 Found 2475 hits with Last Name = 'martin' and Initial = 'n'
Found 2475 hits with Last Name = 'martin' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50041958
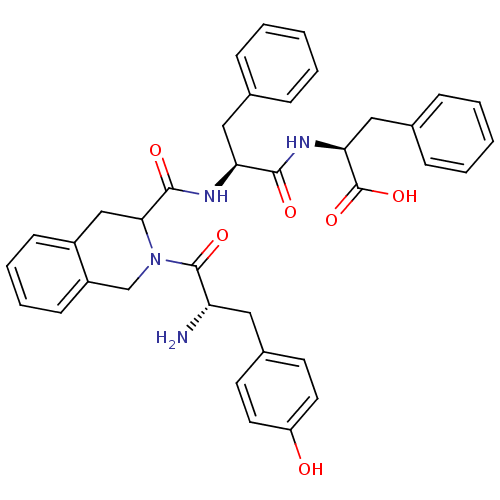
(2-(1-{2-[2-amino-3-(4-hydroxyphenyl)propanoyl]-1,2...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1Cc2ccccc2CC1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C37H38N4O6/c38-30(19-26-15-17-29(42)18-16-26)36(45)41-23-28-14-8-7-13-27(28)22-33(41)35(44)39-31(20-24-9-3-1-4-10-24)34(43)40-32(37(46)47)21-25-11-5-2-6-12-25/h1-18,30-33,42H,19-23,38H2,(H,39,44)(H,40,43)(H,46,47)/t30-,31-,32-,33?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 301: 661-71 (2002)
Article DOI: 10.1124/jpet.301.2.661
BindingDB Entry DOI: 10.7270/Q27D2SQM |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50456223
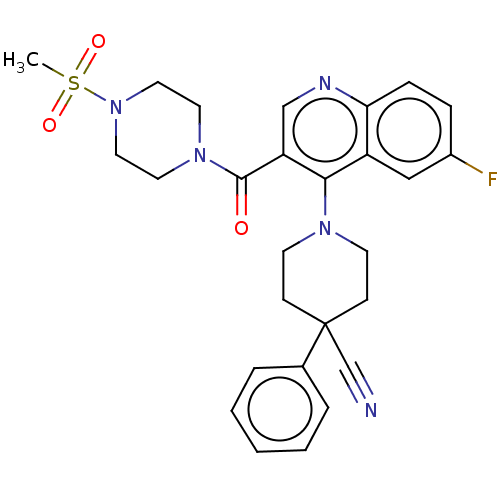
(CHEMBL4206892)Show SMILES CS(=O)(=O)N1CCN(CC1)C(=O)c1cnc2ccc(F)cc2c1N1CCC(CC1)(C#N)c1ccccc1 Show InChI InChI=1S/C27H28FN5O3S/c1-37(35,36)33-15-13-32(14-16-33)26(34)23-18-30-24-8-7-21(28)17-22(24)25(23)31-11-9-27(19-29,10-12-31)20-5-3-2-4-6-20/h2-8,17-18H,9-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A1 using propionaldehyde as substrate and varied concentration of NAD+ as cofactor preincubated for 15 mins followed by subs... |
J Med Chem 61: 4883-4903 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00270
BindingDB Entry DOI: 10.7270/Q2SB48BH |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50041958

(2-(1-{2-[2-amino-3-(4-hydroxyphenyl)propanoyl]-1,2...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1Cc2ccccc2CC1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C37H38N4O6/c38-30(19-26-15-17-29(42)18-16-26)36(45)41-23-28-14-8-7-13-27(28)22-33(41)35(44)39-31(20-24-9-3-1-4-10-24)34(43)40-32(37(46)47)21-25-11-5-2-6-12-25/h1-18,30-33,42H,19-23,38H2,(H,39,44)(H,40,43)(H,46,47)/t30-,31-,32-,33?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 301: 661-71 (2002)
Article DOI: 10.1124/jpet.301.2.661
BindingDB Entry DOI: 10.7270/Q27D2SQM |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50327238
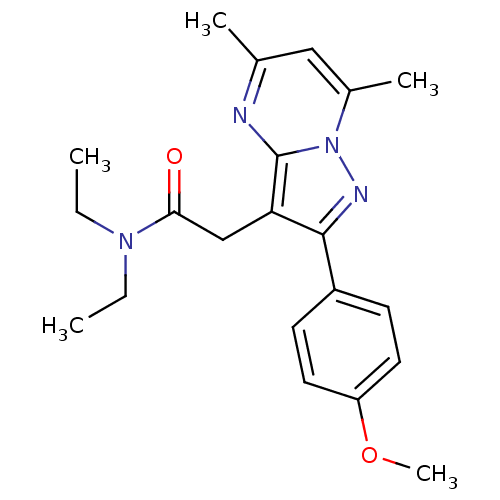
(2-[2-(4'-Methoxyphenyl)-5,7-dimethylpyrazolo[1,5-a...)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)cc(C)nc12)-c1ccc(OC)cc1 Show InChI InChI=1S/C21H26N4O2/c1-6-24(7-2)19(26)13-18-20(16-8-10-17(27-5)11-9-16)23-25-15(4)12-14(3)22-21(18)25/h8-12H,6-7,13H2,1-5H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New South Wales
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in human T98G cell membranes incubated for 90 mins by competition radioligand binding assay |
J Med Chem 58: 8743-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01288
BindingDB Entry DOI: 10.7270/Q2Q24222 |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50456222
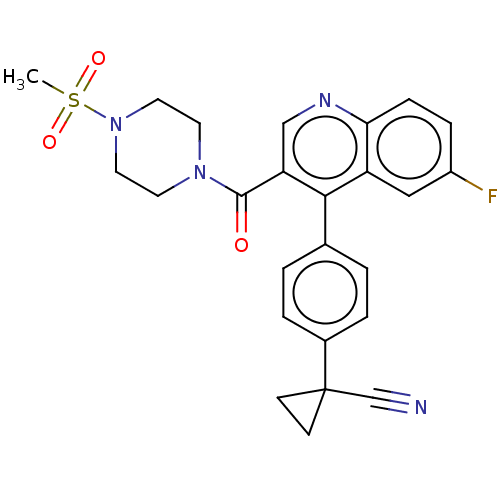
(CHEMBL4206272)Show SMILES CS(=O)(=O)N1CCN(CC1)C(=O)c1cnc2ccc(F)cc2c1-c1ccc(cc1)C1(CC1)C#N Show InChI InChI=1S/C25H23FN4O3S/c1-34(32,33)30-12-10-29(11-13-30)24(31)21-15-28-22-7-6-19(26)14-20(22)23(21)17-2-4-18(5-3-17)25(16-27)8-9-25/h2-7,14-15H,8-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A1 using propionaldehyde as substrate and varied concentration of NAD+ as cofactor preincubated for 15 mins followed by subs... |
J Med Chem 61: 4883-4903 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00270
BindingDB Entry DOI: 10.7270/Q2SB48BH |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50327238

