

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50180036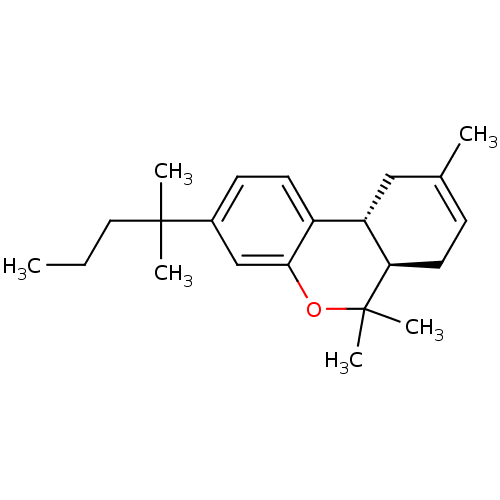 ((6aR,10aR)-3-(1,1-dimethylbutyl)-6a,7,10,10a-tetra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor | J Med Chem 52: 369-78 (2009) Article DOI: 10.1021/jm801044g BindingDB Entry DOI: 10.7270/Q2VQ33M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121507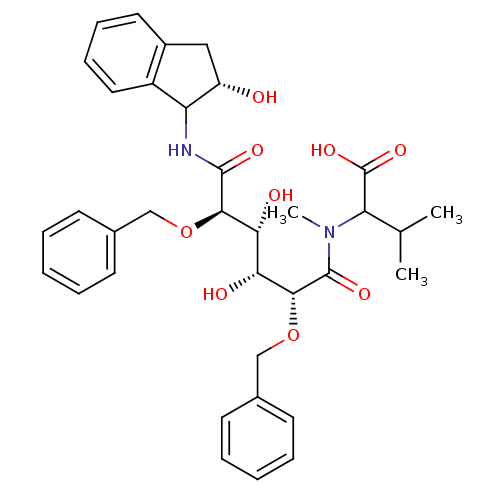 (2-{[2,5-Bis-benzyloxy-3,4-dihydroxy-5-(2-hydroxy-i...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0540 | n/a | n/a | 4.42 | n/a | n/a | 5.97E+6 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM854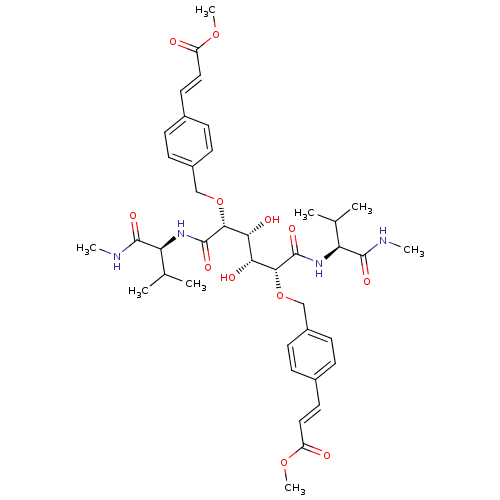 (C2-Symmetric inhibitor 12 | CHEMBL127045 | N1,N6-B...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0900 | n/a | n/a | 1.95 | n/a | n/a | 2.04E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121505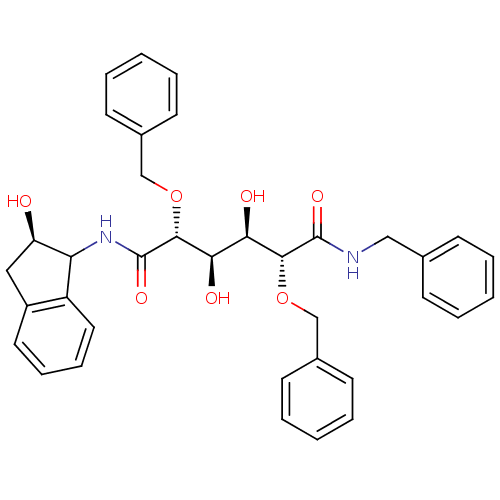 (2,5-Bis-benzyloxy-3,4-dihydroxy-hexanedioic acid b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | 70 | n/a | n/a | 1.01E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50066918 ((2R,3R,4R,5R)-2,5-Bis-benzyloxy-3,4-dihydroxy-hexa...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV-1 protease expressed in E. coli in sensitive fluorometric assay | J Med Chem 41: 3782-92 (1998) Article DOI: 10.1021/jm970777b BindingDB Entry DOI: 10.7270/Q2PK0F93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121499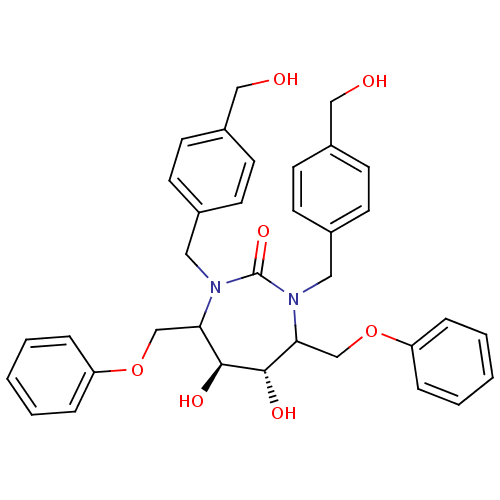 (5,6-Dihydroxy-1,3-bis-(4-hydroxymethyl-benzyl)-4,7...