

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005897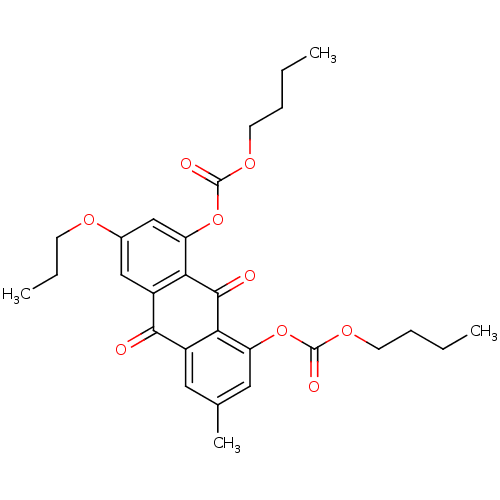 (CHEMBL40840 | Carbonic acid 8-butoxycarbonyloxy-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description HLE-inhibitor (Human Leukocyte Elastase) dissociation constant determined by using Dixon Plot | J Med Chem 35: 1597-605 (1992) BindingDB Entry DOI: 10.7270/Q2QN67D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005903 (CHEMBL44123 | Carbonic acid 8-butoxycarbonyloxy-7-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description Compound was tested for the enzyme inhibitory activity against Human Leukocyte Elastase (HLE) | J Med Chem 35: 1597-605 (1992) BindingDB Entry DOI: 10.7270/Q2QN67D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005900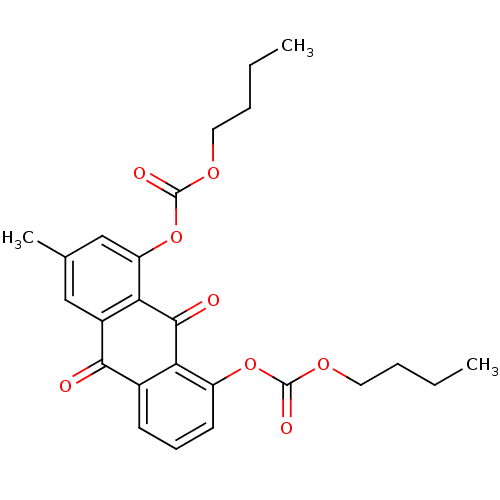 (CHEMBL43983 | Carbonic acid 8-butoxycarbonyloxy-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description HLE-inhibitor (Human Leukocyte Elastase) dissociation constant determined by using Dixon Plot | J Med Chem 35: 1597-605 (1992) BindingDB Entry DOI: 10.7270/Q2QN67D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005887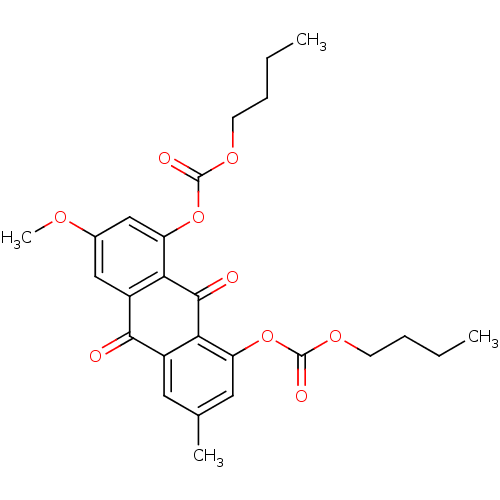 (CHEMBL440290 | Carbonic acid 8-butoxycarbonyloxy-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description HLE-inhibitor (Human Leukocyte Elastase) dissociation constant determined by using Dixon Plot | J Med Chem 35: 1597-605 (1992) BindingDB Entry DOI: 10.7270/Q2QN67D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005889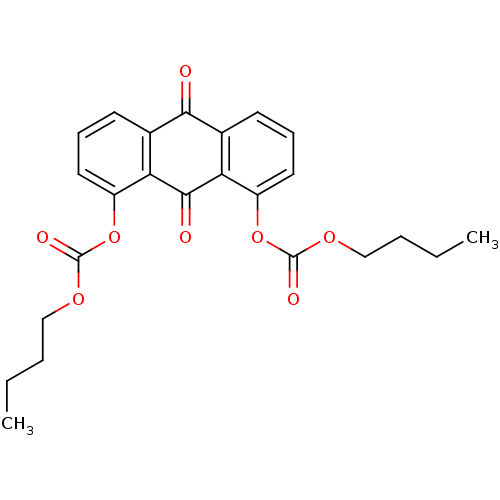 (CHEMBL40878 | Carbonic acid 8-butoxycarbonyloxy-9,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description HLE-inhibitor (Human Leukocyte Elastase) dissociation constant determined by using Dixon Plot | J Med Chem 35: 1597-605 (1992) BindingDB Entry DOI: 10.7270/Q2QN67D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50097532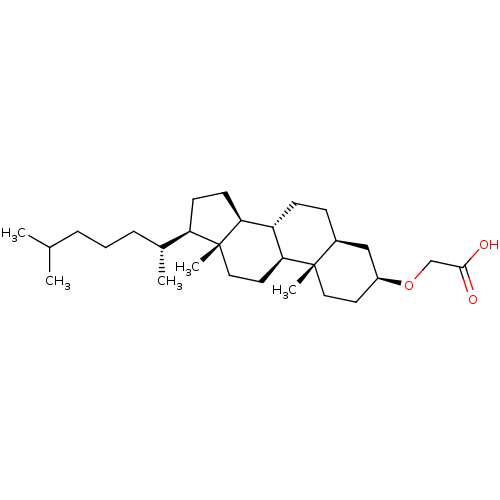 (CHEMBL354794 | [17-(1,5-Dimethyl-hexyl)-10,13-dime...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description Inhibitory activity tested against cell division cycle 25A (assay using fluorescein diphosphate (FDP) as substrate) | J Med Chem 44: 834-48 (2001) BindingDB Entry DOI: 10.7270/Q2C82B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonyl reductase [NADPH] 1 (Homo sapiens (Human)) | BDBM50308752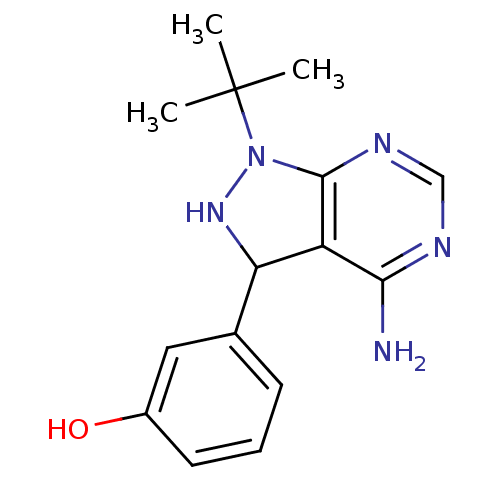 (3-(1-tert-butyl-4-amino-1H-pyrazolo[3,4-d]pyrimidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 759 | n/a | n/a | n/a | n/a | n/a | n/a |