(2-[2-(4'-Methoxyphenyl)-5,7-dimethylpyrazolo[1,5-a...)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)cc(C)nc12)-c1ccc(OC)cc1 Show InChI InChI=1S/C21H26N4O2/c1-6-24(7-2)19(26)13-18-20(16-8-10-17(27-5)11-9-16)23-25-15(4)12-14(3)22-21(18)25/h8-12H,6-7,13H2,1-5H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New South Wales
Curated by ChEMBL
| Assay Description
Inhibition constant against Adenosine A2a receptor |
J Med Chem 58: 8743-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01288
BindingDB Entry DOI: 10.7270/Q2Q24222 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM21008
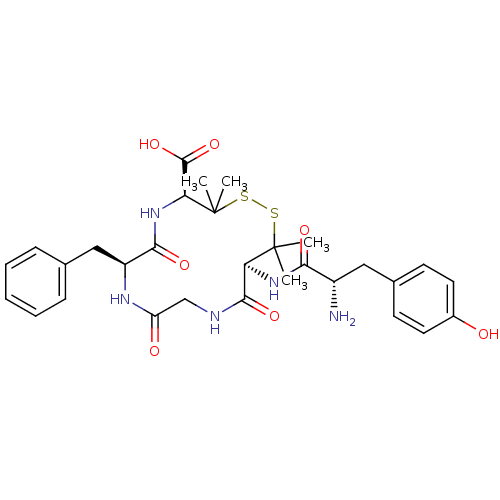
((4S,7S,13S)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H]1C(O)=O Show InChI InChI=1S/C30H39N5O7S2/c1-29(2)23(34-25(38)20(31)14-18-10-12-19(36)13-11-18)27(40)32-16-22(37)33-21(15-17-8-6-5-7-9-17)26(39)35-24(28(41)42)30(3,4)44-43-29/h5-13,20-21,23-24,36H,14-16,31H2,1-4H3,(H,32,40)(H,33,37)(H,34,38)(H,35,39)(H,41,42)/t20-,21-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 301: 661-71 (2002)
Article DOI: 10.1124/jpet.301.2.661
BindingDB Entry DOI: 10.7270/Q27D2SQM |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50456222

(CHEMBL4206272)Show SMILES CS(=O)(=O)N1CCN(CC1)C(=O)c1cnc2ccc(F)cc2c1-c1ccc(cc1)C1(CC1)C#N Show InChI InChI=1S/C25H23FN4O3S/c1-34(32,33)30-12-10-29(11-13-30)24(31)21-15-28-22-7-6-19(26)14-20(22)23(21)17-2-4-18(5-3-17)25(16-27)8-9-25/h2-7,14-15H,8-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A1 using NAD+ as cofactor and varied concentration of propionaldehyde as substrate preincubated for 15 mins followed by subs... |
J Med Chem 61: 4883-4903 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00270
BindingDB Entry DOI: 10.7270/Q2SB48BH |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50456223

(CHEMBL4206892)Show SMILES CS(=O)(=O)N1CCN(CC1)C(=O)c1cnc2ccc(F)cc2c1N1CCC(CC1)(C#N)c1ccccc1 Show InChI InChI=1S/C27H28FN5O3S/c1-37(35,36)33-15-13-32(14-16-33)26(34)23-18-30-24-8-7-21(28)17-22(24)25(23)31-11-9-27(19-29,10-12-31)20-5-3-2-4-6-20/h2-8,17-18H,9-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A1 using NAD+ as cofactor and varied concentration of propionaldehyde as substrate preincubated for 15 mins followed by subs... |
J Med Chem 61: 4883-4903 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00270
BindingDB Entry DOI: 10.7270/Q2SB48BH |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50131400

(CHEMBL3634876)Show SMILES CCN(CC)C(=O)Cn1c(cc2c(C)cc(C)cc12)-c1ccc(OC)cc1 Show InChI InChI=1S/C23H28N2O2/c1-6-24(7-2)23(26)15-25-21(18-8-10-19(27-5)11-9-18)14-20-17(4)12-16(3)13-22(20)25/h8-14H,6-7,15H2,1-5H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New South Wales
Curated by ChEMBL
| Assay Description
Inhibition constant against Adenosine A2a receptor |
J Med Chem 58: 8743-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01288
BindingDB Entry DOI: 10.7270/Q2Q24222 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50131380
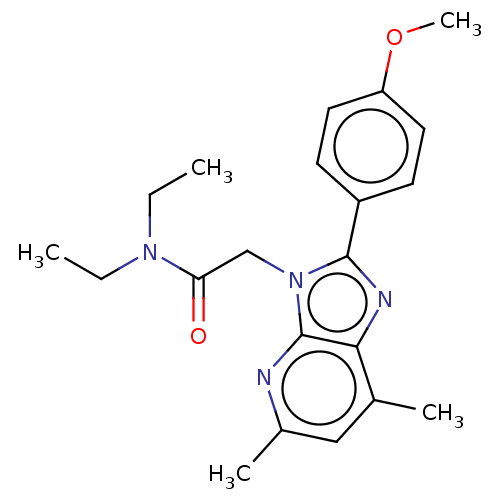
(CHEMBL3634878)Show SMILES CCN(CC)C(=O)Cn1c(nc2c(C)cc(C)nc12)-c1ccc(OC)cc1 Show InChI InChI=1S/C21H26N4O2/c1-6-24(7-2)18(26)13-25-20(16-8-10-17(27-5)11-9-16)23-19-14(3)12-15(4)22-21(19)25/h8-12H,6-7,13H2,1-5H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New South Wales
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in human T98G cell membranes incubated for 90 mins by competition radioligand binding assay |
J Med Chem 58: 8743-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01288
BindingDB Entry DOI: 10.7270/Q2Q24222 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50047420
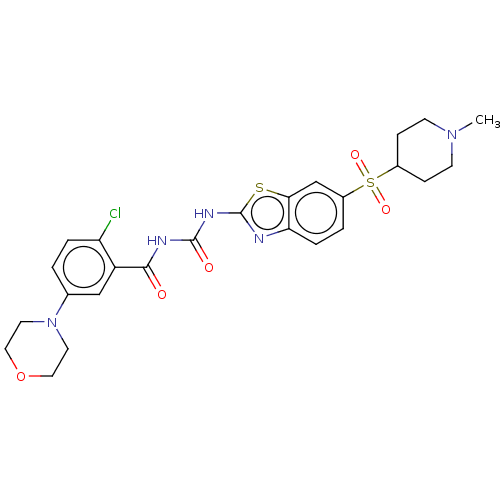
(CHEMBL3319405)Show SMILES CN1CCC(CC1)S(=O)(=O)c1ccc2nc(NC(=O)NC(=O)c3cc(ccc3Cl)N3CCOCC3)sc2c1 Show InChI InChI=1S/C25H28ClN5O5S2/c1-30-8-6-17(7-9-30)38(34,35)18-3-5-21-22(15-18)37-25(27-21)29-24(33)28-23(32)19-14-16(2-4-20(19)26)31-10-12-36-13-11-31/h2-5,14-15,17H,6-13H2,1H3,(H2,27,28,29,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of alpha4 nAChR (unknown origin) |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM15234
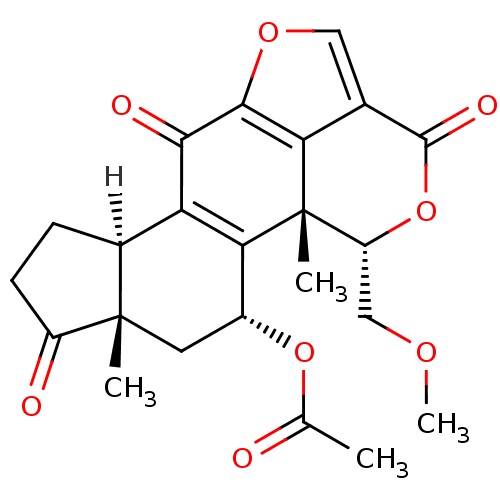
((1R,3R,5S,9R,18S)-18-(methoxymethyl)-1,5-dimethyl-...)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)C[C@@H](OC(C)=O)C1=C2C(=O)c2occ3c2[C@]1(C)[C@@H](COC)OC3=O |r,c:15| Show InChI InChI=1S/C23H24O8/c1-10(24)30-13-7-22(2)12(5-6-14(22)25)16-18(13)23(3)15(9-28-4)31-21(27)11-8-29-20(17(11)23)19(16)26/h8,12-13,15H,5-7,9H2,1-4H3/t12-,13+,15+,22-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University
Curated by ChEMBL
| Assay Description
Binding affinity for DNA dependent protein kinase isolated from HeLa cells; Range is 20-120 |
J Med Chem 48: 569-85 (2005)
Article DOI: 10.1021/jm049526a
BindingDB Entry DOI: 10.7270/Q2BK1BV9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM15234

