

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50304781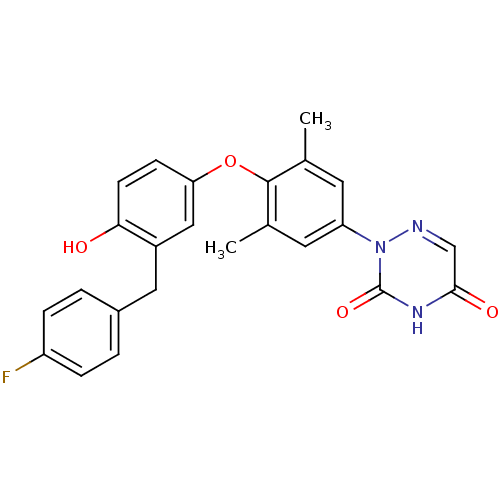 (2-(4-(3-(4-fluorobenzyl)-4-hydroxyphenoxy)-3,5-dim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Binding affinity to thyroid receptor beta | Bioorg Med Chem Lett 20: 306-8 (2010) Article DOI: 10.1016/j.bmcl.2009.10.109 BindingDB Entry DOI: 10.7270/Q2J38SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50304777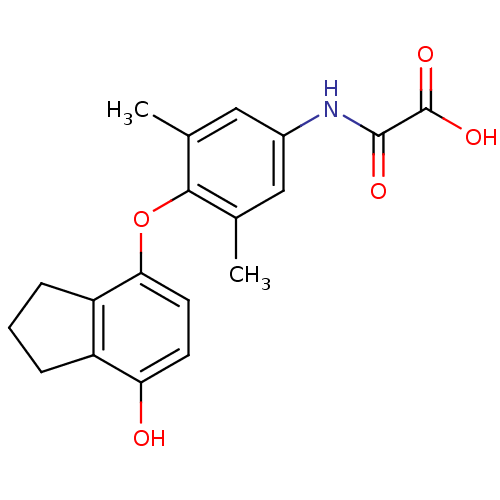 (2-(4-(7-hydroxy-2,3-dihydro-1H-inden-4-yloxy)-3,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Binding affinity to thyroid receptor beta | Bioorg Med Chem Lett 20: 306-8 (2010) Article DOI: 10.1016/j.bmcl.2009.10.109 BindingDB Entry DOI: 10.7270/Q2J38SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50304778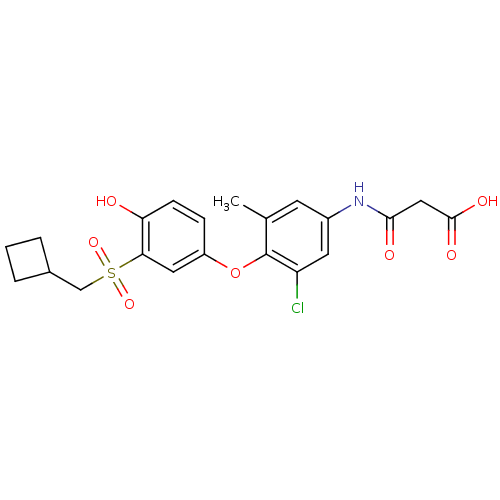 (3-(3-chloro-4-(3-(cyclobutylmethylsulfonyl)-4-hydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Binding affinity to thyroid receptor beta | Bioorg Med Chem Lett 20: 306-8 (2010) Article DOI: 10.1016/j.bmcl.2009.10.109 BindingDB Entry DOI: 10.7270/Q2J38SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50304780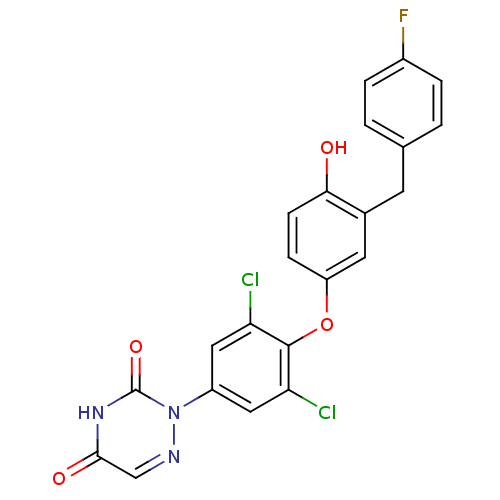 (2-(3,5-dichloro-4-(3-(4-fluorobenzyl)-4-hydroxyphe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Binding affinity to thyroid receptor beta | Bioorg Med Chem Lett 20: 306-8 (2010) Article DOI: 10.1016/j.bmcl.2009.10.109 BindingDB Entry