Howard University Curated by ChEMBL | Assay Description Inhibition of CBR-mediated NADPH-dependent reduction of menadione to menadiol | Bioorg Med Chem 18: 134-41 (2010) Article DOI: 10.1016/j.bmc.2009.11.011 BindingDB Entry DOI: 10.7270/Q2XG9R7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonyl reductase [NADPH] 1 (Homo sapiens (Human)) | BDBM50308752 (3-(1-tert-butyl-4-amino-1H-pyrazolo[3,4-d]pyrimidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 788 | n/a | n/a | n/a | n/a | n/a | n/a |
Howard University Curated by ChEMBL | Assay Description Inhibition of CBR-mediated NADPH-dependent reduction of menadione to menadiol | Bioorg Med Chem 18: 134-41 (2010) Article DOI: 10.1016/j.bmc.2009.11.011 BindingDB Entry DOI: 10.7270/Q2XG9R7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50097529 (CHEMBL354215 | [17-(1,5-Dimethyl-hexyl)-10,13-dime...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description Inhibitory activity tested against cell division cycle 25A (assay using fluorescein diphosphate (FDP) as substrate) | J Med Chem 44: 834-48 (2001) BindingDB Entry DOI: 10.7270/Q2C82B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50097528 (CHEMBL169020 | Dithiocarbonic acid O-[17-(1,5-dime...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description Inhibitory activity tested against cell division cycle 25A (assay using fluorescein diphosphate (FDP) as substrate) | J Med Chem 44: 834-48 (2001) BindingDB Entry DOI: 10.7270/Q2C82B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005895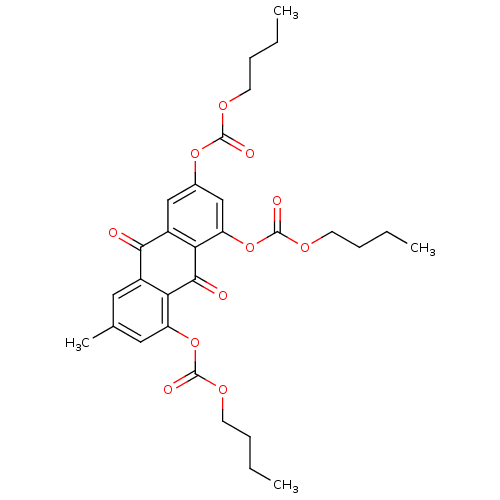 (CHEMBL416614 | Carbonic acid 4,5-bis-butoxycarbony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description In vitro inhibition of human leukocyte elastase. | J Med Chem 35: 1597-605 (1992) BindingDB Entry DOI: 10.7270/Q2QN67D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005897 (CHEMBL40840 | Carbonic acid 8-butoxycarbonyloxy-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description Compound was tested for the enzyme inhibitory activity against Human Leukocyte Elastase (HLE) | J Med Chem 35: 1597-605 (1992) BindingDB Entry DOI: 