((1R,3R,5S,9R,18S)-18-(methoxymethyl)-1,5-dimethyl-...)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)C[C@@H](OC(C)=O)C1=C2C(=O)c2occ3c2[C@]1(C)[C@@H](COC)OC3=O |r,c:15| Show InChI InChI=1S/C23H24O8/c1-10(24)30-13-7-22(2)12(5-6-14(22)25)16-18(13)23(3)15(9-28-4)31-21(27)11-8-29-20(17(11)23)19(16)26/h8,12-13,15H,5-7,9H2,1-4H3/t12-,13+,15+,22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant p110 alpha Phosphatidylinositol 3-kinase |
J Med Chem 48: 569-85 (2005)
Article DOI: 10.1021/jm049526a
BindingDB Entry DOI: 10.7270/Q2BK1BV9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Translocator protein
(Homo sapiens (Human)) | BDBM50131380

(CHEMBL3634878)Show SMILES CCN(CC)C(=O)Cn1c(nc2c(C)cc(C)nc12)-c1ccc(OC)cc1 Show InChI InChI=1S/C21H26N4O2/c1-6-24(7-2)18(26)13-25-20(16-8-10-17(27-5)11-9-16)23-19-14(3)12-15(4)22-21(19)25/h8-12H,6-7,13H2,1-5H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 144 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New South Wales
Curated by ChEMBL
| Assay Description
Inhibition constant against Adenosine A2a receptor |
J Med Chem 58: 8743-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01288
BindingDB Entry DOI: 10.7270/Q2Q24222 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50131399
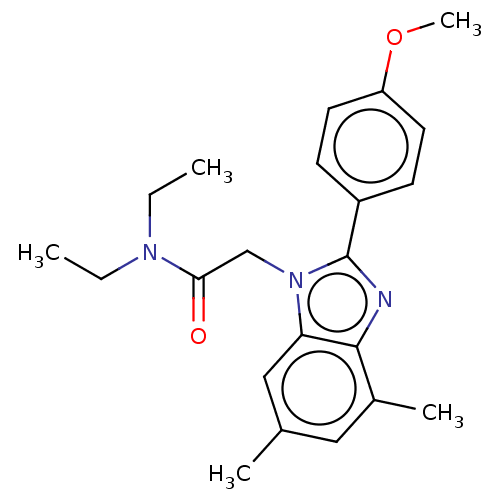
(CHEMBL3634877)Show SMILES CCN(CC)C(=O)Cn1c(nc2c(C)cc(C)cc12)-c1ccc(OC)cc1 Show InChI InChI=1S/C22H27N3O2/c1-6-24(7-2)20(26)14-25-19-13-15(3)12-16(4)21(19)23-22(25)17-8-10-18(27-5)11-9-17/h8-13H,6-7,14H2,1-5H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New South Wales
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in human T98G cell membranes incubated for 90 mins by competition radioligand binding assay |
J Med Chem 58: 8743-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01288
BindingDB Entry DOI: 10.7270/Q2Q24222 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50047420

(CHEMBL3319405)Show SMILES CN1CCC(CC1)S(=O)(=O)c1ccc2nc(NC(=O)NC(=O)c3cc(ccc3Cl)N3CCOCC3)sc2c1 Show InChI InChI=1S/C25H28ClN5O5S2/c1-30-8-6-17(7-9-30)38(34,35)18-3-5-21-22(15-18)37-25(27-21)29-24(33)28-23(32)19-14-16(2-4-20(19)26)31-10-12-36-13-11-31/h2-5,14-15,17H,6-13H2,1H3,(H2,27,28,29,32,33) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of DAT (unknown origin) |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM85944
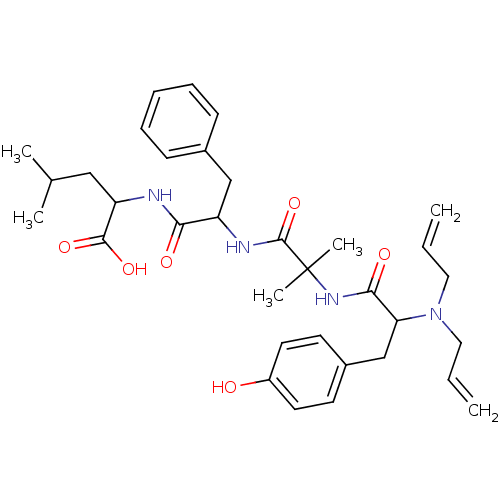
(CAS_107691 | ICI-174864 | NSC_107691)Show SMILES CC(C)CC(NC(=O)C(Cc1ccccc1)NC(=O)C(C)(C)NC(=O)C(Cc1ccc(O)cc1)N(CC=C)CC=C)C(O)=O Show InChI InChI=1S/C34H46N4O6/c1-7-18-38(19-8-2)29(22-25-14-16-26(39)17-15-25)31(41)37-34(5,6)33(44)36-27(21-24-12-10-9-11-13-24)30(40)35-28(32(42)43)20-23(3)4/h7-17,23,27-29,39H,1-2,18-22H2,3-6H3,(H,35,40)(H,36,44)(H,37,41)(H,42,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 304 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 301: 661-71 (2002)
Article DOI: 10.1124/jpet.301.2.661
BindingDB Entry DOI: 10.7270/Q27D2SQM |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50131399

