

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50442630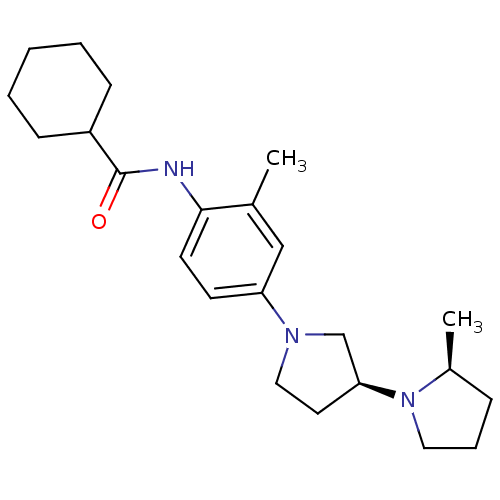 (CHEMBL2441638) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rhesus monkey histamine H3 receptor expressed in CHO cells | Bioorg Med Chem Lett 23: 6141-5 (2013) Article DOI: 10.1016/j.bmcl.2013.09.006 BindingDB Entry DOI: 10.7270/Q24T6KT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50443217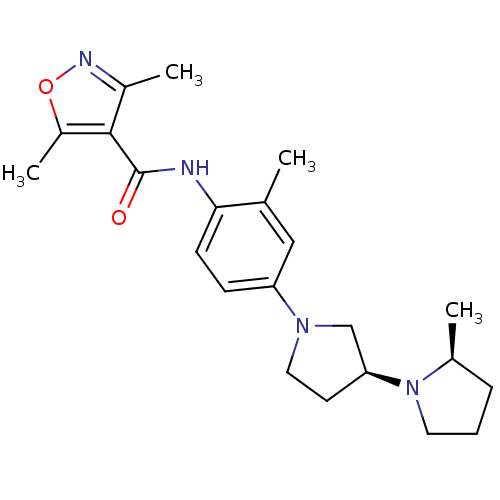 (CHEMBL3087669) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation... | Bioorg Med Chem Lett 23: 6269-73 (2013) Article DOI: 10.1016/j.bmcl.2013.09.081 BindingDB Entry DOI: 10.7270/Q27D2WK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50442628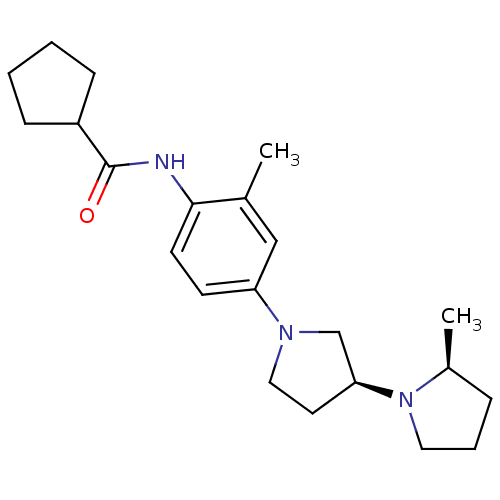 (CHEMBL2441640) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rhesus monkey histamine H3 receptor expressed in CHO cells | Bioorg Med Chem Lett 23: 6141-5 (2013) Article DOI: 10.1016/j.bmcl.2013.09.006 BindingDB Entry DOI: 10.7270/Q24T6KT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50442627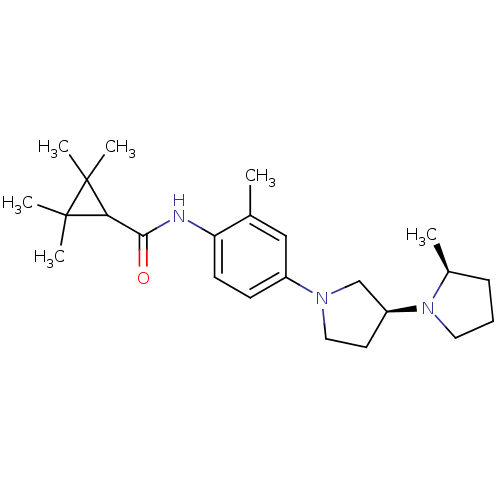 (CHEMBL2441641) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rhesus monkey histamine H3 receptor expressed in CHO cells | Bioorg Med Chem Lett 23: 6141-5 (2013) Article DOI: 10.1016/j.bmcl.2013.09.006 BindingDB Entry DOI: 10.7270/Q24T6KT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50442629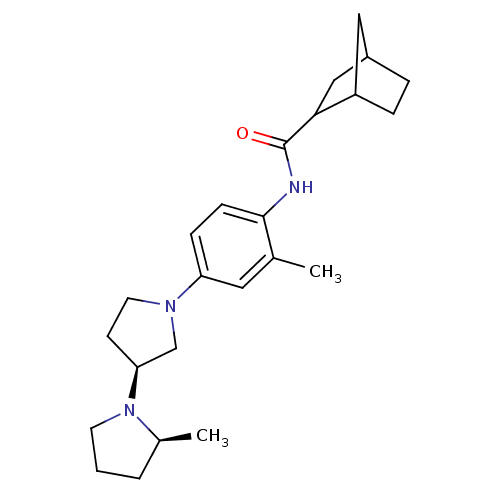 (CHEMBL2441639) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rhesus monkey histamine H3 receptor expressed in CHO cells | Bioorg Med Chem Lett 23: 6141-5 (2013) Article DOI: 10.1016/j.bmcl.2013.09.006 BindingDB Entry DOI: 10.7270/Q24T6KT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM86038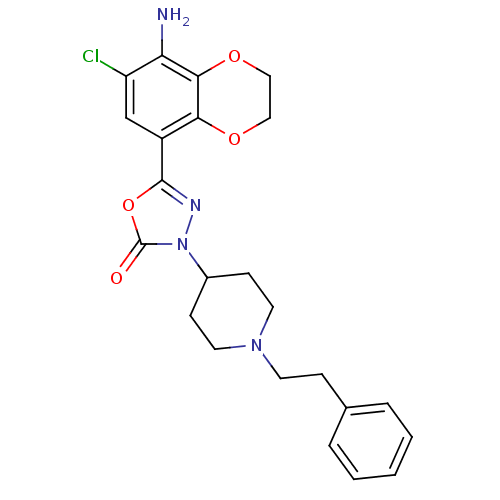 (SL65.0155 | SL650155) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche Curated by PDSP Ki Database | J Pharmacol Exp Ther 302: 731-41 (2002) Article DOI: 10.1124/jpet.102.034249 BindingDB Entry DOI: 10.7270/Q2X065NV | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (RAT) | BDBM26263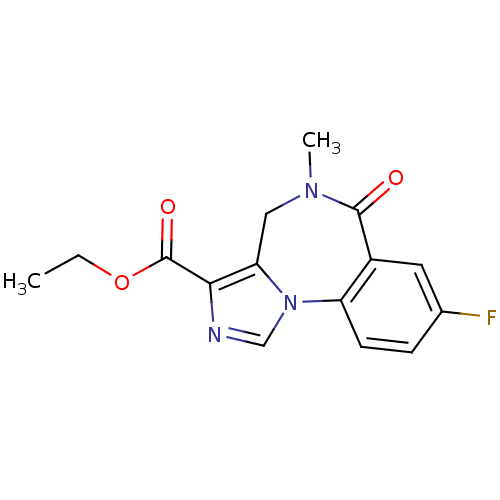 (Anexate | CHEMBL407 | FLUMAZENIL | Ro15-1788 | Rom...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Curated by PDSP Ki Database | J Biol Chem 274: 13370-4 (1999) Article DOI: 10.1074/jbc.274.19.13370 BindingDB Entry DOI: 10.7270/Q2765CWZ | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50442626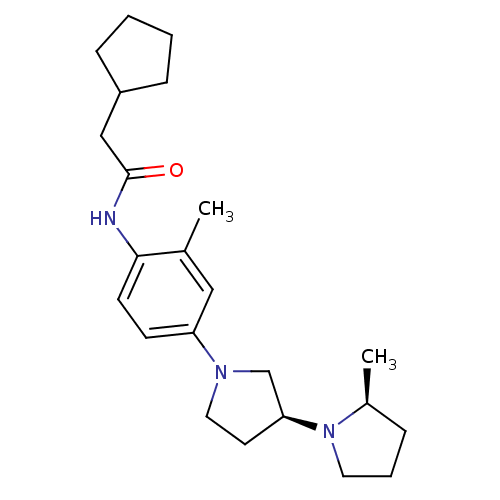 (CHEMBL2441642) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rhesus monkey histamine H3 receptor expressed in CHO cells | Bioorg Med Chem Lett 23: 6141-5 (2013) Article DOI: 10.1016/j.bmcl.2013.09.006 BindingDB Entry DOI: 10.7270/Q24T6KT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50443217 (CHEMBL3087669) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]-methylhistamine from rat histamine H3 receptor (445 amino acid residues) transfected in human 293 cells after 1 hr by scintillat... | Bioorg Med Chem Lett 23: 6269-73 (2013) Article DOI: 10.1016/j.bmcl.2013.09.081 BindingDB Entry DOI: 10.7270/Q27D2WK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50087104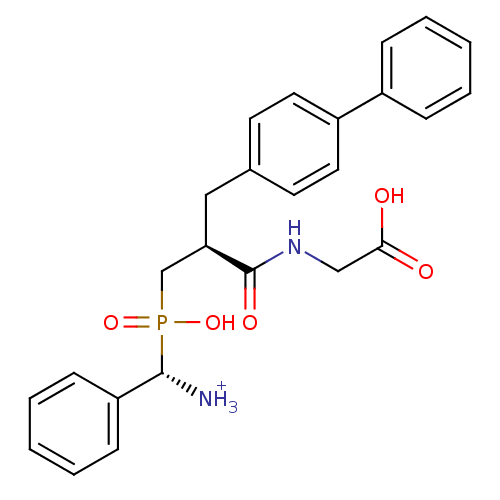 (C-{[3-Biphenyl-4-yl-2-(carboxymethyl-carbamoyl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on neutral endopeptidase (NEP) using DGNPA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50442632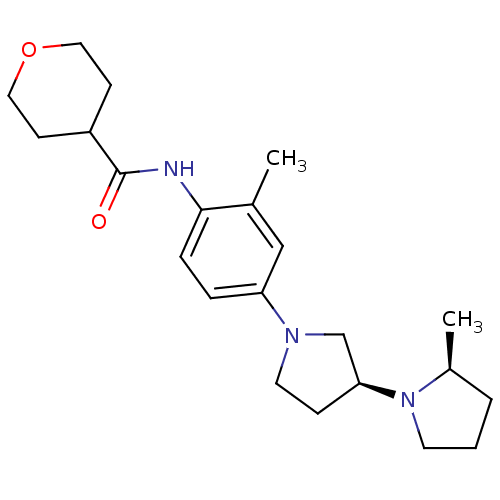 (CHEMBL2441636) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rhesus monkey histamine H3 receptor expressed in CHO cells | Bioorg Med Chem Lett 23: 6141-5 (2013) Article DOI: 10.1016/j.bmcl.2013.09.006 BindingDB Entry DOI: 