DOI: 10.7270/Q2J38SNP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM50304777 (2-(4-(7-hydroxy-2,3-dihydro-1H-inden-4-yloxy)-3,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Binding affinity to thyroid receptor alpha | Bioorg Med Chem Lett 20: 306-8 (2010) Article DOI: 10.1016/j.bmcl.2009.10.109 BindingDB Entry DOI: 10.7270/Q2J38SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123049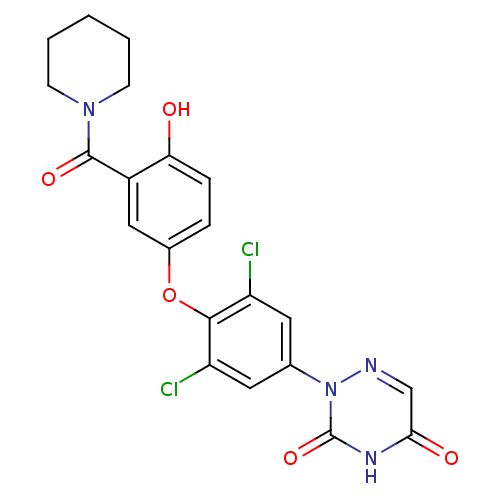 (2-(3,5-dichloro-4-(4-hydroxy-3-(piperidine-1-carbo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Binding affinity to thyroid receptor beta | Bioorg Med Chem Lett 20: 306-8 (2010) Article DOI: 10.1016/j.bmcl.2009.10.109 BindingDB Entry DOI: 10.7270/Q2J38SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378462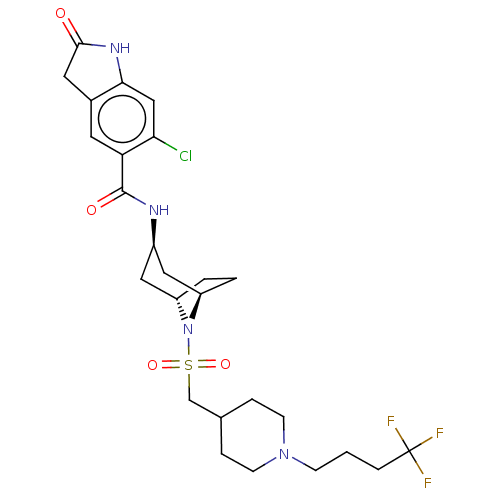 (6-chloro-2-oxo-N-((1R,3r,5S)-8-(((1-(4,4,4-trifluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Non-competitive inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using varyin... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378462 (6-chloro-2-oxo-N-((1R,3r,5S)-8-(((1-(4,4,4-trifluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Non-competitive inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using fixed ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378459 (N-((1R,3r,5S)-8-((4-(benzylamino)piperidin-1-yl)su...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Non-competitive inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using varyin... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50304779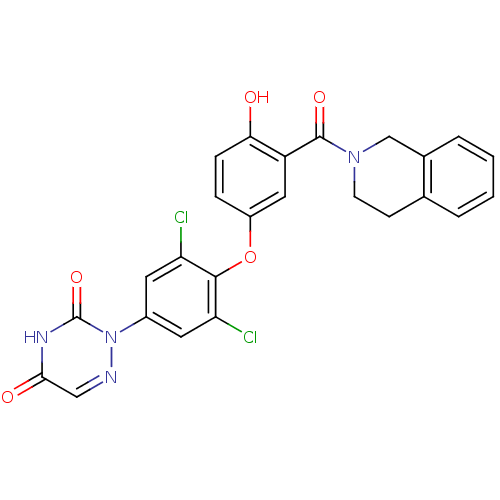 (2-(3,5-dichloro-4-(4-hydroxy-3-(1,2,3,4-tetrahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Binding affinity to thyroid receptor beta | Bioorg Med Chem Lett 20: 306-8 (2010) Article DOI: 10.1016/j.bmcl.2009.10.109 BindingDB Entry DOI: 10.7270/Q2J38SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM50304778 (3-(3-chloro-4-(3-(cyclobutylmethylsulfonyl)-4-hydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Binding affinity to thyroid receptor alpha | Bioorg Med Chem Lett 20: 306-8 (2010) Article DOI: 10.1016/j.bmcl.2009.10.109 BindingDB Entry DOI: 10.7270/Q2J38SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM50304781 (2-(4-(3-(4-fluorobenzyl)-4-hydroxyphenoxy)-3,5-dim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Binding affinity to thyroid receptor alpha | Bioorg Med Chem Lett 20: 306-8 (2010) Article DOI: 10.1016/j.bmcl.2009.10.109 BindingDB Entry DOI: 10.7270/Q2J38SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378459 (N-((1R,3r,5S)-8-((4-(benzylamino)piperidin-1-yl)su...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Mixed type inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using fixed N-ter... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM50304780 (2-(3,5-dichloro-4-(3-(4-fluorobenzyl)-4-hydroxyphe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Binding affinity to thyroid receptor alpha | Bioorg Med Chem Lett 20: 306-8 (2010) Article DOI: 10.1016/j.bmcl.2009.10.109 BindingDB Entry DOI: 10.7270/Q2J38SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM50123049 (2-(3,5-dichloro-4-(4-hydroxy-3-(piperidine-1-carbo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Binding affinity to thyroid receptor alpha | Bioorg Med Chem Lett 20: 306-8 (2010) Article DOI: 10.1016/j.bmcl.2009.10.109 BindingDB Entry DOI: 10.7270/Q2J38SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM50304779 (2-(3,5-dichloro-4-(4-hydroxy-3-(1,2,3,4-tetrahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Binding affinity to thyroid receptor alpha | Bioorg Med Chem Lett 20: 306-8 (2010) Article DOI: 10.1016/j.bmcl.2009.10.109 BindingDB Entry DOI: 10.7270/Q2J38SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433063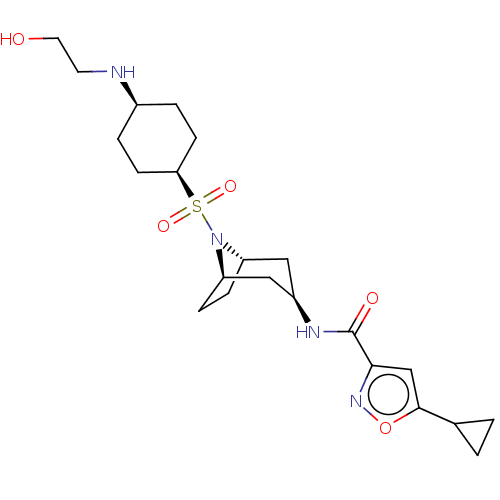 (5-cyclopropyl-N-((1R,3R,5S)-8-(((1S,4S)- 4-((2- hy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378442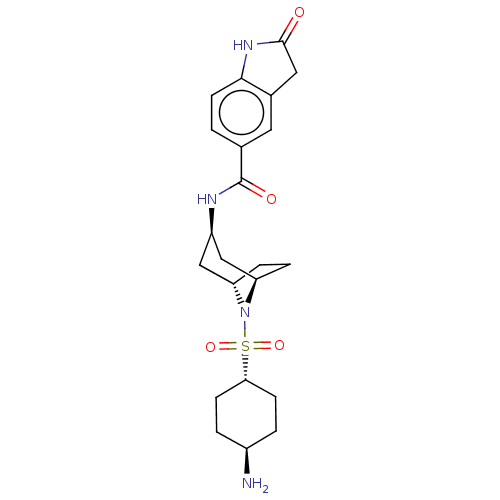 (N-((1R,3R,5S)-8-(((1r,4R)-4-aminocyclohexyl)sulfon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378443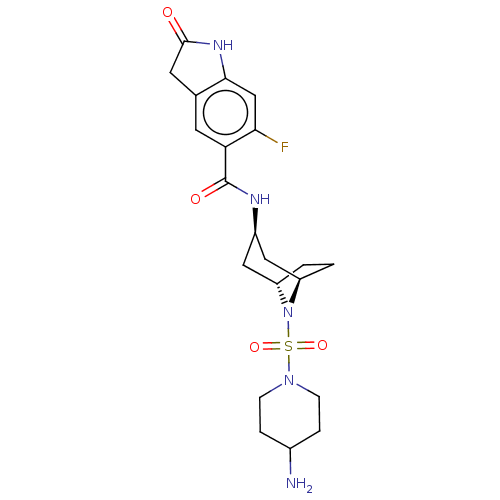 (N-((1R,3r,5S)-8-((4-aminopiperidin-1-yl)sulfonyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432825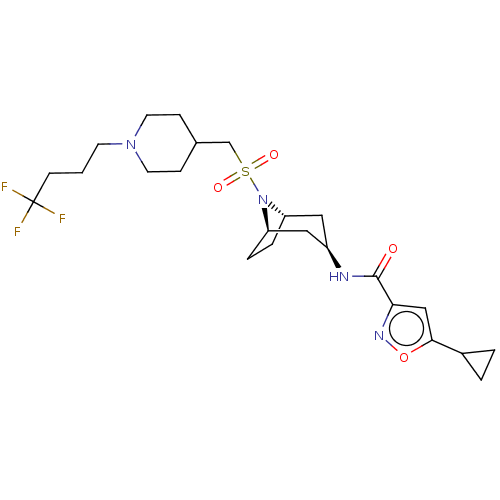 (5-cyclopropyl-N-((1R,3r,5S)-8-(((1-(4,4,4- trifluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378444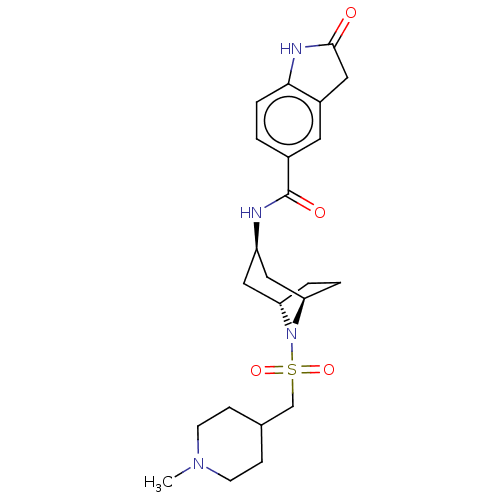 (N-((1R,3r,5S)-8-(((1-methylpiperidin-4-yl)methyl)s...