(CHEMBL3634877)Show SMILES CCN(CC)C(=O)Cn1c(nc2c(C)cc(C)cc12)-c1ccc(OC)cc1 Show InChI InChI=1S/C22H27N3O2/c1-6-24(7-2)20(26)14-25-19-13-15(3)12-16(4)21(19)23-22(25)17-8-10-18(27-5)11-9-17/h8-13H,6-7,14H2,1-5H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New South Wales
Curated by ChEMBL
| Assay Description
Inhibition constant against Adenosine A2a receptor |
J Med Chem 58: 8743-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01288
BindingDB Entry DOI: 10.7270/Q2Q24222 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50131400

(CHEMBL3634876)Show SMILES CCN(CC)C(=O)Cn1c(cc2c(C)cc(C)cc12)-c1ccc(OC)cc1 Show InChI InChI=1S/C23H28N2O2/c1-6-24(7-2)23(26)15-25-21(18-8-10-19(27-5)11-9-18)14-20-17(4)12-16(3)13-22(20)25/h8-14H,6-7,15H2,1-5H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 806 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New South Wales
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in human T98G cell membranes incubated for 90 mins by competition radioligand binding assay |
J Med Chem 58: 8743-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01288
BindingDB Entry DOI: 10.7270/Q2Q24222 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50374677

(CHEMBL405231)Show InChI InChI=1S/C6H15N5O2/c7-4(5(12)13)2-1-3-10-6(8)11-9/h4H,1-3,7,9H2,(H,12,13)(H3,8,10,11)/t4-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of full length iNOS by high-throughput oxymyoglobin assay |
J Med Chem 51: 924-31 (2008)
Article DOI: 10.1021/jm701119v
BindingDB Entry DOI: 10.7270/Q2639QMP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM12915

(2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one |...)Show InChI InChI=1S/C19H17NO3/c21-17-13-18(20-9-11-22-12-10-20)23-19-15(7-4-8-16(17)19)14-5-2-1-3-6-14/h1-8,13H,9-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University
Curated by ChEMBL
| Assay Description
Binding affinity for Phosphatidylinositol-3-kinase isolated from HeLa cells; Range is 20-120 |
J Med Chem 48: 569-85 (2005)
Article DOI: 10.1021/jm049526a
BindingDB Entry DOI: 10.7270/Q2BK1BV9 |
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM12915

(2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one |...)Show InChI InChI=1S/C19H17NO3/c21-17-13-18(20-9-11-22-12-10-20)23-19-15(7-4-8-16(17)19)14-5-2-1-3-6-14/h1-8,13H,9-12H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Newcastle
Curated by ChEMBL
| Assay Description
Affinity for DNA-dependent protein kinase(DNA-PK) from HeLa cell extract |
Bioorg Med Chem Lett 13: 3083-6 (2003)
BindingDB Entry DOI: 10.7270/Q2WS8SP4 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50131379
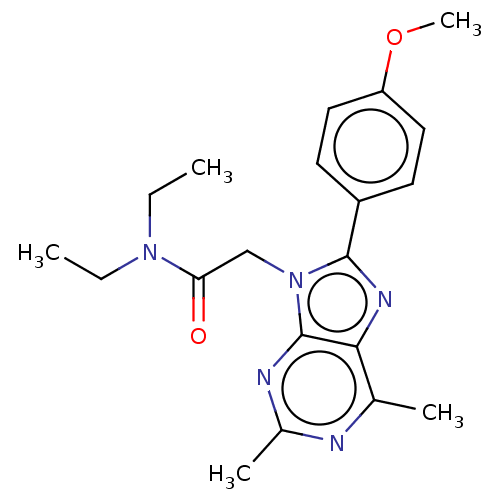
(CHEMBL3634879)Show SMILES CCN(CC)C(=O)Cn1c(nc2c(C)nc(C)nc12)-c1ccc(OC)cc1 Show InChI InChI=1S/C20H25N5O2/c1-6-24(7-2)17(26)12-25-19(15-8-10-16(27-5)11-9-15)23-18-13(3)21-14(4)22-20(18)25/h8-11H,6-7,12H2,1-5H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New South Wales
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in human T98G cell membranes incubated for 90 mins by competition radioligand binding assay |
J Med Chem 58: 8743-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01288
BindingDB Entry DOI: 10.7270/Q2Q24222 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50131379

(CHEMBL3634879)Show SMILES CCN(CC)C(=O)Cn1c(nc2c(C)nc(C)nc12)-c1ccc(OC)cc1 Show InChI InChI=1S/C20H25N5O2/c1-6-24(7-2)17(26)12-25-19(15-8-10-16(27-5)11-9-15)23-18-13(3)21-14(4)22-20(18)25/h8-11H,6-7,12H2,1-5H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New South Wales
Curated by ChEMBL
| Assay Description
Inhibition constant against Adenosine A2a receptor |
J Med Chem 58: 8743-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01288
BindingDB Entry DOI: 10.7270/Q2Q24222 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50374675