10.7270/Q24T6KT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50443216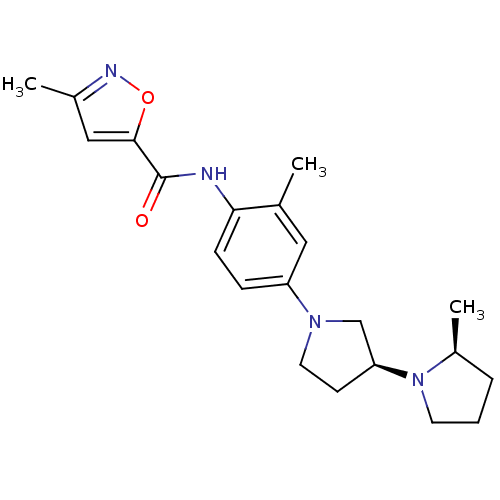 (CHEMBL3087667) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation... | Bioorg Med Chem Lett 23: 6269-73 (2013) Article DOI: 10.1016/j.bmcl.2013.09.081 BindingDB Entry DOI: 10.7270/Q27D2WK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50087106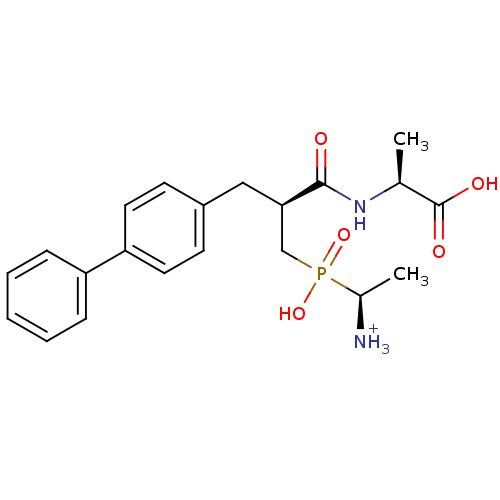 ((2S)-2-[(2S)-3-{[(1S)-1-azaniumylethyl](hydroxy)ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on neutral endopeptidase (NEP) using DGNPA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (RAT) | BDBM26263 (Anexate | CHEMBL407 | FLUMAZENIL | Ro15-1788 | Rom...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Curated by PDSP Ki Database | J Biol Chem 274: 13370-4 (1999) Article DOI: 10.1074/jbc.274.19.13370 BindingDB Entry DOI: 10.7270/Q2765CWZ | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50443227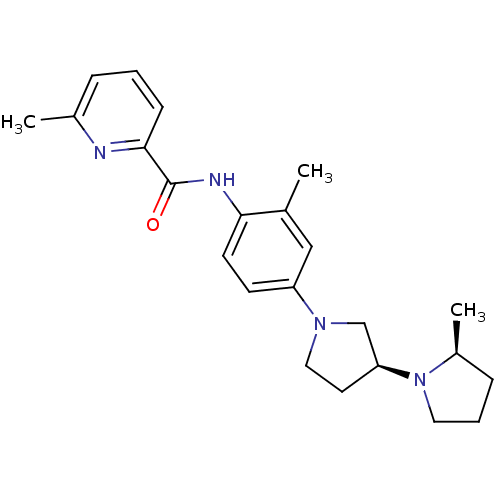 (CHEMBL3087356) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation... | Bioorg Med Chem Lett 23: 6269-73 (2013) Article DOI: 10.1016/j.bmcl.2013.09.081 BindingDB Entry DOI: 10.7270/Q27D2WK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50087088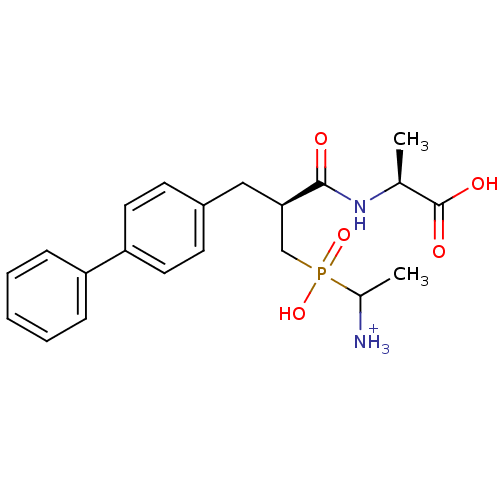 ((2S)-2-[(2S)-3-[(1-azaniumylethyl)(hydroxy)phospho...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on neutral endopeptidase (NEP) using DGNPA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50087089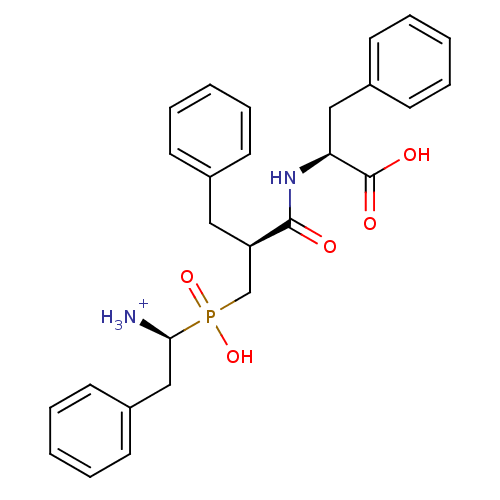 ((2S)-2-[(2S)-3-{[(1S)-1-azaniumyl-2-phenylethyl](h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on aminopeptidase N (APN) using Ala-pNA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (RAT) | BDBM26263 (Anexate | CHEMBL407 | FLUMAZENIL | Ro15-1788 | Rom...