

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50310823 (CHEMBL1078442 | bergamottin) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 assessed as dissociation constant of enzyme-substrate-inhibitor complex using Rh-EVNLDAEFK as substrate by Dixo... | Bioorg Med Chem 20: 784-8 (2012) Article DOI: 10.1016/j.bmc.2011.12.002 BindingDB Entry DOI: 10.7270/Q2F47PKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50494892 (CHEMBL3092601) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate by Dixon plot analysis | Bioorg Med Chem Lett 23: 6580-4 (2013) Article DOI: 10.1016/j.bmcl.2013.10.066 BindingDB Entry DOI: 10.7270/Q2QZ2DW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50361390 (BYAKANGELICIN) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 assessed as dissociation constant of enzyme-substrate-inhibitor complex using Rh-EVNLDAEFK as substrate by Dixo... | Bioorg Med Chem 20: 784-8 (2012) Article DOI: 10.1016/j.bmc.2011.12.002 BindingDB Entry DOI: 10.7270/Q2F47PKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50494892 (CHEMBL3092601) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Mixed inhibition of mushroom tyrosinase using L-tyrosine as substrate by Dixon/Cornish-Bowden plot analysis | Bioorg Med Chem Lett 23: 6580-4 (2013) Article DOI: 10.1016/j.bmcl.2013.10.066 BindingDB Entry DOI: 10.7270/Q2QZ2DW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50308720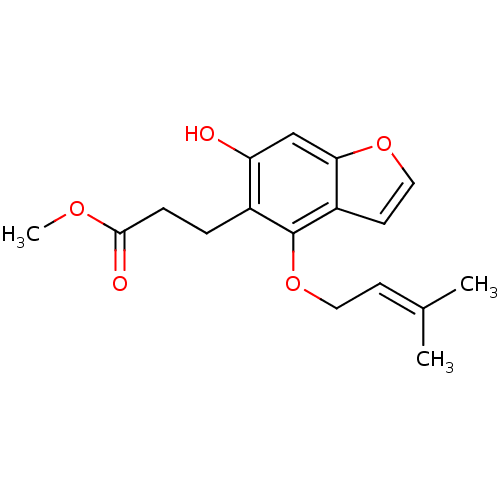 (6,7-Furano-5-prenyloxy hydrocoumaric acid methyl e...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant BACE1 by Lineweaver-Burke plot analysis | Bioorg Med Chem 18: 455-9 (2010) Article DOI: 10.1016/j.bmc.2009.10.004 BindingDB Entry DOI: 10.7270/Q2K64J6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50361388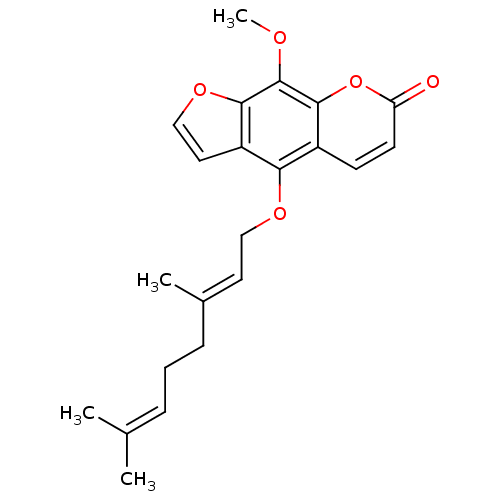 (CHEMBL1934195) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 assessed as dissociation constant of enzyme-substrate-inhibitor complex using Rh-EVNLDAEFK as substrate by Dixo... | Bioorg Med Chem 20: 784-8 (2012) Article DOI: 10.1016/j.bmc.2011.12.002 BindingDB Entry DOI: 10.7270/Q2F47PKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50310823 (CHEMBL1078442 | bergamottin) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 assessed as dissociation constant of enzyme-inhibitor complex using Rh-EVNLDAEFK as substrate by Dixon and Corn... | Bioorg Med Chem 20: 784-8 (2012) Article DOI: 10.1016/j.bmc.2011.12.002 BindingDB Entry DOI: 10.7270/Q2F47PKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50361390 (BYAKANGELICIN) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 assessed as dissociation constant of enzyme-inhibitor complex using Rh-EVNLDAEFK as substrate by Dixon and Corn... | Bioorg Med Chem 20: 784-8 (2012) Article DOI: 10.1016/j.bmc.2011.12.002 BindingDB Entry DOI: 10.7270/Q2F47PKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50361381 (CHEMBL1412710) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 assessed as dissociation constant of enzyme-inhibitor complex using Rh-EVNLDAEFK as substrate by Dixon and Corn... | Bioorg Med Chem 20: 784-8 (2012) Article DOI: 10.1016/j.bmc.2011.12.002 BindingDB Entry DOI: 10.7270/Q2F47PKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50361381 (CHEMBL1412710) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 assessed as dissociation constant of enzyme-substrate-inhibitor complex using Rh-EVNLDAEFK as substrate by Dixo... | Bioorg Med Chem 20: 784-8 (2012) Article DOI: 10.1016/j.bmc.2011.12.002 BindingDB Entry DOI: 10.7270/Q2F47PKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50296247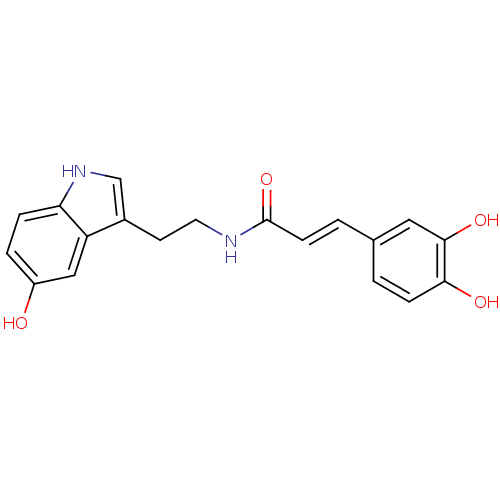 (3-(3,4-Dihydroxyphenyl)-N-[2-(5-hydroxyindol-3-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Uncompetitive inhibition of COX2 by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 22: 2494-6 (2012) Article DOI: 10.1016/j.bmcl.2012.02.002 BindingDB Entry DOI: 10.7270/Q28W3F9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50494895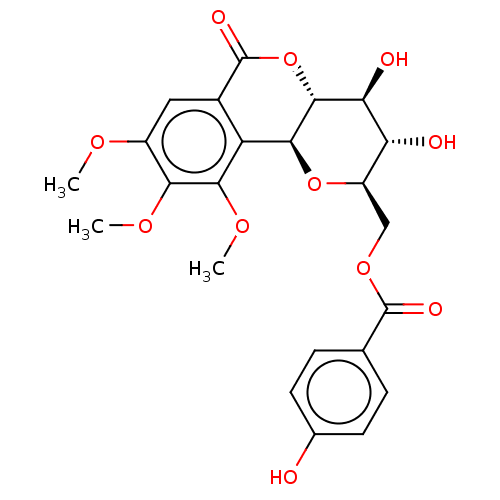 (CHEMBL3092599) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate by Dixon plot analysis | Bioorg Med Chem Lett 23: 6580-4 (2013) Article DOI: 10.1016/j.bmcl.2013.10.066 BindingDB Entry DOI: 10.7270/Q2QZ2DW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50361388 (CHEMBL1934195) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 