(CHEMBL272851)Show InChI InChI=1S/C8H16F3N5O2/c9-8(10,11)4-16(14)7(13)15-3-1-2-5(12)6(17)18/h5H,1-4,12,14H2,(H2,13,15)(H,17,18)/t5-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of full length iNOS by high-throughput oxymyoglobin assay |
J Med Chem 51: 924-31 (2008)
Article DOI: 10.1021/jm701119v
BindingDB Entry DOI: 10.7270/Q2639QMP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50374676
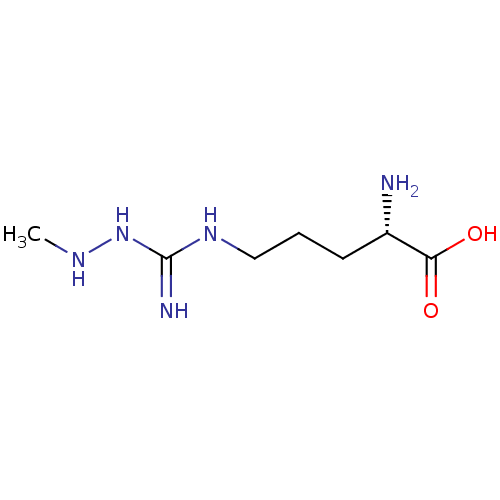
(CHEMBL272814)Show InChI InChI=1S/C7H17N5O2/c1-10-12-7(9)11-4-2-3-5(8)6(13)14/h5,10H,2-4,8H2,1H3,(H,13,14)(H3,9,11,12)/t5-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of full length iNOS by high-throughput oxymyoglobin assay |
J Med Chem 51: 924-31 (2008)
Article DOI: 10.1021/jm701119v
BindingDB Entry DOI: 10.7270/Q2639QMP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50374674
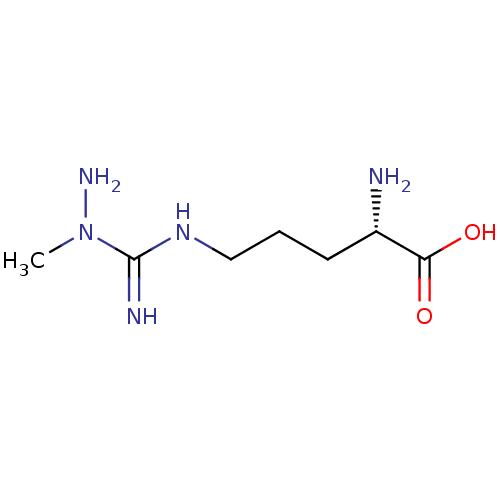
(CHEMBL408756)Show InChI InChI=1S/C7H17N5O2/c1-12(10)7(9)11-4-2-3-5(8)6(13)14/h5H,2-4,8,10H2,1H3,(H2,9,11)(H,13,14)/t5-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of full length iNOS by high-throughput oxymyoglobin assay |
J Med Chem 51: 924-31 (2008)
Article DOI: 10.1021/jm701119v
BindingDB Entry DOI: 10.7270/Q2639QMP |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM13016

(1,2,3,4-tetrahydroisoquinoline | CHEMBL14346 | THI...)Show InChI InChI=1S/C9H11N/c1-2-4-9-7-10-6-5-8(9)3-1/h1-4,10H,5-7H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.70E+7 | -7.88 | n/a | n/a | n/a | n/a | n/a | 8.6 | 37 |
Utrecht University
| Assay Description
Enzyme activity assays were performed as previously described with NNMT (16.25 ug/mL, 550 nM) in 50 mM Tris buffer (pH 8.6) containing 1 mM DTT (all ... |
Biochemistry 55: 5307-15 (2016)
Article DOI: 10.1021/acs.biochem.6b00733
BindingDB Entry DOI: 10.7270/Q2H130TH |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM60920
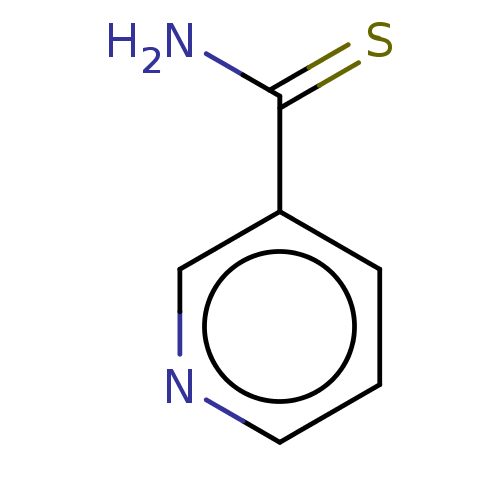
(thionicotinamide)Show InChI InChI=1S/C6H6N2S/c7-6(9)5-2-1-3-8-4-5/h1-4H,(H2,7,9) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60E+7 | -7.01 | n/a | n/a | n/a | n/a | n/a | 8.6 | 37 |
Utrecht University
| Assay Description
Enzyme activity assays were performed as previously described with NNMT (16.25 ug/mL, 550 nM) in 50 mM Tris buffer (pH 8.6) containing 1 mM DTT (all ... |
Biochemistry 55: 5307-15 (2016)
Article DOI: 10.1021/acs.biochem.6b00733
BindingDB Entry DOI: 10.7270/Q2H130TH |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM60924
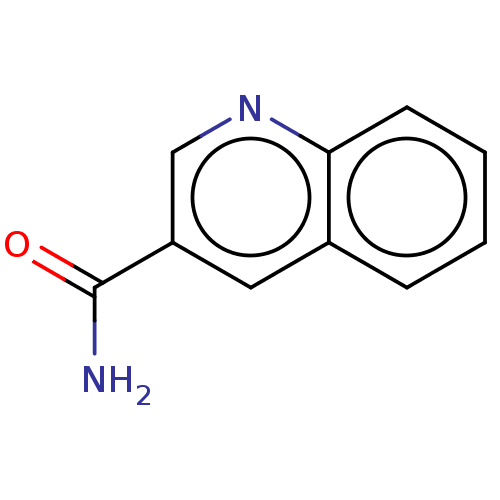
(quinoline 3-carboxamide)Show InChI InChI=1S/C10H8N2O/c11-10(13)8-5-7-3-1-2-4-9(7)12-6-8/h1-6H,(H2,11,13) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.70E+7 | -6.02 | n/a | n/a | n/a | n/a | n/a | 8.6 | 37 |
Utrecht University
| Assay Description
Enzyme activity assays were performed as previously described with NNMT (16.25 ug/mL, 550 nM) in 50 mM Tris buffer (pH 8.6) containing 1 mM DTT (all ... |
Biochemistry 55: 5307-15 (2016)
Article DOI: 10.1021/acs.biochem.6b00733
BindingDB Entry DOI: 10.7270/Q2H130TH |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50047015