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Curated by PDSP Ki Database | J Biol Chem 274: 13370-4 (1999) Article DOI: 10.1074/jbc.274.19.13370 BindingDB Entry DOI: 10.7270/Q2765CWZ | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-1 (Rattus norvegicus (Rat)) | BDBM26263 (Anexate | CHEMBL407 | FLUMAZENIL | Ro15-1788 | Rom...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Curated by PDSP Ki Database | J Biol Chem 274: 13370-4 (1999) Article DOI: 10.1074/jbc.274.19.13370 BindingDB Entry DOI: 10.7270/Q2765CWZ | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (RAT) | BDBM26263 (Anexate | CHEMBL407 | FLUMAZENIL | Ro15-1788 | Rom...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synthélabo Curated by PDSP Ki Database | J Biol Chem 274: 13370-4 (1999) Article DOI: 10.1074/jbc.274.19.13370 BindingDB Entry DOI: 10.7270/Q2765CWZ | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50443226 (CHEMBL3087357) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation... | Bioorg Med Chem Lett 23: 6269-73 (2013) Article DOI: 10.1016/j.bmcl.2013.09.081 BindingDB Entry DOI: 10.7270/Q27D2WK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50434389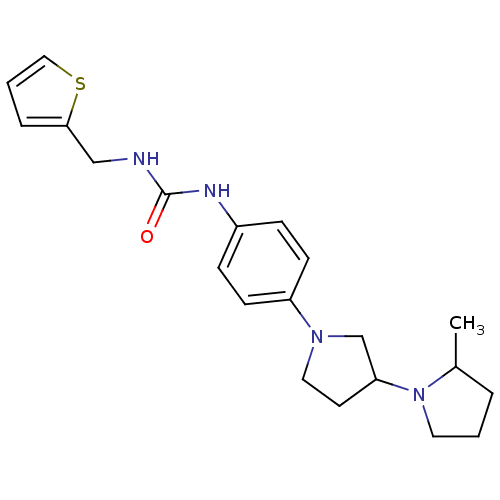 (CHEMBL2387288) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from cloned rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation coun... | Bioorg Med Chem Lett 23: 3416-20 (2013) Article DOI: 10.1016/j.bmcl.2013.03.080 BindingDB Entry DOI: 10.7270/Q2RB75Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50087095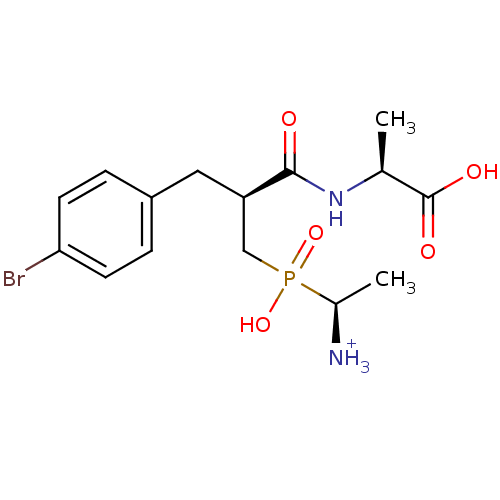 (1-{[3-(4-Bromo-phenyl)-2-(1-carboxy-ethylcarbamoyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on aminopeptidase N (APN) using Ala-pNA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50087113 ((2S)-2-[(2S)-3-{[(S)-azaniumyl(phenyl)methyl](hydr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on