assessed as dissociation constant of enzyme-inhibitor complex using Rh-EVNLDAEFK as substrate by Dixon and Corn... | Bioorg Med Chem 20: 784-8 (2012) Article DOI: 10.1016/j.bmc.2011.12.002 BindingDB Entry DOI: 10.7270/Q2F47PKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50494897 (CHEMBL3092604) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.07E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Uncompetitive inhibition of mushroom tyrosinase using L-tyrosine as substrate by Dixon/Cornish-Bowden plot analysis | Bioorg Med Chem Lett 23: 6580-4 (2013) Article DOI: 10.1016/j.bmcl.2013.10.066 BindingDB Entry DOI: 10.7270/Q2QZ2DW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50494895 (CHEMBL3092599) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.21E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Mixed inhibition of mushroom tyrosinase using L-tyrosine as substrate by Dixon/Cornish-Bowden plot analysis | Bioorg Med Chem Lett 23: 6580-4 (2013) Article DOI: 10.1016/j.bmcl.2013.10.066 BindingDB Entry DOI: 10.7270/Q2QZ2DW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50100436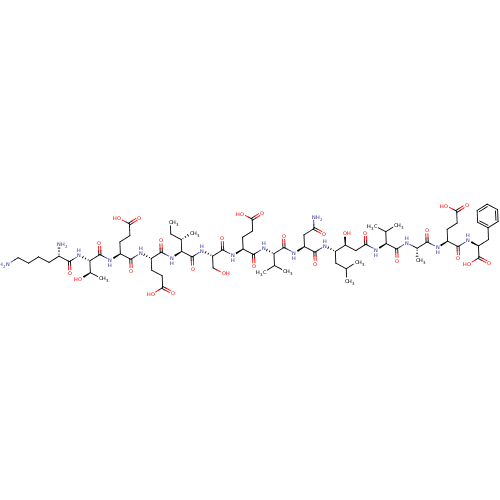 (CHEMBL412768 | NH2-Lys-Thr-Glu-Glu-Ile-Ser-Glu-Val...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 using Rh-EVNLDAEFK as substrate after 60 mins by fluorescence quenching assay | Bioorg Med Chem 20: 784-8 (2012) Article DOI: 10.1016/j.bmc.2011.12.002 BindingDB Entry DOI: 10.7270/Q2F47PKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of human erythrocytes acetylcholinesterase after 15 mins | J Nat Prod 74: 86-9 (2011) Article DOI: 10.1021/np100416v BindingDB Entry DOI: 10.7270/Q2154J2P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50341137 (CHEMBL1760549 | N-Isoferuloyl serotonin) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase after 25 mins by spectrophotometry | Bioorg Med Chem Lett 21: 1983-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.028 BindingDB Entry DOI: 10.7270/Q2T43TCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50296246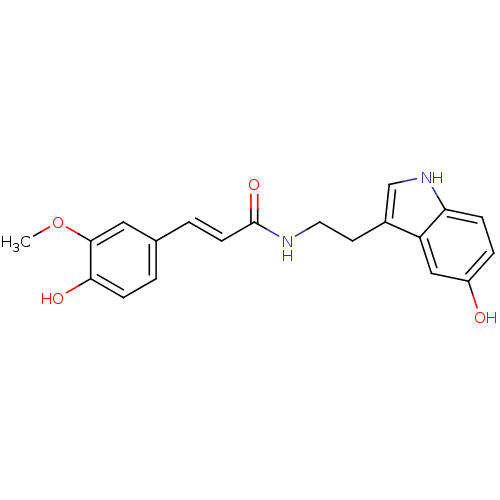 (CHEMBL564482 | N-Feruloylserotonin) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase after 25 mins by