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378445 (N-((1R,3r,5S)-8-((4-aminopiperidin-1-yl)sulfonyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433064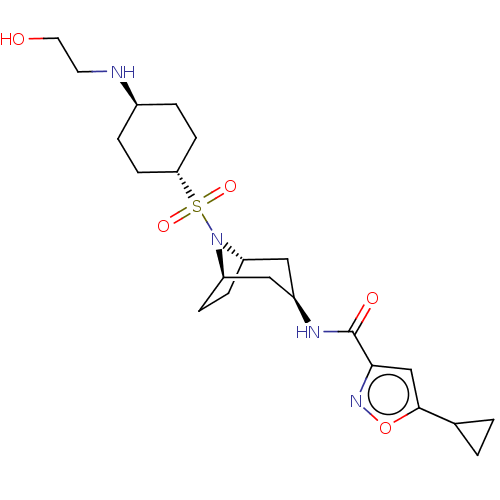 (5-cyclopropyl-N-((1R,3R,5S)-8-(((1R,4R)- 4-((2- hy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433065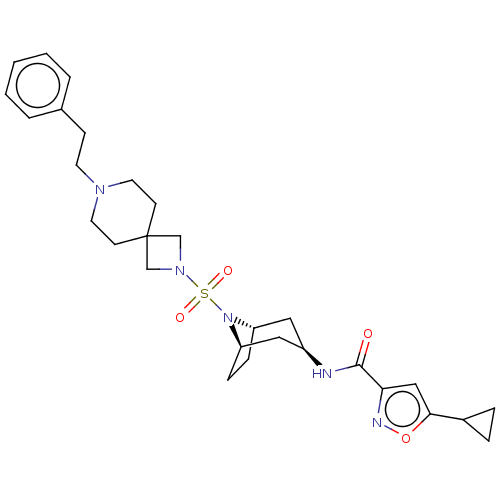 (5-cyclopropyl-N-((1R,3r,5S)-8-((7- phenethyl-2,7-d...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432826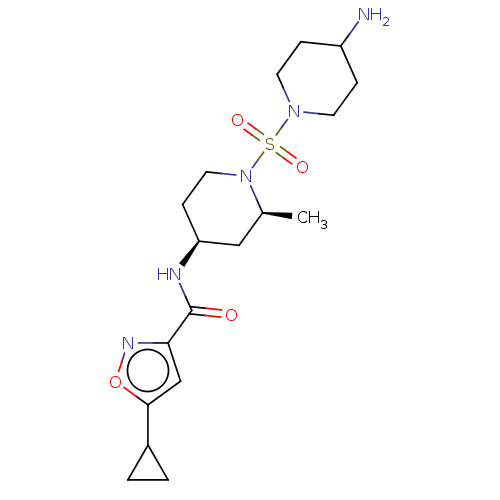 (N-((2S,4S)-1-((4-aminopiperidin-1- yl)sulfonyl)-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432822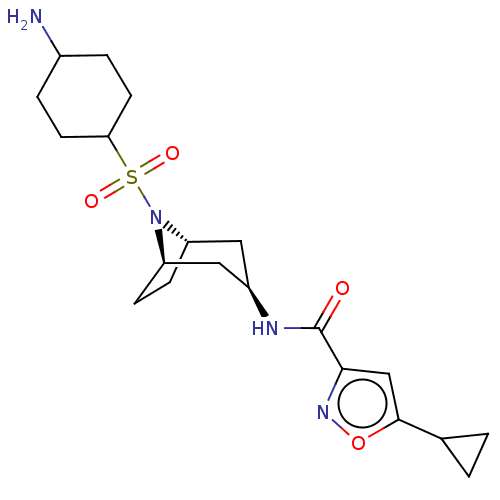 (N-((1R,3r,5S)-8-((4- aminocyclohexyl)sulfonyl)-8- ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378446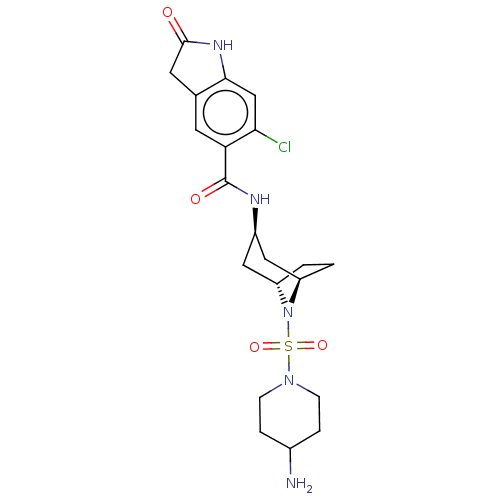 (N-((1R,3r,5S)-8-((4-aminopiperidin-1-yl)sulfonyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433066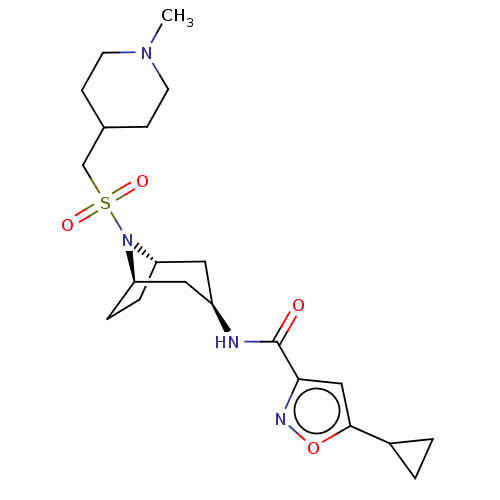 (5-cyclopropyl-N-((1R,3r,5S)-8-(((1- methylpiperidi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432829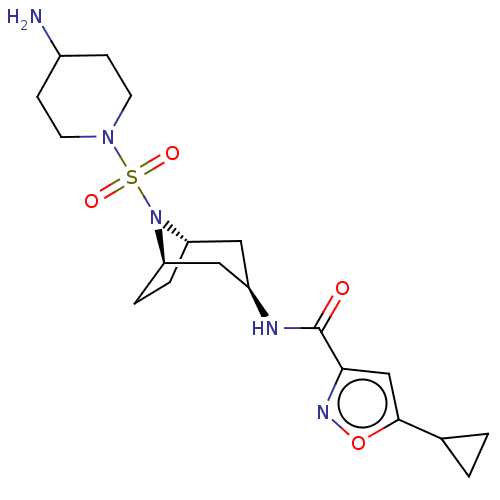 (N-((1R,3r,5S)-8-((4-aminopiperidin-1- yl)sulfonyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432828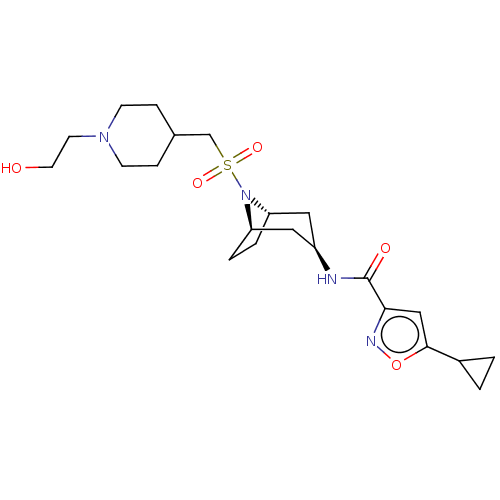 (5-cyclopropyl-N-((1R,3r,5S)-8-(((1-(2- hydroxyethy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432827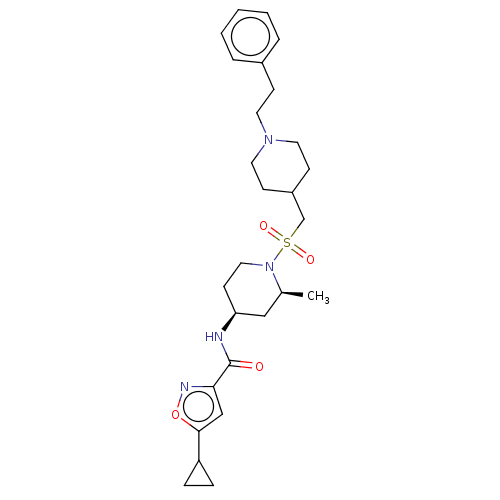 (5-cyclopropyl-N-((2S,4S)-2-methyl-1- (((1-phenethy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378447 (N-((2S,4S)-1-((4-aminopiperidin-1-yl)sulfonyl)-2-m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433067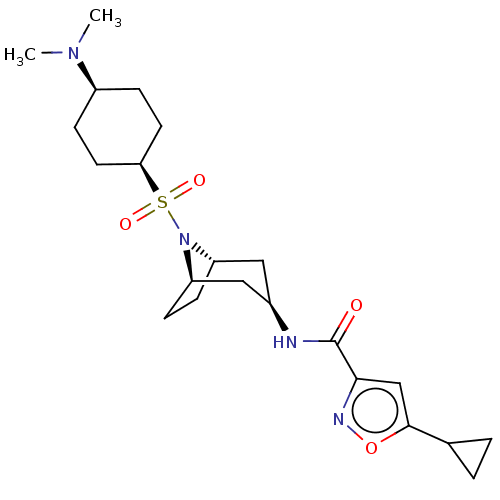 (5-cyclopropyl-N-((1R,3R,5S)-8-(((1s,4S)- 4-(dimeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378448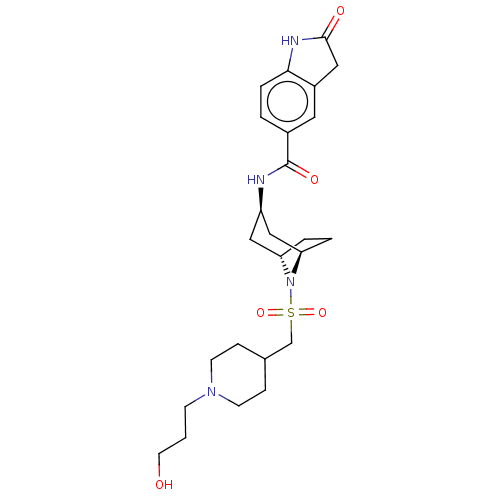 (N-((1R,3r,5S)-8-(((1-(3-hydroxypropyl)piperidin-4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433068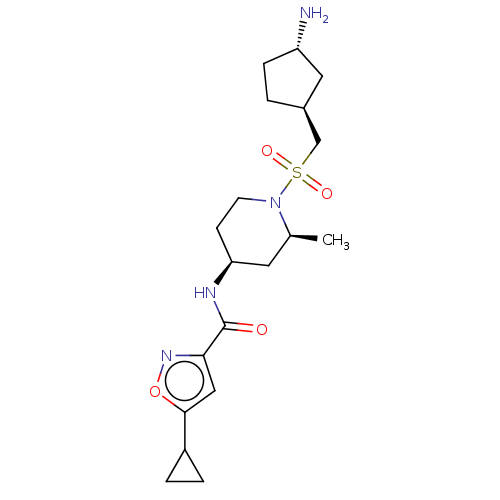 (N-((2S,4S)-1-((((1S,3S)-3- aminocyclopentyl)methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378449 (N-((2S,4S)-1-((4-aminopiperidin-1-yl)sulfonyl)-2-m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378445 (N-((1R,3r,5S)-8-((4-aminopiperidin-1-yl)sulfonyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using N-terminal GST-tagged ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432823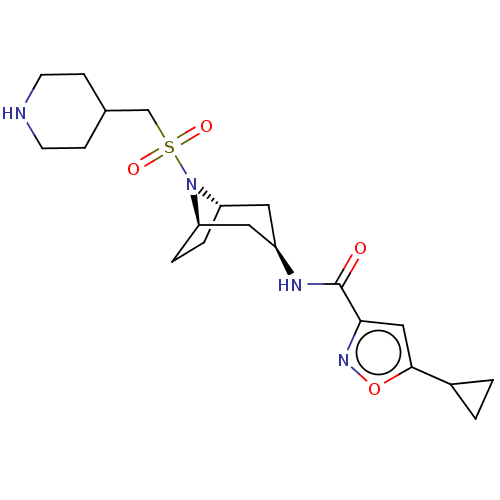 (5-cyclopropyl-N-((1R,3r,5S)-8- ((piperidin-4-ylmet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378444 (N-((1R,3r,5S)-8-(((1-methylpiperidin-4-yl)methyl)s...