10.7270/Q2QN67D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005903 (CHEMBL44123 | Carbonic acid 8-butoxycarbonyloxy-7-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description In vitro inhibition of human leukocyte elastase. | J Med Chem 35: 1597-605 (1992) BindingDB Entry DOI: 10.7270/Q2QN67D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50097542 (CHEMBL354816 | Phenyl-thiocarbamic acid O-[17-(1,5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description Inhibitory activity tested against cell division cycle 25A (assay using fluorescein diphosphate (FDP) as substrate) | J Med Chem 44: 834-48 (2001) BindingDB Entry DOI: 10.7270/Q2C82B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50097530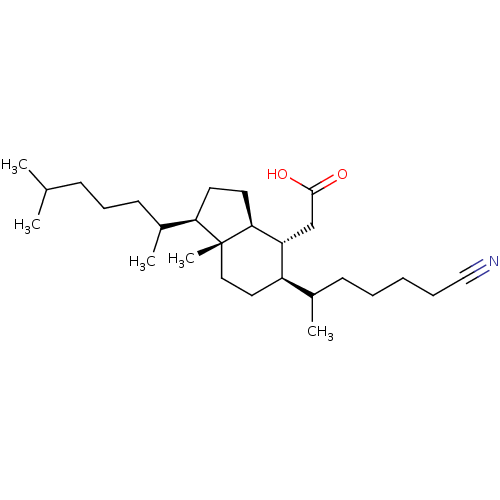 (CHEMBL168868 | [5-(5-Cyano-1-methyl-pentyl)-1-(1,5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description Inhibitory activity tested against cell division cycle 25A (assay using fluorescein diphosphate (FDP) as substrate) | J Med Chem 44: 834-48 (2001) BindingDB Entry DOI: 10.7270/Q2C82B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50068031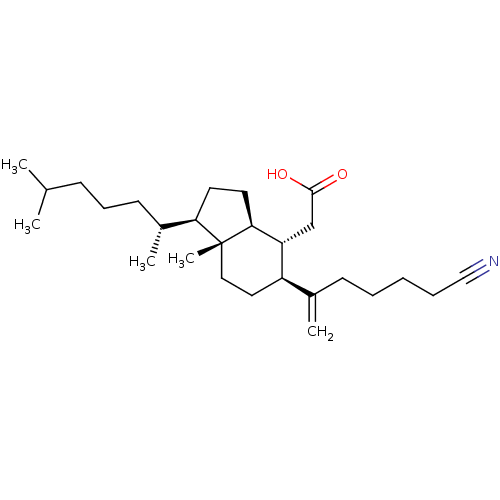 (CHEMBL142455 | [(1R,3aS,4R,5S,7aR)-5-(5-Cyano-1-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Cdc25A phosphatase by using fluorescein diphosphate as substrate | J Med Chem 41: 4677-80 (1998) Article DOI: 10.1021/jm980500r BindingDB Entry DOI: 10.7270/Q2PC31JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50097530 (CHEMBL168868 | [5-(5-Cyano-1-methyl-pentyl)-1-(1,5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description Inhibitory activity tested against cell division cycle 25A (assay using fluorescein diphosphate (FDP) as substrate) | J Med Chem 44: 834-48 (2001) BindingDB Entry DOI: 10.7270/Q2C82B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50068031 (CHEMBL142455 | [(1R,3aS,4R,5S,7aR)-5-(5-Cyano-1-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description Inhibitory activity tested against cell division cycle 25A (assay using fluorescein diphosphate (FDP) as substrate) | J Med Chem 44: 834-48 (2001) BindingDB Entry DOI: 10.7270/Q2C82B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50097539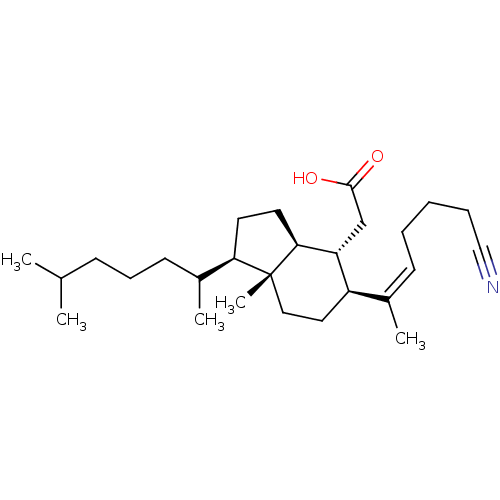 (CHEMBL354578 | [5-(5-Cyano-1-methyl-pent-1-enyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description