spectrophotometry | Bioorg Med Chem Lett 21: 1983-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.028 BindingDB Entry DOI: 10.7270/Q2T43TCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50341135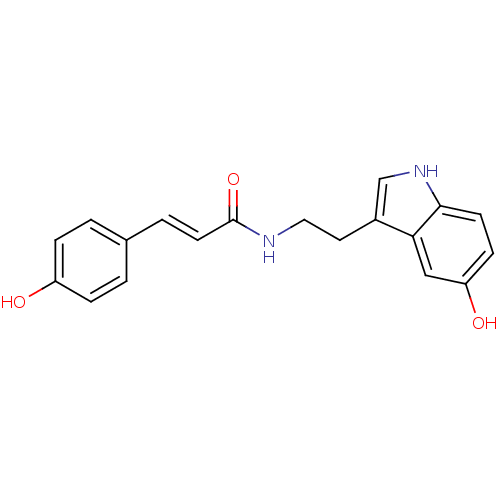 (CHEMBL1760547 | N-p-Coumaroyl serotonin) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase after 25 mins by spectrophotometry | Bioorg Med Chem Lett 21: 1983-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.028 BindingDB Entry DOI: 10.7270/Q2T43TCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50494892 (CHEMBL3092601) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins followed by enzyme addition measured after 20 mins by microp... | Bioorg Med Chem Lett 23: 6580-4 (2013) Article DOI: 10.1016/j.bmcl.2013.10.066 BindingDB Entry DOI: 10.7270/Q2QZ2DW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50361388 (CHEMBL1934195) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 using Rh-EVNLDAEFK as substrate after 60 mins by fluorescence quenching assay | Bioorg Med Chem 20: 784-8 (2012) Article DOI: 10.1016/j.bmc.2011.12.002 BindingDB Entry DOI: 10.7270/Q2F47PKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50361391 (CHEMBL1934197) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 using Rh-EVNLDAEFK as substrate after 60 mins by fluorescence quenching assay | Bioorg Med Chem 20: 784-8 (2012) Article DOI: 10.1016/j.bmc.2011.12.002 BindingDB Entry DOI: 10.7270/Q2F47PKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50308717 (6,7-Furano-8a-methoxy-5-prenyloxy hydrocoumaric ac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 after 60 mins by fluorescence assay | Bioorg Med Chem 18: 455-9 (2010) Article DOI: 10.1016/j.bmc.2009.10.004 BindingDB Entry DOI: 10.7270/Q2K64J6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50494902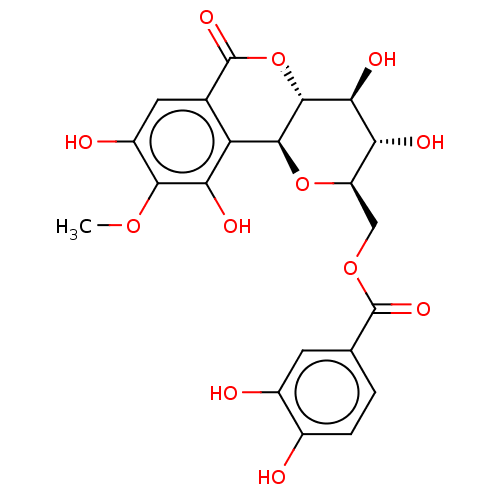 (CHEMBL3092597) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins followed by enzyme addition measured after 20 mins by microp... | Bioorg Med Chem Lett 23: 6580-4 (2013) Article DOI: 10.1016/j.bmcl.2013.10.066 BindingDB Entry DOI: 10.7270/Q2QZ2DW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50361381 (CHEMBL1412710) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 using Rh-EVNLDAEFK as substrate after 60 mins by fluorescence quenching assay | Bioorg Med Chem 20: 784-8 (2012) Article DOI: 10.1016/j.bmc.2011.12.002 BindingDB Entry DOI: 10.7270/Q2F47PKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50341136 (CHEMBL1760548 | N-p-Methoxy cinnamoyl serotonin) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase after 25 mins by spectrophotometry | Bioorg Med Chem Lett 21: 1983-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.028 BindingDB Entry DOI: 10.7270/Q2T43TCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM243068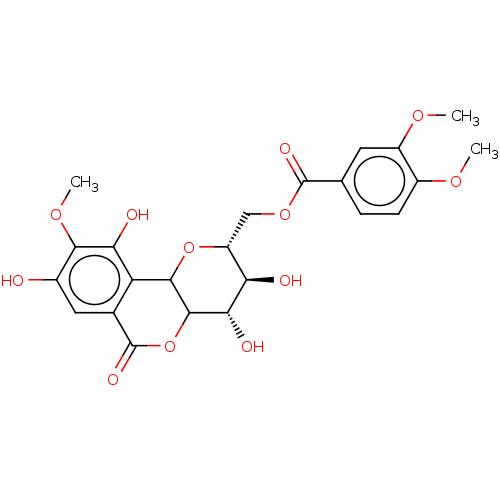 (11-O-(3',4'-dimethoxybenzoyl)-bergenin (6)) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.46E+4 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Kinki University | Assay Description α-glucosidase (25 μL, 0.2 U/mL), 25 μL of various concentrations of samples, and 175 μL of 50 mM sodium phosphate buffer (pH 7.0)... | J Enzyme Inhib Med Chem 28: 1162-70 (2013) Article DOI: 10.3109/14756366.2012.719503 BindingDB Entry DOI: 10.7270/Q22F7MCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50341142 ((E)-3-(4-Hydroxy-3,5-dimethoxy-phenyl)-acrylic aci...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of COX2 after 5 mins by spectrophotometric analysis | Bioorg Med Chem Lett 22: 2494-6 (2012) Article DOI: 10.1016/j.bmcl.2012.02.002 BindingDB Entry DOI: 10.7270/Q28W3F9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50308719 ((9-[(3-methyl-2-buten-1-yl)oxy]-7H-furo[3,2-g][1]b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 after 60 mins by fluorescence assay | Bioorg Med Chem 18: 455-9 (2010) Article DOI: 10.1016/j.bmc.2009.10.004 BindingDB Entry DOI: 10.7270/Q2K64J6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50494895 (CHEMBL3092599) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins followed by enzyme addition measured after 20 mins by microp... | Bioorg Med Chem Lett 23: 6580-4 (2013) Article DOI: 10.1016/j.bmcl.2013.10.066 BindingDB Entry DOI: 10.7270/Q2QZ2DW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50310823 (CHEMBL1078442 | bergamottin) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 using Rh-EVNLDAEFK as substrate after 60 mins by fluorescence quenching assay | Bioorg Med Chem 20: 784-8 (2012) Article DOI: 10.1016/j.bmc.2011.12.002 BindingDB Entry DOI: 10.7270/Q2F47PKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50494897 (CHEMBL3092604) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins followed by enzyme addition measured after 20 mins by microp... | Bioorg Med Chem Lett 23: 6580-4 (2013) Article DOI: 10.1016/j.bmcl.2013.10.066 BindingDB Entry DOI: 10.7270/Q2QZ2DW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50341142 ((E)-3-(4-Hydroxy-3,5-dimethoxy-phenyl)-acrylic aci...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of COX1 after 5 mins by spectrophotometric analysis | Bioorg Med Chem Lett 22: 2494-6 (2012) Article DOI: 10.1016/j.bmcl.2012.02.002 BindingDB Entry DOI: 10.7270/Q28W3F9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50341134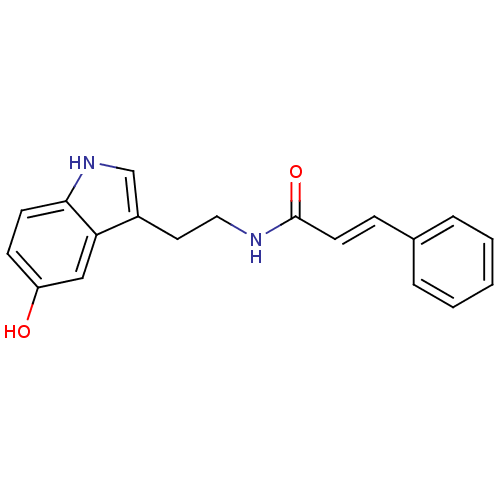 (CHEMBL1760546 | N-Cinnamoyl serotonin) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase after 25 mins by spectrophotometry | Bioorg Med Chem Lett 21: 1983-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.028 BindingDB Entry DOI: 10.7270/Q2T43TCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50296247 (3-(3,4-Dihydroxyphenyl)-N-[2-(5-hydroxyindol-3-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of COX2 after 5 mins by spectrophotometric analysis | Bioorg Med Chem Lett 22: 2494-6 (2012) Article DOI: 10.1016/j.bmcl.2012.02.002 BindingDB Entry DOI: 10.7270/Q28W3F9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50031467 (5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins followed by enzyme addition measured after 20 mins by microp... | Bioorg Med Chem Lett 23: 6580-4 (2013) Article DOI: 10.1016/j.bmcl.2013.10.066 BindingDB Entry DOI: 10.7270/Q2QZ2DW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50031467 (5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase after 25 mins by spectrophotometry | Bioorg Med Chem Lett 21: 1983-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.028 BindingDB Entry DOI: 