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.230 | n/a | n/a | 7 | n/a | n/a | 7.06E+9 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.230 | n/a | n/a | 0.315 | n/a | n/a | 8.17E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM18137 (AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.230 | n/a | n/a | 1.13 | n/a | n/a | 4.43E+6 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM150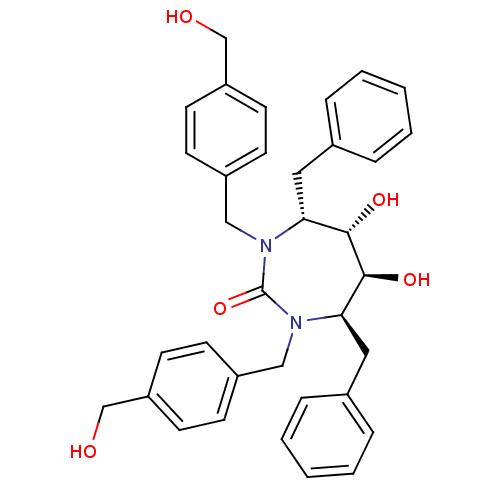 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.270 | n/a | n/a | 3.83 | n/a | n/a | 2.52E+10 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM845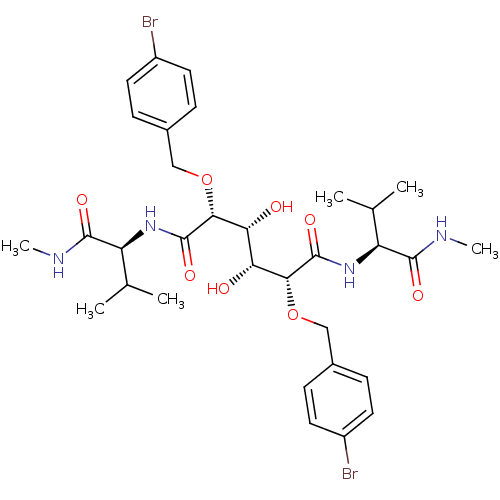 ((2R,3R,4R,5R)-2,5-bis[(4-bromophenyl)methoxy]-3,4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.300 | n/a | n/a | 21 | n/a | n/a | 8.89E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM855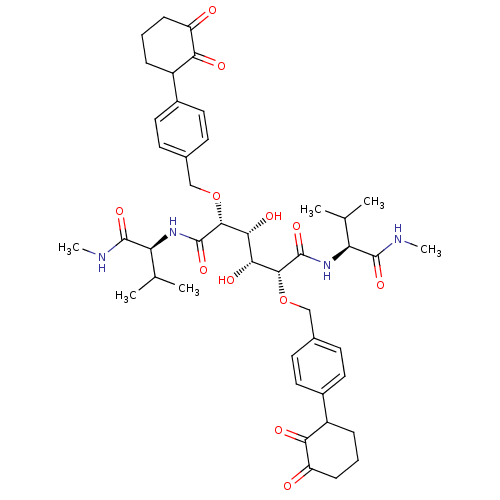 ((2R,3R,4R,5R)-3,4-dihydroxy-2,5-bis({[4-(2-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.300 | n/a | n/a | 18 | n/a | n/a | 2.93E+4 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.310 | n/a | n/a | 1.07 | n/a | n/a | 1.53E+6 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM348 ((2R,3R,4R,5R)-2,5-bis(benzyloxy)-3,4-dihydroxy-N,N...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.400 | n/a | n/a | 11 | n/a | n/a | 3.55E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM348 ((2R,3R,4R,5R)-2,5-bis(benzyloxy)-3,4-dihydroxy-N,N...