Inhibitory activity tested against cell division cycle 25A (assay using fluorescein diphosphate (FDP) as substrate) | J Med Chem 44: 834-48 (2001) BindingDB Entry DOI: 10.7270/Q2C82B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005887 (CHEMBL440290 | Carbonic acid 8-butoxycarbonyloxy-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description Compound was tested for the enzyme inhibitory activity against Human Leukocyte Elastase (HLE) | J Med Chem 35: 1597-605 (1992) BindingDB Entry DOI: 10.7270/Q2QN67D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50097526 (CHEMBL172392 | [5-(5-Cyano-4-ethoxy-1-methylene-pe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description Inhibitory activity tested against cell division cycle 25A (assay using fluorescein diphosphate (FDP) as substrate) | J Med Chem 44: 834-48 (2001) BindingDB Entry DOI: 10.7270/Q2C82B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonyl reductase [NADPH] 1 (Homo sapiens (Human)) | BDBM50308753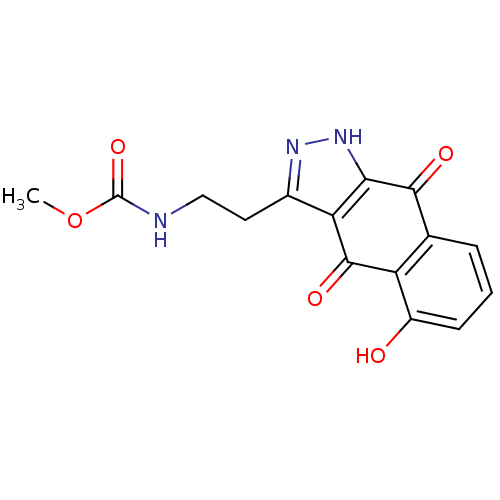 (3-(Methyl-N-ethylcarbamate)-5-hydroxybenz[f]indazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Howard University Curated by ChEMBL | Assay Description Displacement of menadione from human recombinant CBR expressed in Escherichia coli BL21 (DE3) | Bioorg Med Chem 18: 134-41 (2010) Article DOI: 10.1016/j.bmc.2009.11.011 BindingDB Entry DOI: 10.7270/Q2XG9R7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005883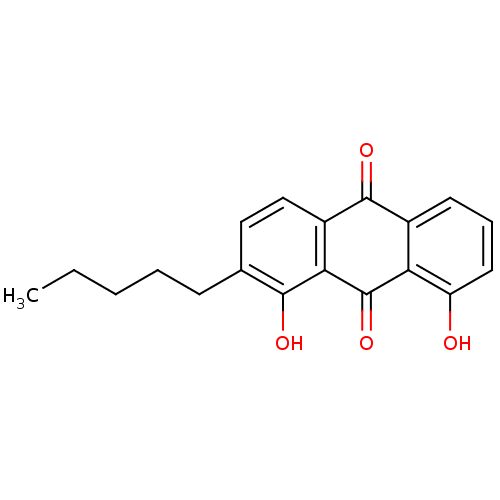 (1,8-Dihydroxy-2-pentyl-anthraquinone | CHEMBL40884) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description In vitro inhibition of human leukocyte elastase. | J Med Chem 35: 1597-605 (1992) BindingDB Entry DOI: 10.7270/Q2QN67D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonyl reductase [NADPH] 1 (Homo sapiens (Human)) | BDBM50308751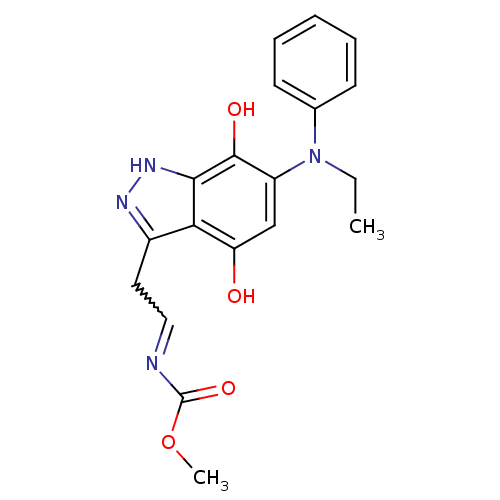 (3-(methyl-N-ethylcarbamate)-6-(N0-ethylphenylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Howard University Curated by ChEMBL | Assay Description Displacement of menadione from human recombinant CBR expressed in Escherichia coli BL21 (DE3) | Bioorg Med Chem 18: 134-41 (2010) Article DOI: 10.1016/j.bmc.2009.11.011 BindingDB Entry DOI: 10.7270/Q2XG9R7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonyl reductase [NADPH] 1 (Homo sapiens (Human)) | BDBM50308750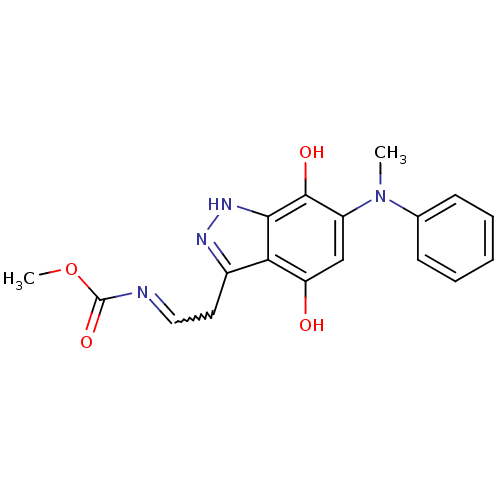 (3-(Methyl-N-ethylcarbamate)-6-(N0-methylphenylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Howard University Curated by ChEMBL | Assay Description Displacement of menadione from human recombinant CBR expressed in Escherichia coli BL21 (DE3) | Bioorg Med Chem 18: 134-41 (2010) Article DOI: 10.1016/j.bmc.2009.11.011 BindingDB Entry DOI: 10.7270/Q2XG9R7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50068037 (5-(Benzoyl-{2-[(2,5-diphenyl-oxazole-4-carbonyl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Cdc25A phosphatase by using p-nitrophenylphosphate as substrate | J Med Chem 41: 4677-80 (1998) Article DOI: 10.1021/jm980500r BindingDB Entry DOI: 10.7270/Q2PC31JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005884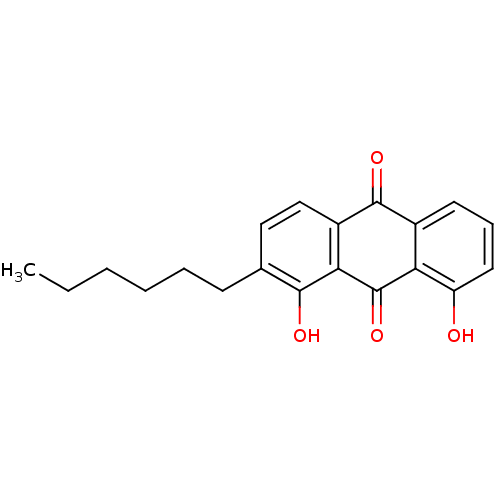 (2-Hexyl-1,8-dihydroxy-anthraquinone | CHEMBL41317) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description In vitro inhibition of human leukocyte elastase. | J Med Chem 35: 1597-605 (1992) BindingDB Entry DOI: 10.7270/Q2QN67D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005882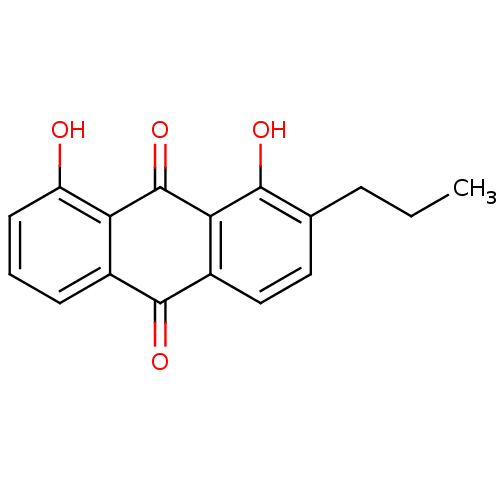 (1,8-Dihydroxy-2-propyl-anthraquinone | CHEMBL40292) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description In vitro inhibition of human leukocyte elastase. | J Med Chem 35: 1597-605 (1992) BindingDB Entry DOI: 10.7270/Q2QN67D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonyl reductase [NADPH] 1 (Homo sapiens (Human)) | BDBM50308754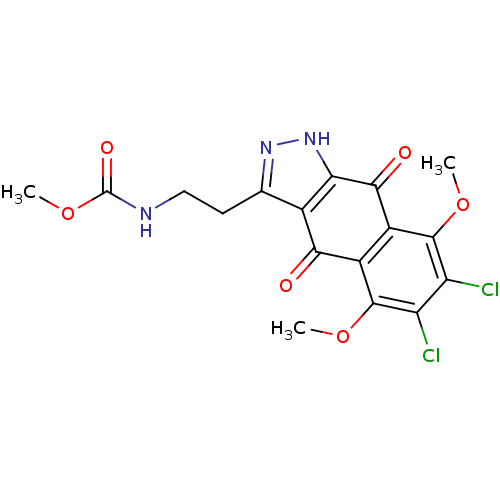 (3-(Methyl-N-ethylcarbamate)-6,7-dichloro-5,8-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Howard University Curated by ChEMBL | Assay Description Displacement of menadione from human recombinant CBR expressed in Escherichia coli BL21 (DE3) | Bioorg Med Chem 18: 134-41 (2010) Article DOI: 10.1016/j.bmc.2009.11.011 BindingDB Entry DOI: 10.7270/Q2XG9R7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50097534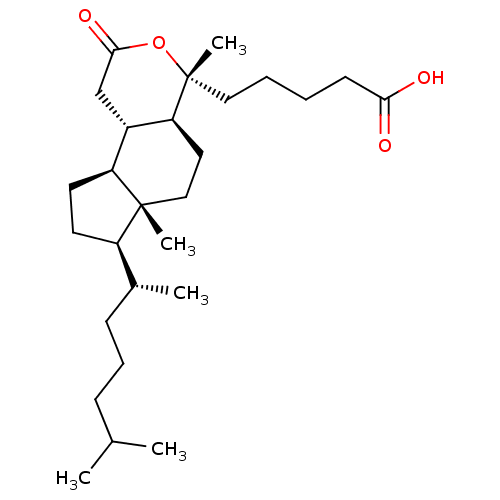 (5-[3-(1,5-Dimethyl-hexyl)-3a,6-dimethyl-8-oxo-dode...