(CHEMBL14474 | Chinolin | benzo[b]pyridine | quinol...)Show InChI InChI=1S/C9H7N/c1-2-6-9-8(4-1)5-3-7-10-9/h1-7H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.02E+8 | -5.89 | n/a | n/a | n/a | n/a | n/a | 8.6 | 37 |
Utrecht University
| Assay Description
Enzyme activity assays were performed as previously described with NNMT (16.25 ug/mL, 550 nM) in 50 mM Tris buffer (pH 8.6) containing 1 mM DTT (all ... |
Biochemistry 55: 5307-15 (2016)
Article DOI: 10.1021/acs.biochem.6b00733
BindingDB Entry DOI: 10.7270/Q2H130TH |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM60921
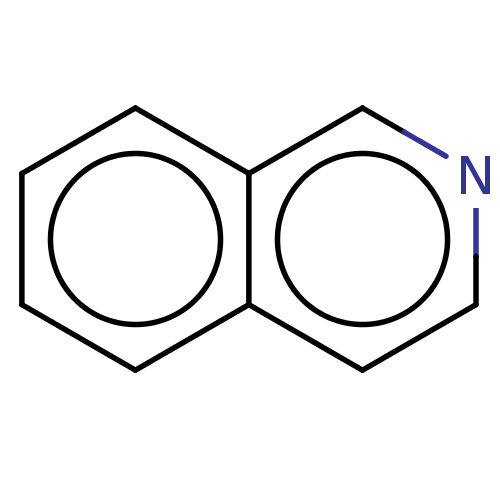
(isoquinoline)Show InChI InChI=1S/C9H7N/c1-2-4-9-7-10-6-5-8(9)3-1/h1-7H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2.02E+8 | -4.12 | n/a | n/a | n/a | n/a | n/a | 8.6 | 37 |
Utrecht University
| Assay Description
Enzyme activity assays were performed as previously described with NNMT (16.25 ug/mL, 550 nM) in 50 mM Tris buffer (pH 8.6) containing 1 mM DTT (all ... |
Biochemistry 55: 5307-15 (2016)
Article DOI: 10.1021/acs.biochem.6b00733
BindingDB Entry DOI: 10.7270/Q2H130TH |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047485
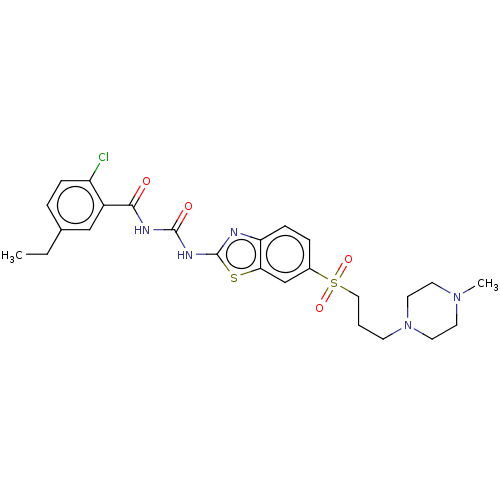
(CHEMBL3319217)Show SMILES CCc1ccc(Cl)c(c1)C(=O)NC(=O)Nc1nc2ccc(cc2s1)S(=O)(=O)CCCN1CCN(C)CC1 Show InChI InChI=1S/C25H30ClN5O4S2/c1-3-17-5-7-20(26)19(15-17)23(32)28-24(33)29-25-27-21-8-6-18(16-22(21)36-25)37(34,35)14-4-9-31-12-10-30(2)11-13-31/h5-8,15-16H,3-4,9-14H2,1-2H3,(H2,27,28,29,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50348452
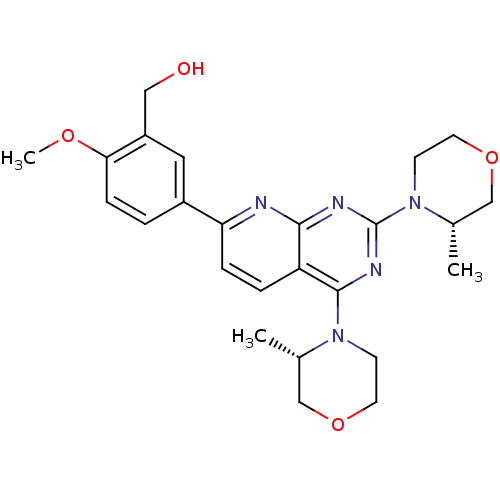
(AZD-8055 | CHEMBL1801204 | US9102670, 1a)Show SMILES COc1ccc(cc1CO)-c1ccc2c(nc(nc2n1)N1CCOC[C@@H]1C)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C25H31N5O4/c1-16-14-33-10-8-29(16)24-20-5-6-21(18-4-7-22(32-3)19(12-18)13-31)26-23(20)27-25(28-24)30-9-11-34-15-17(30)2/h4-7,12,16-17,31H,8-11,13-15H2,1-3H3/t16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FLAG-tagged mTOR (1362 to 2549) (unknown origin) expressed in HEK293 cells |
Bioorg Med Chem Lett 23: 1212-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.019
BindingDB Entry DOI: 10.7270/Q2N29Z97 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM174595

(US9102670, 14a)Show SMILES COc1ccc(cc1C(O)=O)-c1ccc2c(nc(nc2n1)N1CCOC[C@@H]1C)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C25H29N5O5/c1-15-13-34-10-8-29(15)23-18-5-6-20(17-4-7-21(33-3)19(12-17)24(31)32)26-22(18)27-25(28-23)30-9-11-35-14-16(30)2/h4-7,12,15-16H,8-11,13-14H2,1-3H3,(H,31,32)/t15-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED
US Patent
| Assay Description
The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... |
US Patent US9102670 (2015)
BindingDB Entry DOI: 10.7270/Q26M35MZ |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047481