neutral endopeptidase (NEP) using DGNPA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50087085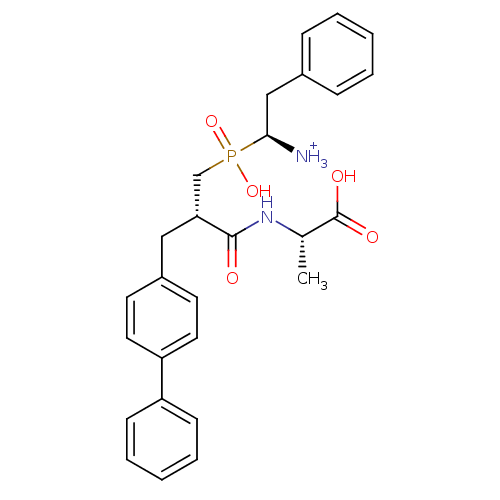 ((2S)-2-[(2S)-3-{[(1S)-1-azaniumyl-2-phenylethyl](h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on neutral endopeptidase (NEP) using DGNPA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50087097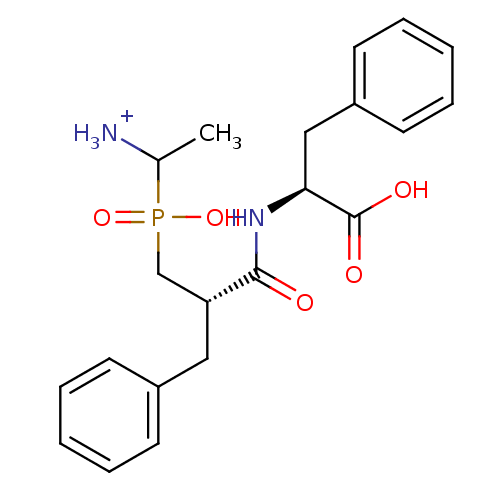 (1-{[2-(1-Carboxy-2-phenyl-ethylcarbamoyl)-3-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on aminopeptidase N (APN) using Ala-pNA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50087084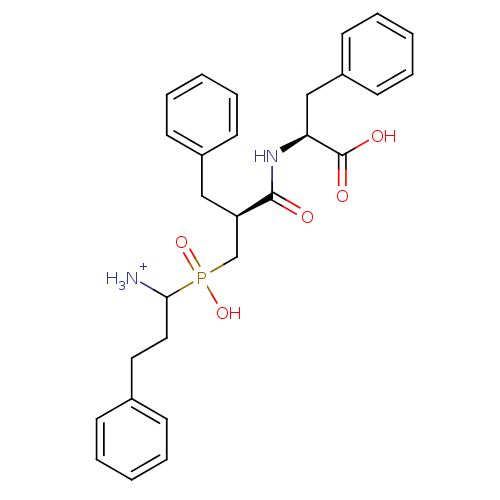 (1-{[2-(1-Carboxy-2-phenyl-ethylcarbamoyl)-3-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on aminopeptidase N (APN) using Ala-pNA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50087085 ((2S)-2-[(2S)-3-{[(1S)-1-azaniumyl-2-phenylethyl](h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on neutral endopeptidase (NEP) using DGNPA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50087094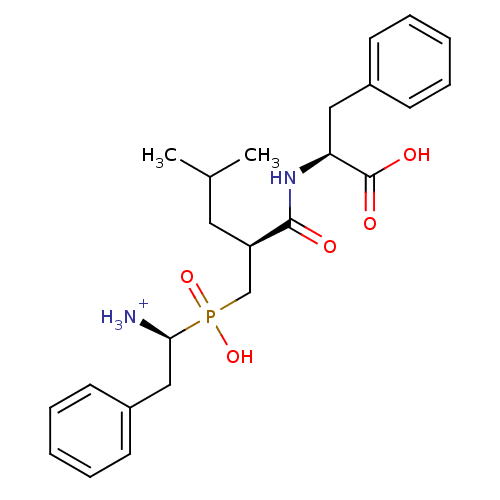 ((R,S,S)1-{[2-(1-Carboxy-2-phenyl-ethylcarbamoyl)-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on aminopeptidase N (APN) using Ala-pNA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50087093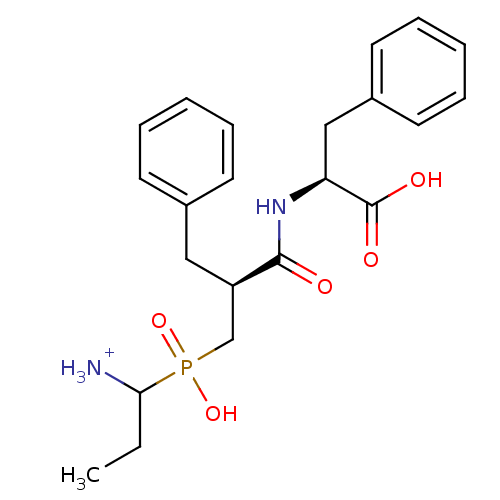 (1-{[2-(1-Carboxy-2-phenyl-ethylcarbamoyl)-3-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on aminopeptidase N (APN) using Ala-pNA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50437067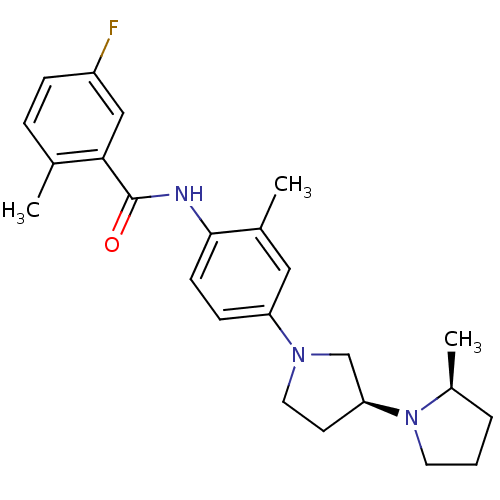 (CHEMBL2403550) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant rhesus monkey histamine H3 receptor transfected in CHO cell membranes after 1 hr by scin... | Bioorg Med Chem Lett 23: 4044-7 (2013) Article DOI: 10.1016/j.bmcl.2013.05.068 BindingDB Entry