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description Inhibitory activity tested against cell division cycle 25A (assay using fluorescein diphosphate (FDP) as substrate) | J Med Chem 44: 834-48 (2001) BindingDB Entry DOI: 10.7270/Q2C82B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005900 (CHEMBL43983 | Carbonic acid 8-butoxycarbonyloxy-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description In vitro inhibition of human leukocyte elastase. | J Med Chem 35: 1597-605 (1992) BindingDB Entry DOI: 10.7270/Q2QN67D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50097540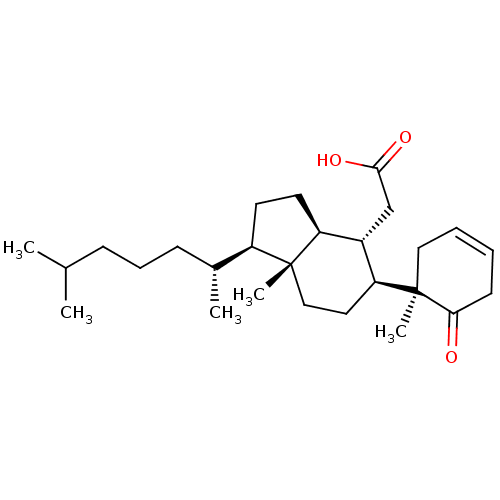 (CHEMBL434693 | [1-(1,5-Dimethyl-hexyl)-7a-methyl-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description Inhibitory activity tested against cell division cycle 25A (assay using fluorescein diphosphate (FDP) as substrate) | J Med Chem 44: 834-48 (2001) BindingDB Entry DOI: 10.7270/Q2C82B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005898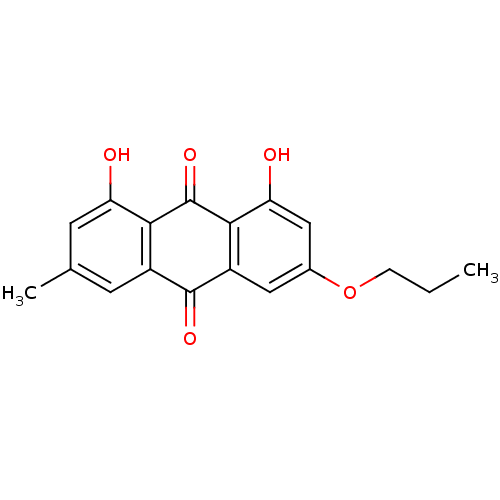 (1,8-Dihydroxy-3-methyl-6-propoxy-anthraquinone | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description Compound was tested for the enzyme inhibitory activity against Human Leukocyte Elastase (HLE) at 62 uM concentration | J Med Chem 35: 1597-605 (1992) BindingDB Entry DOI: 10.7270/Q2QN67D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005905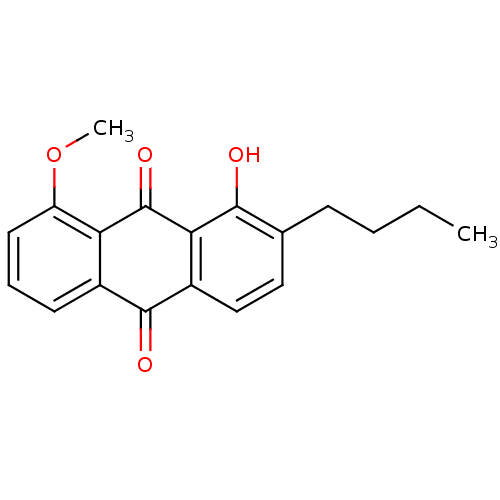 (2-Butyl-1-hydroxy-8-methoxy-anthraquinone | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description In vitro inhibition of human leukocyte elastase. | J Med Chem 35: 1597-605 (1992) BindingDB Entry DOI: 10.7270/Q2QN67D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50097544 (CHEMBL169414 | [1-(1,5-Dimethyl-hexyl)-7a-methyl-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description Inhibitory activity tested against cell division cycle 25A (assay using fluorescein diphosphate (FDP) as substrate) | J Med Chem 44: 834-48 (2001) BindingDB Entry DOI: 10.7270/Q2C82B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005885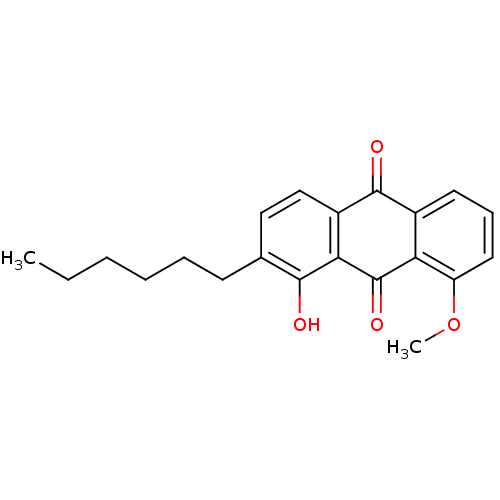 (2-Hexyl-1-hydroxy-8-methoxy-anthraquinone | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description Compound was tested for the enzyme inhibitory activity against Human Leukocyte elastase | J Med Chem 35: 1597-605 (1992) BindingDB Entry DOI: 10.7270/Q2QN67D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005888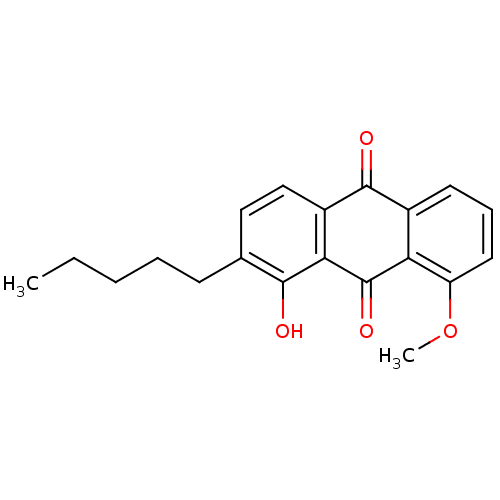 (1-Hydroxy-8-methoxy-2-pentyl-anthraquinone | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description In vitro inhibition of human