(CHEMBL3319221)Show SMILES CN1CCN(CCCS(=O)(=O)c2ccc3nc(NC(=O)NC(=O)c4cc(ccc4Cl)-n4cccc4)sc3c2)CC1 Show InChI InChI=1S/C27H29ClN6O4S2/c1-32-12-14-33(15-13-32)9-4-16-40(37,38)20-6-8-23-24(18-20)39-27(29-23)31-26(36)30-25(35)21-17-19(5-7-22(21)28)34-10-2-3-11-34/h2-3,5-8,10-11,17-18H,4,9,12-16H2,1H3,(H2,29,30,31,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50028503
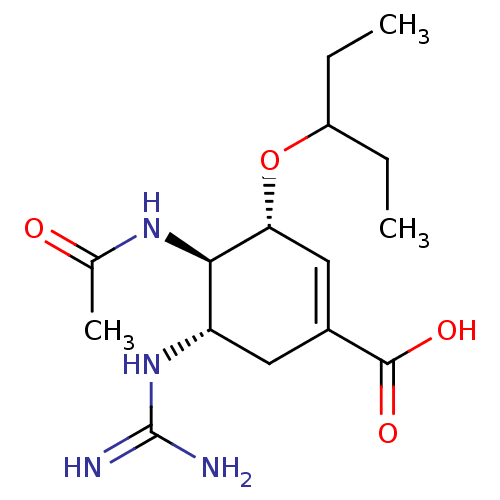
(CHEMBL81717 | Guanidino-Oseltamivir Carboxylicacid)Show SMILES CCC(CC)O[C@@H]1C=C(C[C@H](NC(N)=N)[C@H]1NC(C)=O)C(O)=O |r,c:7| Show InChI InChI=1S/C15H26N4O4/c1-4-10(5-2)23-12-7-9(14(21)22)6-11(19-15(16)17)13(12)18-8(3)20/h7,10-13H,4-6H2,1-3H3,(H,18,20)(H,21,22)(H4,16,17,19)/t11-,12+,13+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Utrecht University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza virus neuraminidase |
J Med Chem 57: 3154-60 (2014)
Article DOI: 10.1021/jm401977j
BindingDB Entry DOI: 10.7270/Q2377CP4 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047429
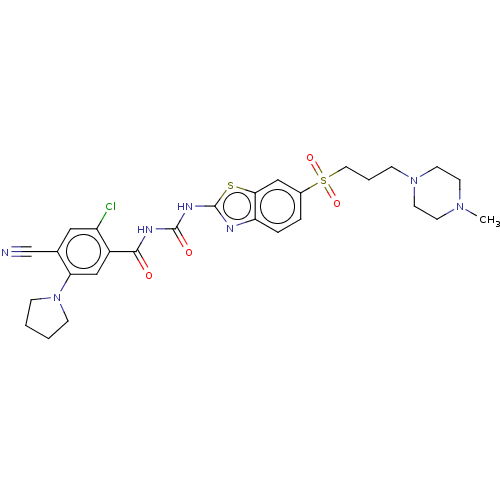
(CHEMBL3319398)Show SMILES CN1CCN(CCCS(=O)(=O)c2ccc3nc(NC(=O)NC(=O)c4cc(N5CCCC5)c(cc4Cl)C#N)sc3c2)CC1 Show InChI InChI=1S/C28H32ClN7O4S2/c1-34-10-12-35(13-11-34)7-4-14-42(39,40)20-5-6-23-25(16-20)41-28(31-23)33-27(38)32-26(37)21-17-24(36-8-2-3-9-36)19(18-30)15-22(21)29/h5-6,15-17H,2-4,7-14H2,1H3,(H2,31,32,33,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM174368
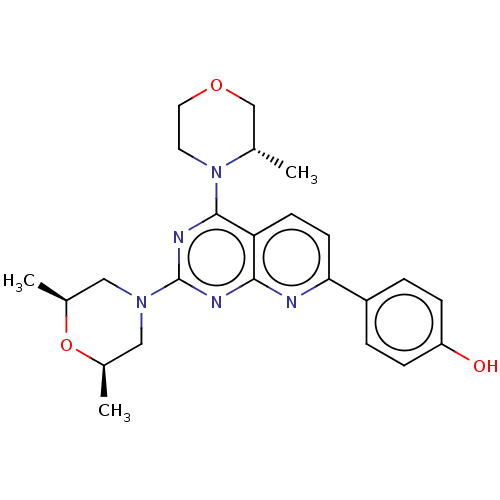
(US9102670, 1cg)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1nc(N2CCOC[C@@H]2C)c2ccc(nc2n1)-c1ccc(O)cc1 |r| Show InChI InChI=1S/C24H29N5O3/c1-15-14-31-11-10-29(15)23-20-8-9-21(18-4-6-19(30)7-5-18)25-22(20)26-24(27-23)28-12-16(2)32-17(3)13-28/h4-9,15-17,30H,10-14H2,1-3H3/t15-,16-,17+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED
US Patent
| Assay Description
The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... |
US Patent US9102670 (2015)
BindingDB Entry DOI: 10.7270/Q26M35MZ |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047480
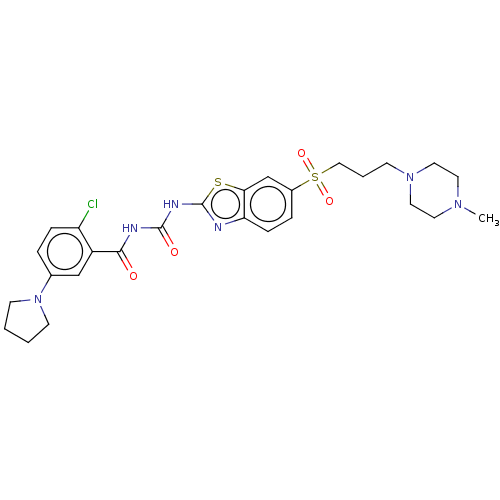
(CHEMBL3319222)Show SMILES CN1CCN(CCCS(=O)(=O)c2ccc3nc(NC(=O)NC(=O)c4cc(ccc4Cl)N4CCCC4)sc3c2)CC1 Show InChI InChI=1S/C27H33ClN6O4S2/c1-32-12-14-33(15-13-32)9-4-16-40(37,38)20-6-8-23-24(18-20)39-27(29-23)31-26(36)30-25(35)21-17-19(5-7-22(21)28)34-10-2-3-11-34/h5-8,17-18H,2-4,9-16H2,1H3,(H2,29,30,31,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047431
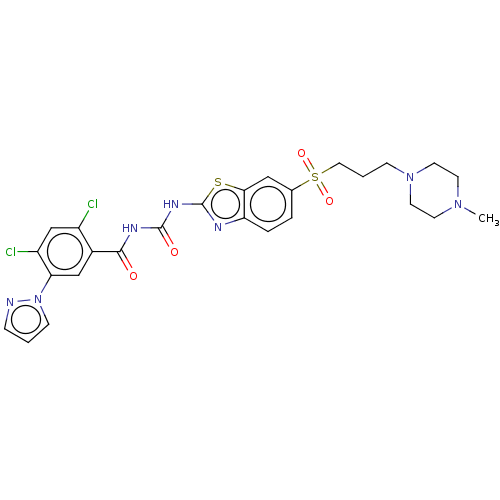
(CHEMBL3319397)Show SMILES CN1CCN(CCCS(=O)(=O)c2ccc3nc(NC(=O)NC(=O)c4cc(c(Cl)cc4Cl)-n4cccn4)sc3c2)CC1 Show InChI InChI=1S/C26H27Cl2N7O4S2/c1-33-9-11-34(12-10-33)7-3-13-41(38,39)17-4-5-21-23(14-17)40-26(30-21)32-25(37)31-24(36)18-15-22(20(28)16-19(18)27)35-8-2-6-29-35/h2,4-6,8,14-16H,3,7,9-13H2,1H3,(H2,30,31,32,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047482

(CHEMBL3319220)Show SMILES CCOc1ccc(Cl)c(c1)C(=O)NC(=O)Nc1nc2ccc(cc2s1)S(=O)(=O)CCCN1CCN(C)CC1 Show InChI InChI=1S/C25H30ClN5O5S2/c1-3-36-17-5-7-20(26)19(15-17)23(32)28-24(33)29-25-27-21-8-6-18(16-22(21)37-25)38(34,35)14-4-9-31-12-10-30(2)11-13-31/h5-8,15-16H,3-4,9-14H2,1-2H3,(H2,27,28,29,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047483

