6-24(22)32)31-16-12-21(13-17-31)37(2,34)35/h4-6,11,14-15,18-21H,3,7-10,12-13,16-17H2,1-2H3,(H,28,29,30)/t19-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Roche Palo Alto LLC
US Patent
| Assay Description
JNK activity was measured by phosphorylation of GST-ATF2 (19-96) with [gamma-33P] ATP. |
US Patent US8536172 (2013)
BindingDB Entry DOI: 10.7270/Q23N222K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50492032

(CHEMBL2392831)Show SMILES CS(=O)(=O)N[C@H]1CC[C@@H](CC1)Nc1nccc(n1)-n1ccc2c(OCC3CCS(=O)(=O)C3)cccc12 |r,wU:8.11,wD:5.4,(17.22,-45.97,;18.56,-46.73,;17.79,-48.07,;19.33,-48.07,;19.89,-45.97,;19.89,-44.43,;21.22,-43.66,;21.22,-42.12,;19.89,-41.35,;18.56,-42.12,;18.56,-43.66,;19.89,-39.81,;21.22,-39.04,;21.22,-37.51,;22.55,-36.73,;23.89,-37.5,;23.89,-39.04,;22.56,-39.81,;25.34,-39.55,;26.61,-38.67,;27.83,-39.61,;27.33,-41.06,;28.06,-42.4,;29.6,-42.44,;30.37,-43.77,;31.91,-43.77,;32.81,-42.52,;34.28,-43,;34.28,-44.54,;34.9,-45.95,;35.81,-44.7,;32.81,-45.02,;27.26,-43.71,;25.72,-43.68,;24.99,-42.33,;25.79,-41.02,)| Show InChI InChI=1S/C24H31N5O5S2/c1-35(30,31)28-19-7-5-18(6-8-19)26-24-25-12-9-23(27-24)29-13-10-20-21(29)3-2-4-22(20)34-15-17-11-14-36(32,33)16-17/h2-4,9-10,12-13,17-19,28H,5-8,11,14-16H2,1H3,(H,25,26,27)/t17?,18-,19- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of human JNK1alpha1 using GST-tagged ATF2 as substrate preincubated for 10 mins prior to substrate addition measured after 30 mins by micr... |
Bioorg Med Chem Lett 23: 3565-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.029
BindingDB Entry DOI: 10.7270/Q2MG7SF1 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM102564

(US8536172, I-13)Show SMILES CC(=O)N[C@H]1CC[C@@H](CC1)Nc1nccc(n1)-n1ccc2c(cccc12)N1CCC(CC1)S(C)(=O)=O |r,wU:7.10,wD:4.3,(-3.75,-3.47,;-5.08,-4.24,;-5.08,-5.78,;-6.41,-3.47,;-6.41,-1.93,;-5.08,-1.16,;-5.08,.38,;-6.41,1.15,;-7.75,.38,;-7.75,-1.16,;-6.41,2.69,;-5.08,3.46,;-5.08,5,;-3.75,5.78,;-2.41,5,;-2.41,3.46,;-3.75,2.69,;-1.08,2.69,;.39,3.17,;1.29,1.92,;.39,.68,;.71,-.83,;-.44,-1.86,;-1.9,-1.38,;-2.22,.12,;-1.08,1.15,;2.19,-1.23,;2.59,-2.71,;4.08,-3.11,;5.17,-2.02,;4.77,-.54,;3.28,-.14,;6.66,-2.42,;5.57,-3.51,;7.75,-1.33,;7.06,-3.91,)| Show InChI InChI=1S/C26H34N6O3S/c1-18(33)28-19-6-8-20(9-7-19)29-26-27-14-10-25(30-26)32-17-13-22-23(4-3-5-24(22)32)31-15-11-21(12-16-31)36(2,34)35/h3-5,10,13-14,17,19-21H,6-9,11-12,15-16H2,1-2H3,(H,28,33)(H,27,29,30)/t19-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of human JNK1alpha1 using GST-tagged ATF2 as substrate preincubated for 10 mins prior to substrate addition measured after 30 mins by micr... |
Bioorg Med Chem Lett 23: 3565-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.029
BindingDB Entry DOI: 10.7270/Q2MG7SF1 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM102571