10.7270/Q2T43TCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50335906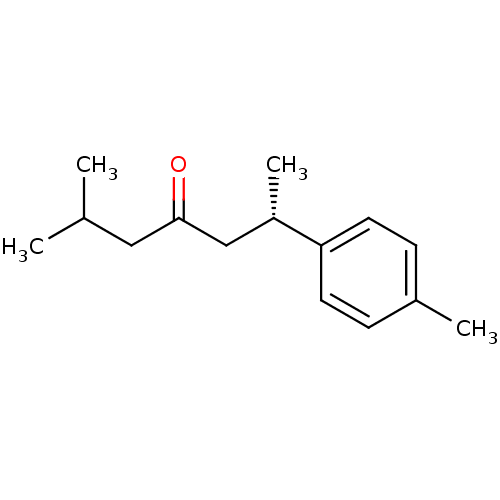 ((+)-(S)-dihydro-ar-turmerone | CHEMBL1668334) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of human erythrocytes acetylcholinesterase after 15 mins | J Nat Prod 74: 86-9 (2011) Article DOI: 10.1021/np100416v BindingDB Entry DOI: 10.7270/Q2154J2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50308719 ((9-[(3-methyl-2-buten-1-yl)oxy]-7H-furo[3,2-g][1]b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 using Rh-EVNLDAEFK as substrate after 60 mins by fluorescence quenching assay | Bioorg Med Chem 20: 784-8 (2012) Article DOI: 10.1016/j.bmc.2011.12.002 BindingDB Entry DOI: 10.7270/Q2F47PKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50494894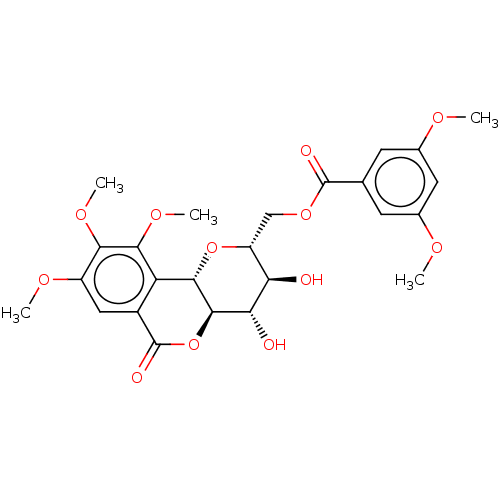 (CHEMBL3092605) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins followed by enzyme addition measured after 20 mins by microp... | Bioorg Med Chem Lett 23: 6580-4 (2013) Article DOI: 10.1016/j.bmcl.2013.10.066 BindingDB Entry DOI: 10.7270/Q2QZ2DW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM26194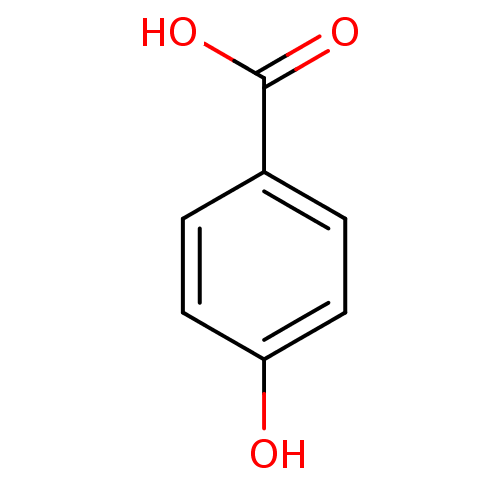 (4-Hydroxybenzoate, III | 4-hydroxybenzoic acid | C...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins followed by enzyme addition measured after 20 mins by microp... | Bioorg Med Chem Lett 23: 6580-4 (2013) Article DOI: 10.1016/j.bmcl.2013.10.066 BindingDB Entry DOI: 10.7270/Q2QZ2DW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50337364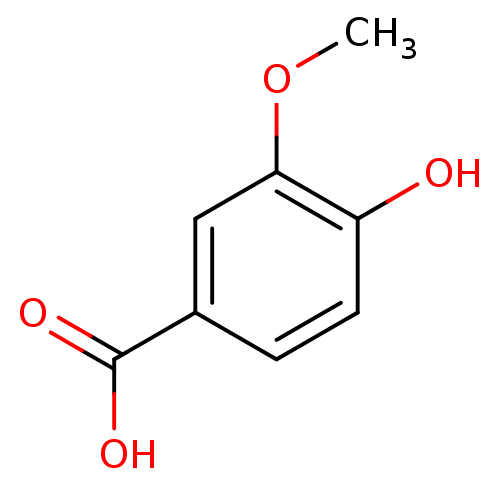 (3-methoxy-4-hydroxybenzoic acid | 4-Hydroxy-3-meth...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins followed by enzyme addition measured after 20 mins by microp... | Bioorg Med Chem Lett 23: 6580-4 (2013) Article DOI: 10.1016/j.bmcl.2013.10.066 BindingDB Entry DOI: 10.7270/Q2QZ2DW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM197302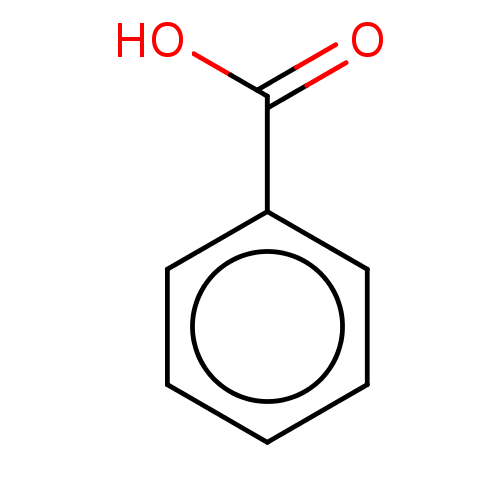 (Benzoic acid | SAMPL4, O1) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins followed by enzyme addition measured after 20 mins by microp... | Bioorg Med Chem Lett 23: 6580-4 (2013) Article DOI: 10.1016/j.bmcl.2013.10.066 BindingDB Entry DOI: 