(CHEMBL3319219)Show SMILES CN1CCN(CCCS(=O)(=O)c2ccc3nc(NC(=O)NC(=O)c4cc(ccc4Cl)C4CC4)sc3c2)CC1 Show InChI InChI=1S/C26H30ClN5O4S2/c1-31-10-12-32(13-11-31)9-2-14-38(35,36)19-6-8-22-23(16-19)37-26(28-22)30-25(34)29-24(33)20-15-18(17-3-4-17)5-7-21(20)27/h5-8,15-17H,2-4,9-14H2,1H3,(H2,28,29,30,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM174414
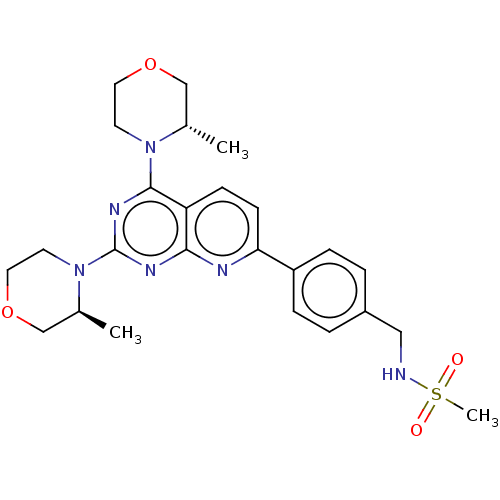
(US9102670, 1du)Show SMILES C[C@H]1COCCN1c1nc(N2CCOC[C@@H]2C)c2ccc(nc2n1)-c1ccc(CNS(C)(=O)=O)cc1 |r| Show InChI InChI=1S/C25H32N6O4S/c1-17-15-34-12-10-30(17)24-21-8-9-22(20-6-4-19(5-7-20)14-26-36(3,32)33)27-23(21)28-25(29-24)31-11-13-35-16-18(31)2/h4-9,17-18,26H,10-16H2,1-3H3/t17-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED
US Patent
| Assay Description
The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... |
US Patent US9102670 (2015)
BindingDB Entry DOI: 10.7270/Q26M35MZ |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047688

(CHEMBL3319407)Show SMILES CN1CCC(CC1)S(=O)(=O)c1ccc2nc(NC(=O)NC(=O)c3cc(N4CCCC4)c(cc3Cl)C#N)sc2c1 Show InChI InChI=1S/C26H27ClN6O4S2/c1-32-10-6-17(7-11-32)39(36,37)18-4-5-21-23(13-18)38-26(29-21)31-25(35)30-24(34)19-14-22(33-8-2-3-9-33)16(15-28)12-20(19)27/h4-5,12-14,17H,2-3,6-11H2,1H3,(H2,29,30,31,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047426
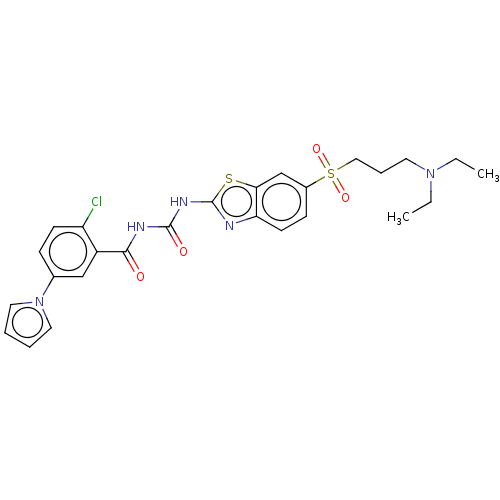
(CHEMBL3319400)Show SMILES CCN(CC)CCCS(=O)(=O)c1ccc2nc(NC(=O)NC(=O)c3cc(ccc3Cl)-n3cccc3)sc2c1 Show InChI InChI=1S/C26H28ClN5O4S2/c1-3-31(4-2)12-7-15-38(35,36)19-9-11-22-23(17-19)37-26(28-22)30-25(34)29-24(33)20-16-18(8-10-21(20)27)32-13-5-6-14-32/h5-6,8-11,13-14,16-17H,3-4,7,12,15H2,1-2H3,(H2,28,29,30,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047474

(CHEMBL3319228)Show SMILES CN1CCN(CCCS(=O)(=O)c2ccc3nc(NC(=O)NC(=O)c4ccc(cc4Cl)-n4ccc(C)n4)sc3c2)CC1 Show InChI InChI=1S/C27H30ClN7O4S2/c1-18-8-10-35(32-18)19-4-6-21(22(28)16-19)25(36)30-26(37)31-27-29-23-7-5-20(17-24(23)40-27)41(38,39)15-3-9-34-13-11-33(2)12-14-34/h4-8,10,16-17H,3,9,11-15H2,1-2H3,(H2,29,30,31,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047475
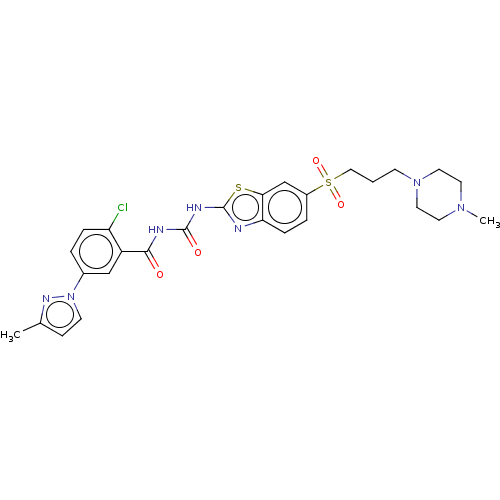
(CHEMBL3319227)Show SMILES CN1CCN(CCCS(=O)(=O)c2ccc3nc(NC(=O)NC(=O)c4cc(ccc4Cl)-n4ccc(C)n4)sc3c2)CC1 Show InChI InChI=1S/C27H30ClN7O4S2/c1-18-8-10-35(32-18)19-4-6-22(28)21(16-19)25(36)30-26(37)31-27-29-23-7-5-20(17-24(23)40-27)41(38,39)15-3-9-34-13-11-33(2)12-14-34/h4-8,10,16-17H,3,9,11-15H2,1-2H3,(H2,29,30,31,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50047428
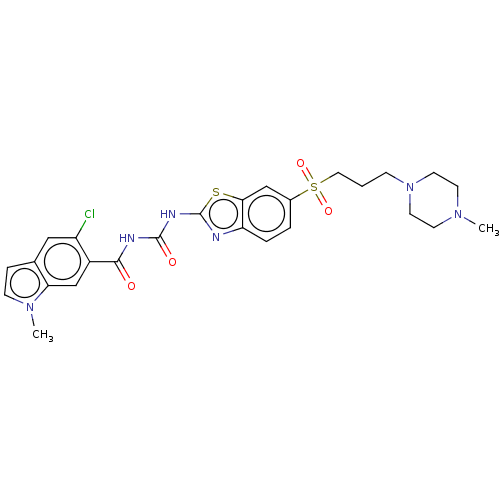
(CHEMBL3319399)Show SMILES CN1CCN(CCCS(=O)(=O)c2ccc3nc(NC(=O)NC(=O)c4cc5n(C)ccc5cc4Cl)sc3c2)CC1 Show InChI InChI=1S/C26H29ClN6O4S2/c1-31-9-11-33(12-10-31)7-3-13-39(36,37)18-4-5-21-23(15-18)38-26(28-21)30-25(35)29-24(34)19-16-22-17(14-20(19)27)6-8-32(22)2/h4-6,8,14-16H,3,7,9-13H2,1-2H3,(H2,28,29,30,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method |
J Med Chem 57: 6128-40 (2014)
Article DOI: 10.1021/jm500610n
BindingDB Entry DOI: 10.7270/Q279469X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data