leukocyte elastase. | J Med Chem 35: 1597-605 (1992) BindingDB Entry DOI: 10.7270/Q2QN67D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005886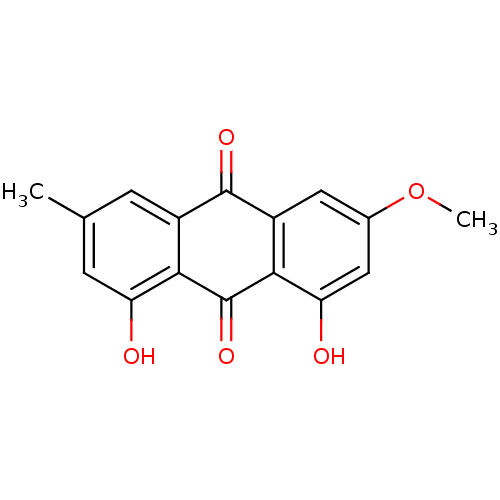 (1,8-Dihydroxy-3-methoxy-6-methylanthraquinone | 1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description Compound was tested for the enzyme inhibitory activity against Human Leukocyte Elastase (HLE) at 65 uM concentration | J Med Chem 35: 1597-605 (1992) BindingDB Entry DOI: 10.7270/Q2QN67D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005896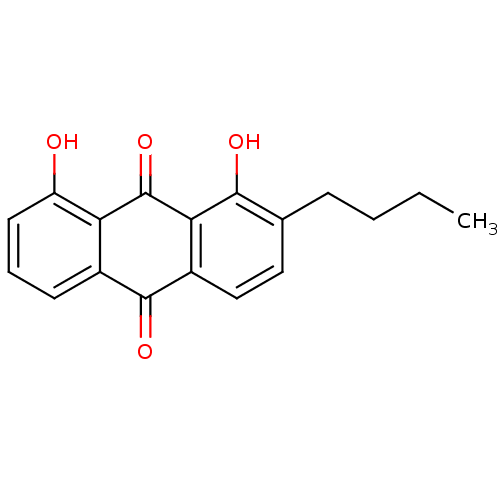 (2-Butyl-1,8-dihydroxy-anthraquinone | CHEMBL41504) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description In vitro inhibition of human leukocyte elastase. | J Med Chem 35: 1597-605 (1992) BindingDB Entry DOI: 10.7270/Q2QN67D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 3 (Homo sapiens (Human)) | BDBM50068031 (CHEMBL142455 | [(1R,3aS,4R,5S,7aR)-5-(5-Cyano-1-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description Inhibitory activity tested against Human cell division cycle 25 degree C | J Med Chem 44: 834-48 (2001) BindingDB Entry DOI: 10.7270/Q2C82B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005902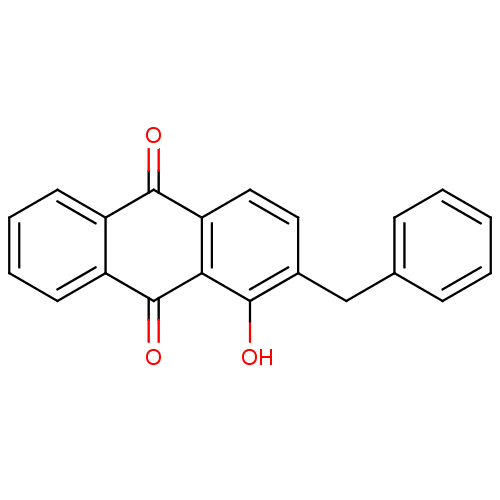 (2-Benzyl-1-hydroxy-anthraquinone | CHEMBL43177) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description Compound was tested for the enzyme inhibitory activity against Human Leukocyte elastase | J Med Chem 35: 1597-605 (1992) BindingDB Entry DOI: 10.7270/Q2QN67D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005915 (2-Benzyl-1-hydroxy-8-methoxy-anthraquinone | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description In vitro inhibition of human leukocyte elastase. | J Med Chem 35: 1597-605 (1992) BindingDB Entry DOI: 10.7270/Q2QN67D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50097546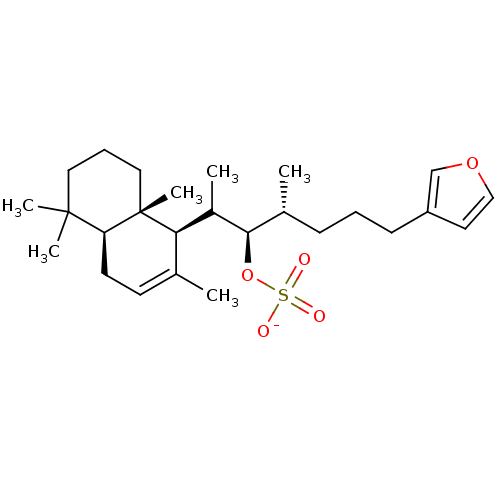 (Sulfuric acidmono-{5-furan-3-yl-2-methyl-1-[1-(2,5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description Inhibitory activity tested against cell division cycle 25A (assay using fluorescein diphosphate (FDP) as substrate) | J Med Chem 44: 834-48 (2001) BindingDB Entry DOI: 10.7270/Q2C82B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005891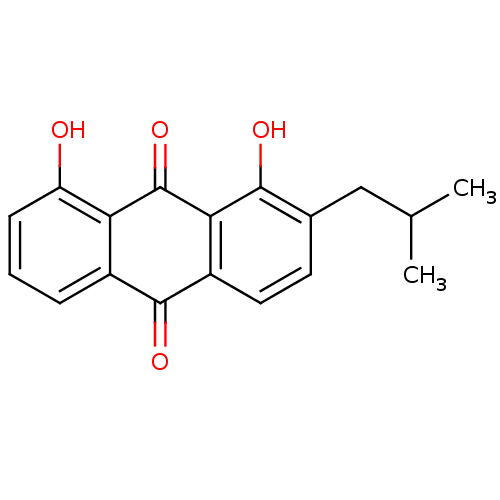 (1,8-Dihydroxy-2-isobutyl-anthraquinone | CHEMBL434...