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using N-terminal GST-tagged ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378446 (N-((1R,3r,5S)-8-((4-aminopiperidin-1-yl)sulfonyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. Curated by ChEMBL | Assay Description Inhibition of full length N-terminal His-tagged SMYD3 (1 to 428 residues) (unknown origin) expressed in Escherichia coli using N-terminal GST-tagged ... | ACS Med Chem Lett 7: 134-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00272 BindingDB Entry DOI: 10.7270/Q2NG4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432830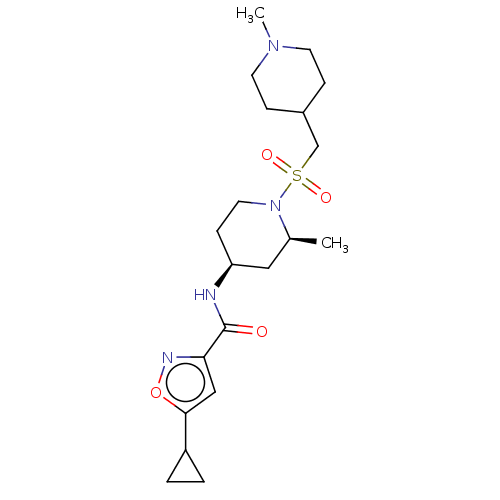 (5-cyclopropyl-N-((2S,4S)-2-methyl-1- (((1-methylpi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433069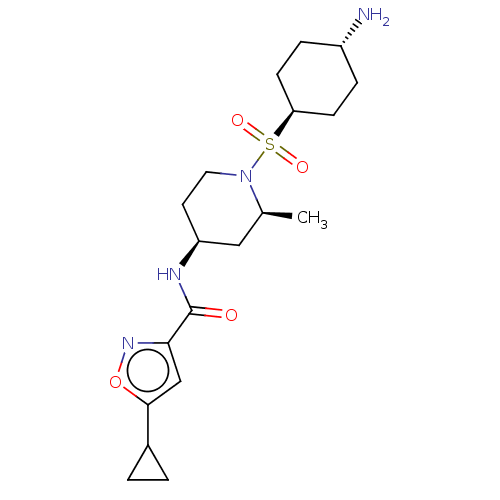 (N-((2S,4S)-1-(((1r,4S)-4- aminocyclohexyl)sulfonyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.03 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433070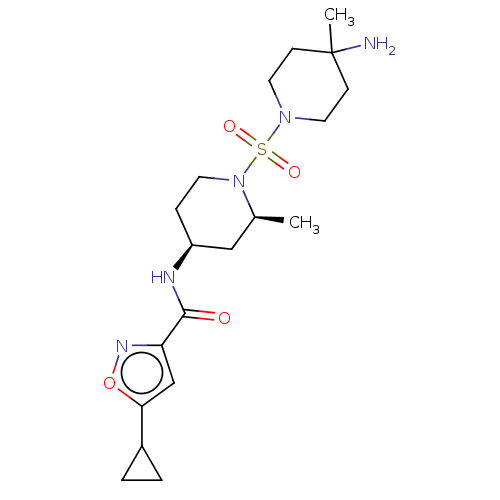 (N-((2S,4S)-1-((4-amino-4-methylpiperidin- l-yl)sul...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.04 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433071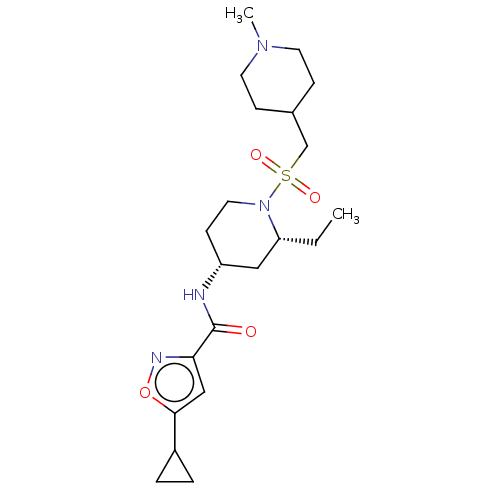 (5-cyclopropyl-N-((2R,4R)-2-ethyl-1-(((1- methylpip...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.07 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432831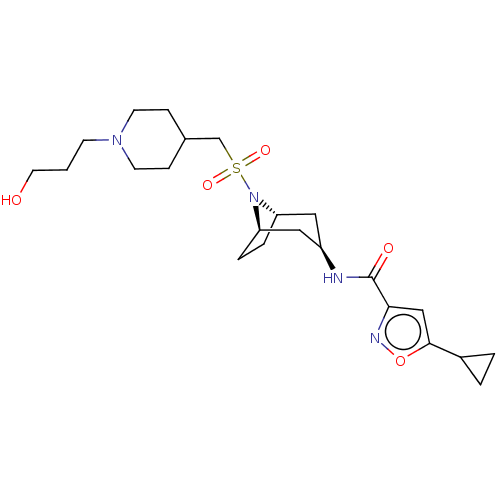 (5-cyclopropyl-N-((1R,3r,5S)-8-(((1-(3- hydroxyprop...