DOI: 10.7270/Q28C9XN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50437067 (CHEMBL2403550) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation... | Bioorg Med Chem Lett 23: 6269-73 (2013) Article DOI: 10.1016/j.bmcl.2013.09.081 BindingDB Entry DOI: 10.7270/Q27D2WK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50443225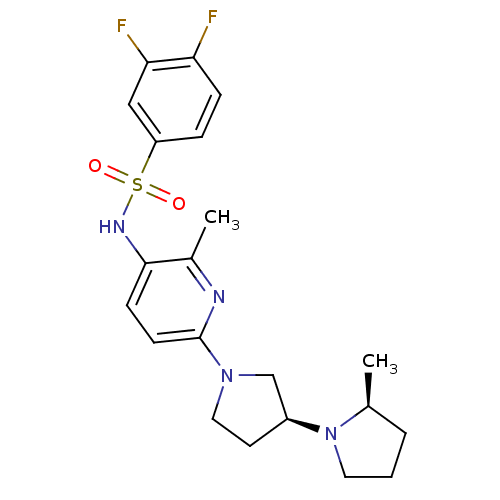 (CHEMBL3087671) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation... | Bioorg Med Chem Lett 23: 6269-73 (2013) Article DOI: 10.1016/j.bmcl.2013.09.081 BindingDB Entry DOI: 10.7270/Q27D2WK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50087106 ((2S)-2-[(2S)-3-{[(1S)-1-azaniumylethyl](hydroxy)ph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on aminopeptidase N (APN) using Ala-pNA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50087089 ((2S)-2-[(2S)-3-{[(1S)-1-azaniumyl-2-phenylethyl](h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on aminopeptidase N (APN) using Ala-pNA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50087100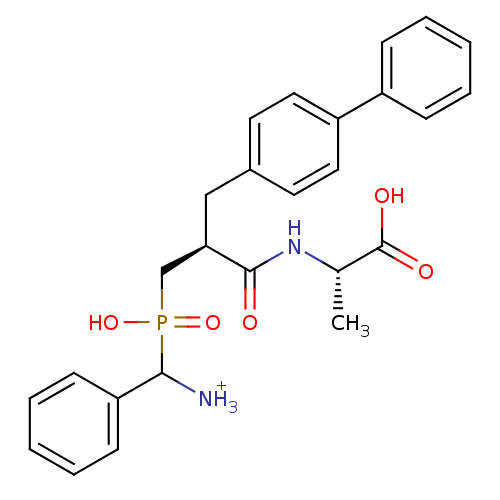 ((2S)-2-[(2S)-3-{[azaniumyl(phenyl)methyl](hydroxy)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on neutral endopeptidase (NEP) using DGNPA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50437067 (CHEMBL2403550) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant rat histamine H3 receptor transfected in CHO cell membranes after 1 hr by scintillation ... | Bioorg Med Chem Lett 23: 4044-7 (2013) Article DOI: 10.1016/j.bmcl.2013.05.068 BindingDB Entry DOI: 10.7270/Q28C9XN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50443224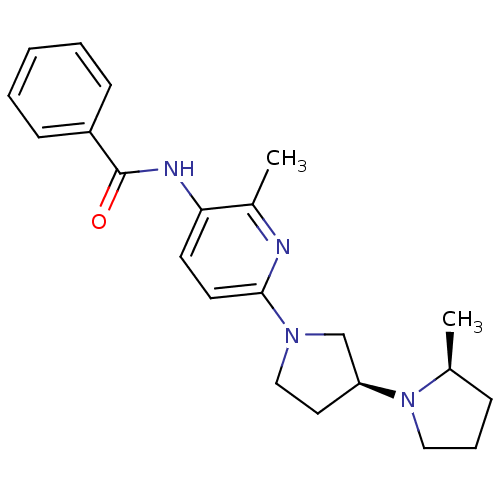 (CHEMBL3087670) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation... | Bioorg Med Chem Lett 23: 6269-73 (2013) Article DOI: 10.1016/j.bmcl.2013.09.081 