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description In vitro inhibition of human leukocyte elastase. | J Med Chem 35: 1597-605 (1992) BindingDB Entry DOI: 10.7270/Q2QN67D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division control protein 45 homolog (Homo sapiens (Human)) | BDBM50097530 (CHEMBL168868 | [5-(5-Cyano-1-methyl-pentyl)-1-(1,5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description Inhibitory activity tested against human cell division cycle 45-like 2 | J Med Chem 44: 834-48 (2001) BindingDB Entry DOI: 10.7270/Q2C82B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005892 (1-Hydroxy-8-methoxy-2-propyl-anthraquinone | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description In vitro inhibition of human leukocyte elastase. | J Med Chem 35: 1597-605 (1992) BindingDB Entry DOI: 10.7270/Q2QN67D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50097530 (CHEMBL168868 | [5-(5-Cyano-1-methyl-pentyl)-1-(1,5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description Inhibitory activity tested against Human CD45 Phosphatase (assay using fluorescein diphosphate (FDP) as substrate) | J Med Chem 44: 834-48 (2001) BindingDB Entry DOI: 10.7270/Q2C82B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50097543 (CHEMBL165813 | [5-(4-Acetoxy-1-methyl-2-oxo-cycloh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description Inhibitory activity tested against cell division cycle 25A (assay using fluorescein diphosphate (FDP) as substrate) | J Med Chem 44: 834-48 (2001) BindingDB Entry DOI: 10.7270/Q2C82B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50341997 (CHEMBL1765353 | Dysidiolide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry Curated by ChEMBL | Assay Description Inhibitory activity tested against cell division cycle 25A (assay using fluorescein diphosphate (FDP) as substrate) | J Med Chem 44: 834-48 (2001) BindingDB Entry DOI: 10.7270/Q2C82B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50341997 (CHEMBL1765353 | Dysidiolide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Cdc25A phosphatase by using p-nitrophenylphosphate as substrate | J Med Chem 41: 4677-80 (1998) Article DOI: 10.1021/jm980500r BindingDB Entry DOI: 10.7270/Q2PC31JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 108 total ) | Next | Last >> |