(US8536172, I-20)Show SMILES CCOC(=O)[C@H]1CC[C@@H](CC1)Nc1nccc(n1)-n1ccc2c(cccc12)N1CCC(CC1)S(C)(=O)=O |r,wU:8.11,wD:5.4,(-2.41,-5.01,;-3.75,-4.24,;-5.08,-5.01,;-6.41,-4.24,;-7.75,-5.01,;-6.41,-2.7,;-5.08,-1.93,;-5.08,-.39,;-6.41,.38,;-7.75,-.39,;-7.75,-1.93,;-6.41,1.92,;-5.08,2.69,;-5.08,4.23,;-3.75,5.01,;-2.41,4.23,;-2.41,2.69,;-3.75,1.92,;-1.08,1.92,;.39,2.4,;1.29,1.15,;.39,-.09,;.71,-1.6,;-.44,-2.63,;-1.9,-2.15,;-2.22,-.65,;-1.08,.38,;2.19,-2,;2.59,-3.48,;4.08,-3.88,;5.17,-2.79,;4.77,-1.31,;3.28,-.91,;6.66,-3.19,;5.57,-4.28,;7.75,-2.1,;7.06,-4.68,)| Show InChI InChI=1S/C27H35N5O4S/c1-3-36-26(33)19-7-9-20(10-8-19)29-27-28-15-11-25(30-27)32-18-14-22-23(5-4-6-24(22)32)31-16-12-21(13-17-31)37(2,34)35/h4-6,11,14-15,18-21H,3,7-10,12-13,16-17H2,1-2H3,(H,28,29,30)/t19-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of human JNK1alpha1 using GST-tagged ATF2 as substrate preincubated for 10 mins prior to substrate addition measured after 30 mins by micr... |
Bioorg Med Chem Lett 23: 3565-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.029
BindingDB Entry DOI: 10.7270/Q2MG7SF1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313011
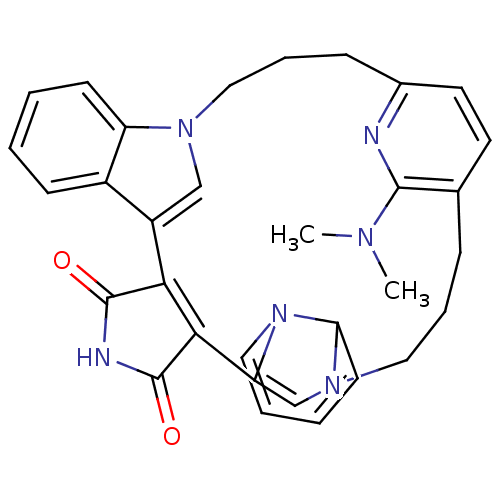
(31-(dimethylamino)-5,11,15,25,30-pentaazaheptacycl...)Show SMILES CN(C)c1nc2CCCn3cc(C4=C(C(=O)NC4=O)C4=CN(CCCc1cc2)C1C=CC=CN41)c1ccccc31 |c:32,34,t:12,20| Show InChI InChI=1S/C32H32N6O2/c1-35(2)30-21-9-7-17-37-20-26(38-18-6-5-13-27(37)38)29-28(31(39)34-32(29)40)24-19-36(25-12-4-3-11-23(24)25)16-8-10-22(33-30)15-14-21/h3-6,11-15,18-20,27H,7-10,16-17H2,1-2H3,(H,34,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... |
Bioorg Med Chem Lett 20: 1693-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.038
BindingDB Entry DOI: 10.7270/Q2ZK5HNS |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against MMP2 |
J Med Chem 49: 456-8 (2006)
Article DOI: 10.1021/jm051101g
BindingDB Entry DOI: 10.7270/Q2WQ03C3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data