10.7270/Q2QZ2DW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50336487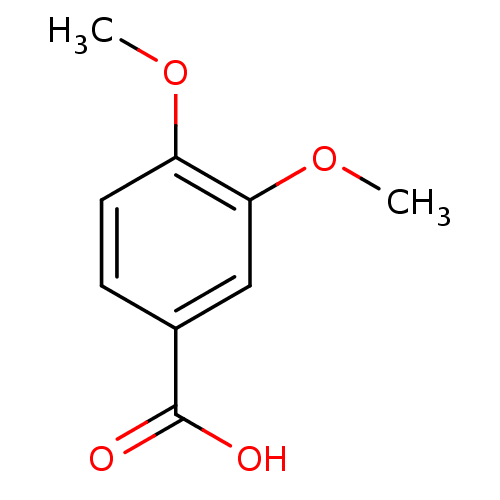 (3,4-Dimethoxy-benzoic acid | 3,4-dimethoxybenzoic ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins followed by enzyme addition measured after 20 mins by microp... | Bioorg Med Chem Lett 23: 6580-4 (2013) Article DOI: 10.1016/j.bmcl.2013.10.066 BindingDB Entry DOI: 10.7270/Q2QZ2DW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50494901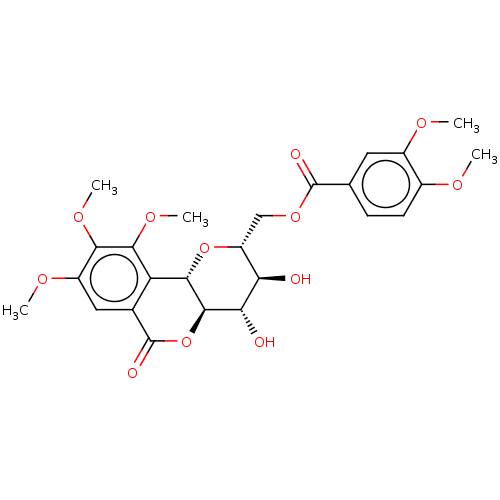 (CHEMBL3092602) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins followed by enzyme addition measured after 20 mins by microp... | Bioorg Med Chem Lett 23: 6580-4 (2013) Article DOI: 10.1016/j.bmcl.2013.10.066 BindingDB Entry DOI: 10.7270/Q2QZ2DW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50494900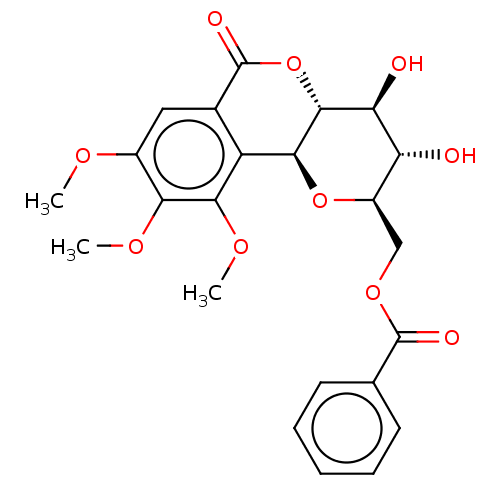 (CHEMBL3092598) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins followed by enzyme addition measured after 20 mins by microp... | Bioorg Med Chem Lett 23: 6580-4 (2013) Article DOI: 10.1016/j.bmcl.2013.10.066 BindingDB Entry DOI: 10.7270/Q2QZ2DW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50494899 (CHEMBL3092606) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins followed by enzyme addition measured after 20 mins by microp... | Bioorg Med Chem Lett 23: 6580-4 (2013) Article DOI: 10.1016/j.bmcl.2013.10.066 BindingDB Entry DOI: 10.7270/Q2QZ2DW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM243072 (Anisic acid) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins followed by enzyme addition measured after 20 mins by microp... | Bioorg Med Chem Lett 23: 6580-4 (2013) Article DOI: 10.1016/j.bmcl.2013.10.066 BindingDB Entry DOI: 10.7270/Q2QZ2DW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50100861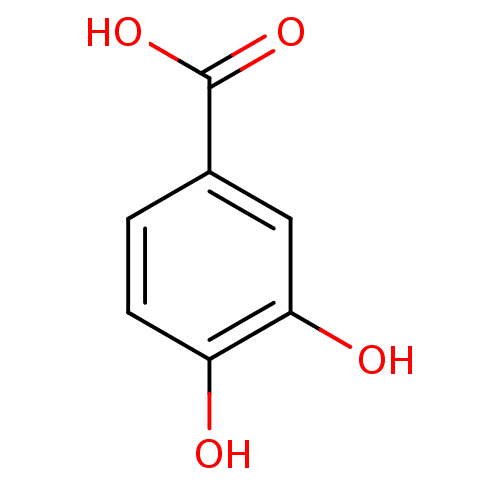 (3,4-Dihydroxybenzoate, VIII | 3,4-dihydroxybenzoic...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins followed by enzyme addition measured after 20 mins by microp... | Bioorg Med Chem Lett 23: 6580-4 (2013) Article DOI: 10.1016/j.bmcl.2013.10.066 BindingDB Entry DOI: 10.7270/Q2QZ2DW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 143 total ) | Next | Last >> |