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM378450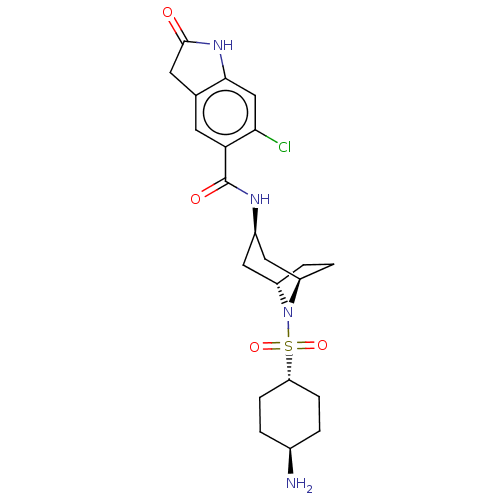 (N-((1R,3R,5S)-8-(((1r,4R)-4-aminocyclohexyl)sulfon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | Bioorg Med Chem 14: 7241-57 (2006) BindingDB Entry DOI: 10.7270/Q2DJ5HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433072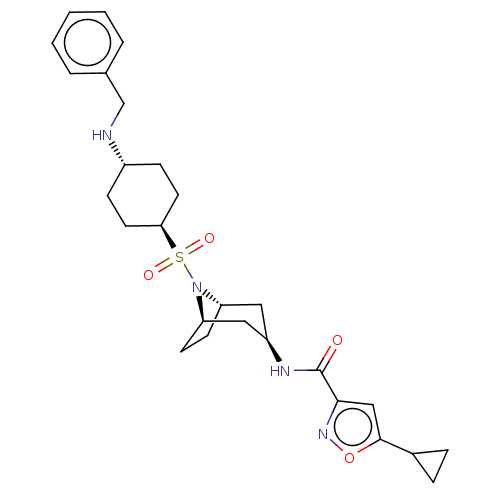 (N-((1R,3R,5S)-8-(((1r,4R)-4- (benzylamino)cyclohex...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.12 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433073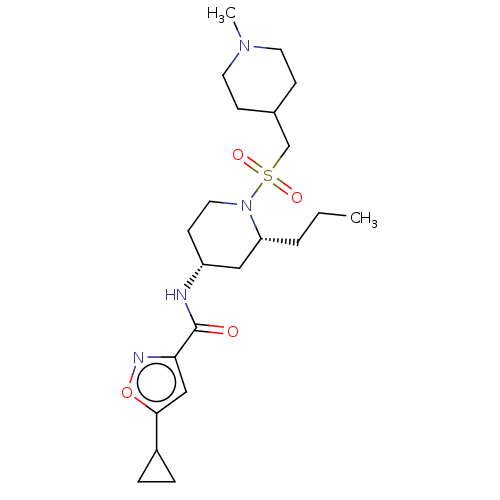 (5-cyclopropyl-N-((2R,4R)-1-(((1- methylpiperidin-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.14 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM433074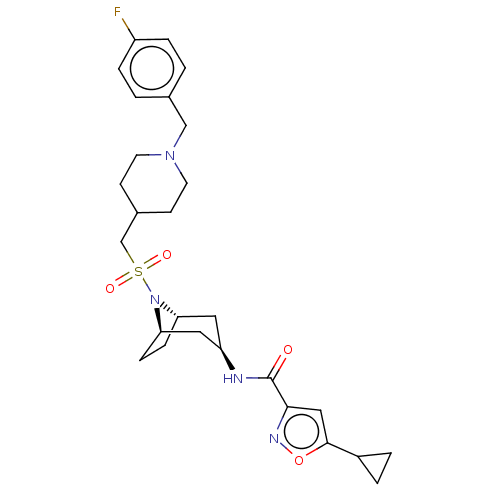 (5-cyclopropyl-N-((1R,3r,5S)-8-(((1-(4- fluorobenzy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.18 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM432833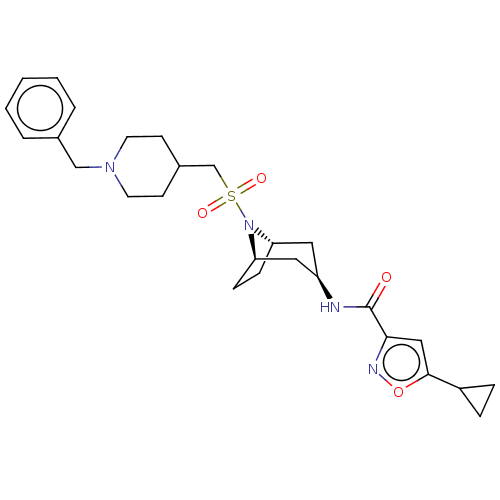 (N-((1R,3r,5S)-8-(((1-benzylpiperidin-4- yl)methyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
EPIZYME, INC. US Patent | Assay Description The assays were all performed in a buffer consisting of 25 mM Tris-Cl pH 8.0, 1 mM TCEP, 0.005% BSG, and 0.005% Tween 20, prepared on the day of use.... | US Patent US10577363 (2020) BindingDB Entry DOI: 10.7270/Q2BZ68G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2420 total ) | Next | Last >> |