BindingDB Entry DOI: 10.7270/Q27D2WK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50087114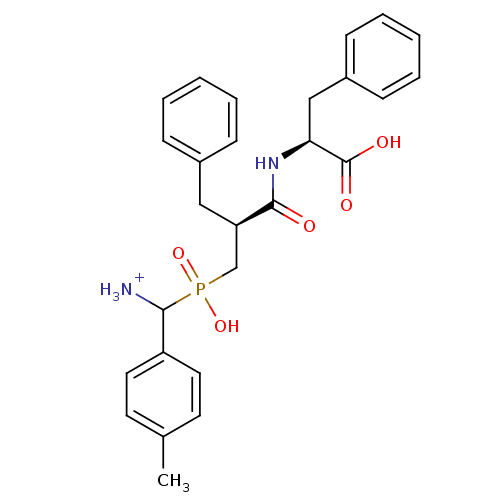 (C-{[2-(1-Carboxy-2-phenyl-ethylcarbamoyl)-3-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on aminopeptidase N (APN) using Ala-pNA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50442640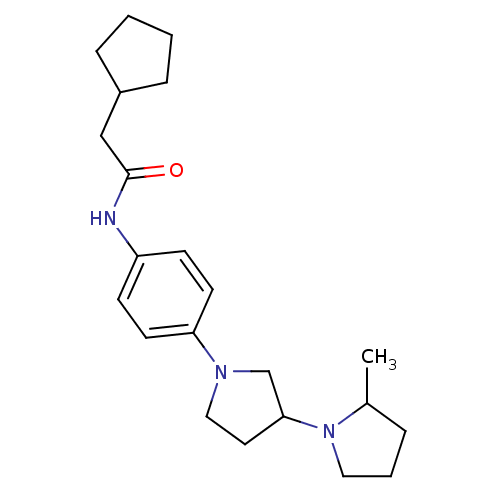 (CHEMBL2441653) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rhesus monkey histamine H3 receptor expressed in CHO cells | Bioorg Med Chem Lett 23: 6141-5 (2013) Article DOI: 10.1016/j.bmcl.2013.09.006 BindingDB Entry DOI: 10.7270/Q24T6KT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50087096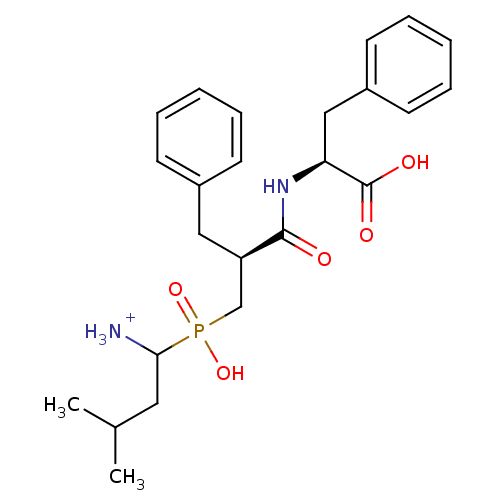 (1-{[2-(1-Carboxy-2-phenyl-ethylcarbamoyl)-3-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on aminopeptidase N (APN) using Ala-pNA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50087101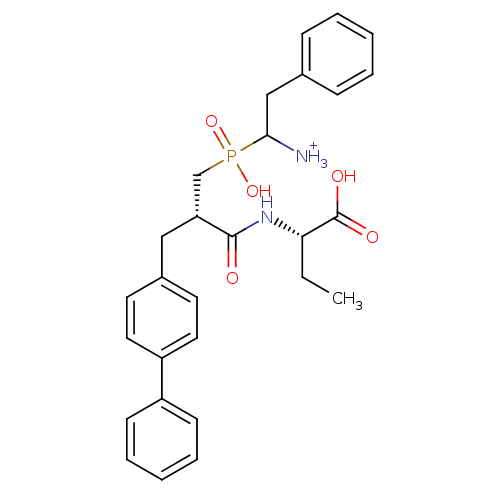 (1-{[3-Biphenyl-4-yl-2-(1-carboxy-propylcarbamoyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on neutral endopeptidase (NEP) using DGNPA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50434382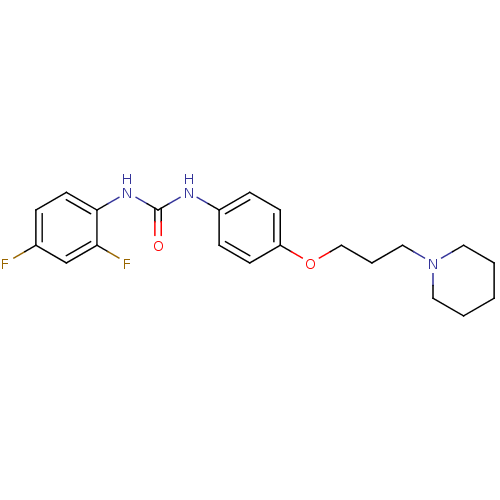 (CHEMBL2387309) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from cloned rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation coun... | Bioorg Med Chem Lett 23: 3416-20 (2013) Article DOI: 10.1016/j.bmcl.2013.03.080 BindingDB Entry DOI: 10.7270/Q2RB75Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50434405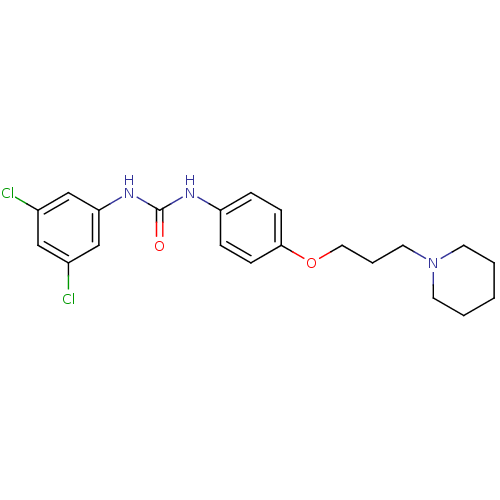 (CHEMBL2387306) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from cloned rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation coun... | Bioorg Med Chem Lett 23: 3416-20 (2013) Article DOI: 10.1016/j.bmcl.2013.03.080 BindingDB Entry DOI: 10.7270/Q2RB75Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50087105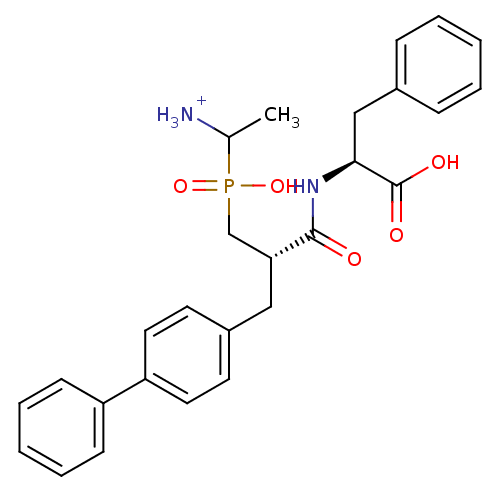 (1-{[3-Biphenyl-4-yl-2-(1-carboxy-2-phenyl-ethylcar...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on aminopeptidase N (APN) using Ala-pNA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50442631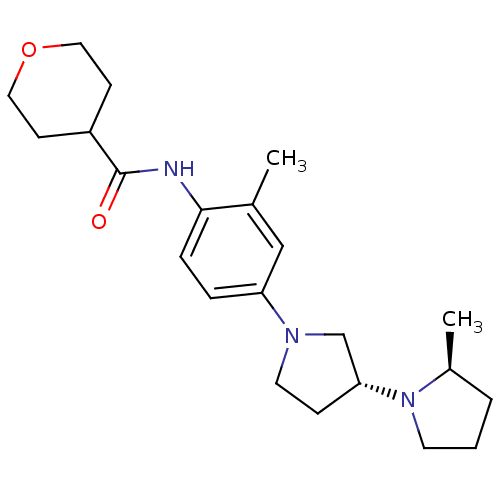 (CHEMBL2441637) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rhesus monkey histamine H3 receptor expressed in CHO cells | Bioorg Med Chem Lett 23: 6141-5 (2013) Article DOI: 10.1016/j.bmcl.2013.09.006 BindingDB Entry DOI: 10.7270/Q24T6KT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50443223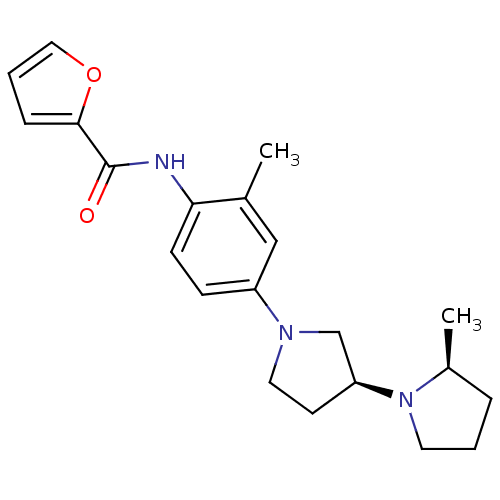 (CHEMBL3087358) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation... | Bioorg Med Chem Lett 23: 6269-73 (2013) Article DOI: 10.1016/j.bmcl.2013.09.081 BindingDB Entry DOI: 10.7270/Q27D2WK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50434401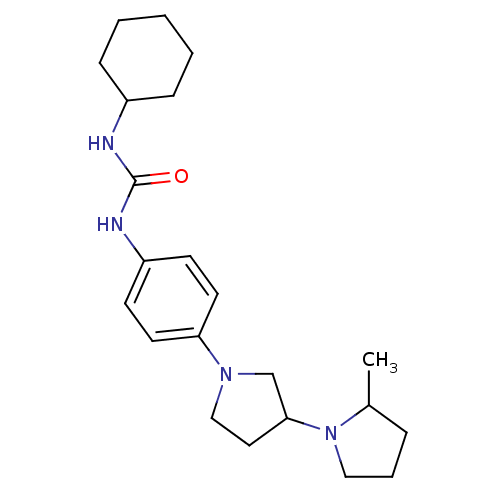 (CHEMBL2387314) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from cloned rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation coun... | Bioorg Med Chem Lett 23: 3416-20 (2013) Article DOI: 10.1016/j.bmcl.2013.03.080 BindingDB Entry DOI: 10.7270/Q2RB75Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50087098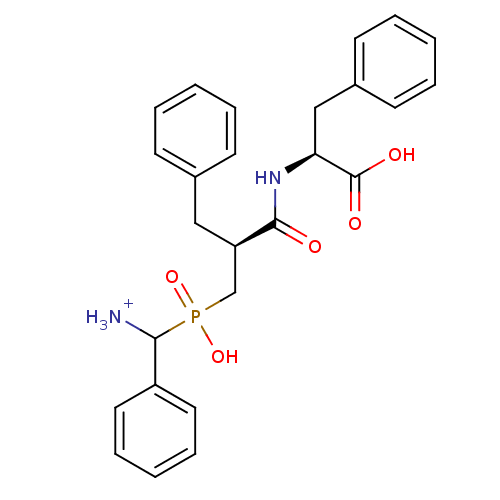 ((2S)-2-[(2S)-3-{[azaniumyl(phenyl)methyl](hydroxy)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on aminopeptidase N (APN) using Ala-pNA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50087111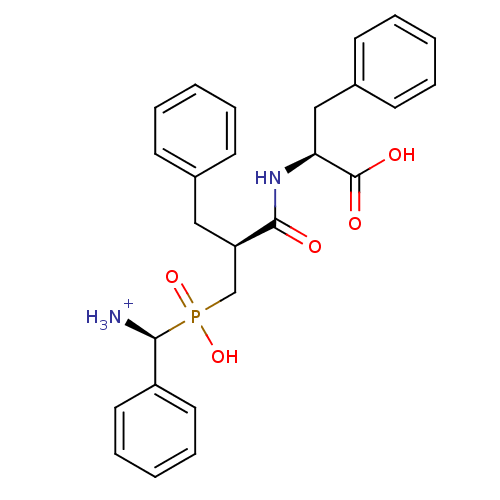 ((2S)-2-[(2S)-3-{[(S)-azaniumyl(phenyl)methyl](hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on aminopeptidase N (APN) using Ala-pNA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1184 total ) | Next | Last >> |