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV-1 protease expressed in E. coli in sensitive fluorometric assay | J Med Chem 41: 3782-92 (1998) Article DOI: 10.1021/jm970777b BindingDB Entry DOI: 10.7270/Q2PK0F93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50366785 (NELFINAVIR) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.540 | n/a | n/a | 1.64 | n/a | n/a | 6.63E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM853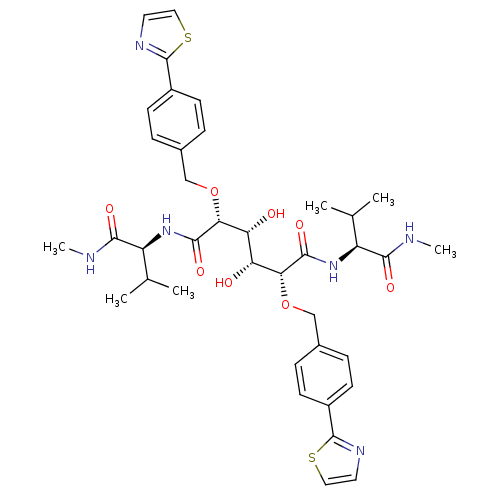 ((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S)-2-methyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.550 | n/a | n/a | 0.643 | n/a | n/a | 4.77E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.590 | n/a | n/a | 0.608 | n/a | n/a | 3.92E+6 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121502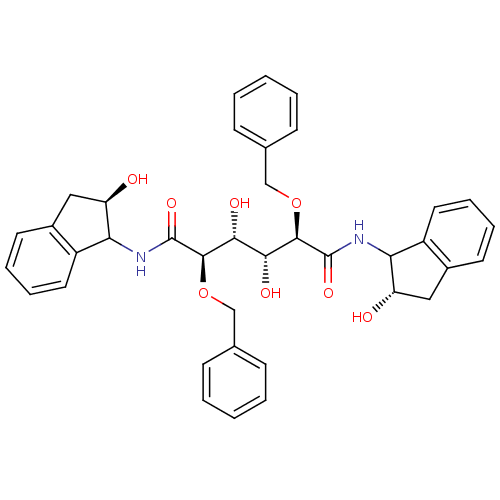 (2,5-Bis-benzyloxy-3,4-dihydroxy-hexanedioic acid b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | 14 | n/a | n/a | 6.39E+6 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM852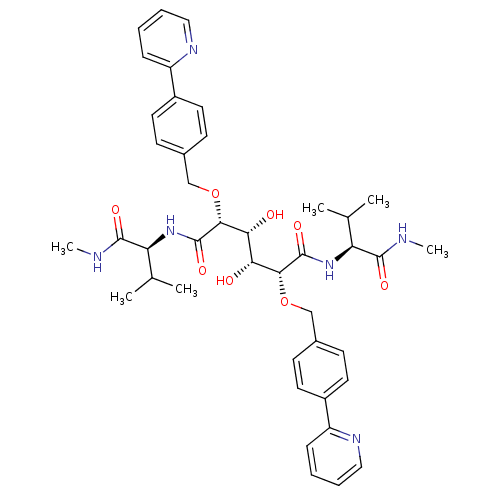 ((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S)-2-methyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.610 | n/a | n/a | 1.18 | n/a | n/a | 3.23E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50180036 ((6aR,10aR)-3-(1,1-dimethylbutyl)-6a,7,10,10a-tetra...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.797 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB1 receptor | J Med Chem 52: 369-78 (2009) Article DOI: 10.1021/jm801044g BindingDB Entry DOI: 10.7270/Q2VQ33M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM850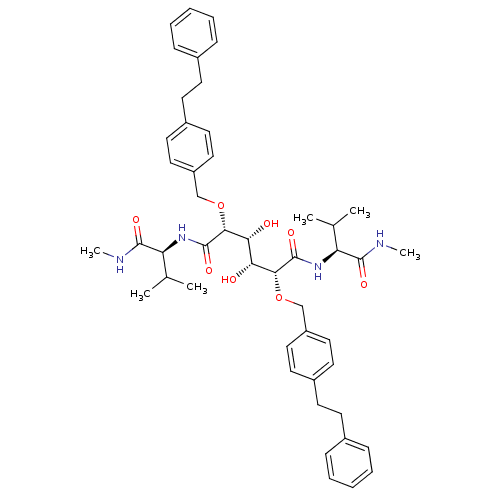 ((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S)-2-methyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.800 | n/a | n/a | 34 | n/a | n/a | 8.11E+4 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50228072 (1,9-dihydroxy-3-(1',1'-dimethylheptyl)-6H-benzo[c]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor | J Med Chem 52: 369-78 (2009) Article DOI: 10.1021/jm801044g BindingDB Entry DOI: 10.7270/Q2VQ33M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50066915 ((2R,3R,4R,5R)-2,5-Bis-benzyloxy-3,4-dihydroxy-hexa...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV-1 protease expressed in E. coli in sensitive fluorometric assay | J Med Chem 41: 3782-92 (1998) Article DOI: 10.1021/jm970777b BindingDB Entry DOI: 10.7270/Q2PK0F93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121521 (2-({2,5-Bis-benzyloxy-5-[(1-carboxy-2-methyl-butyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.910 | n/a | n/a | 48 | n/a | n/a | 1.85E+6 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM848 ((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S)-2-methyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 1.20 | n/a | n/a | 3 | n/a | n/a | 3.48E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50265650 (2-(N-(2,5-dimethoxyphenyl)-4-methylphenylsulfonami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor | J Med Chem 52: 369-78 (2009) Article DOI: 10.1021/jm801044g BindingDB Entry DOI: 10.7270/Q2VQ33M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121506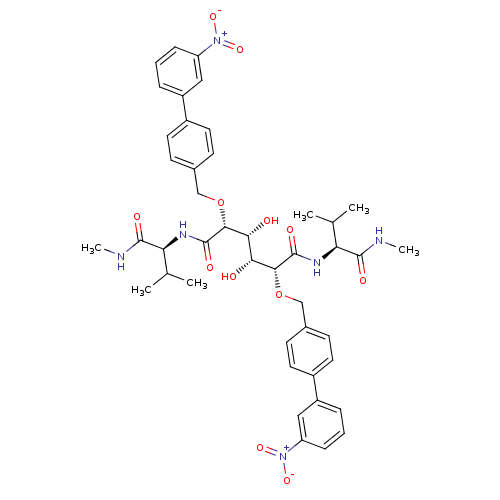 ((2R,3R,4R,5R)-3,4-Dihydroxy-2,5-bis-(3'-nitro-biph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | 18 | n/a | n/a | 1.81E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121500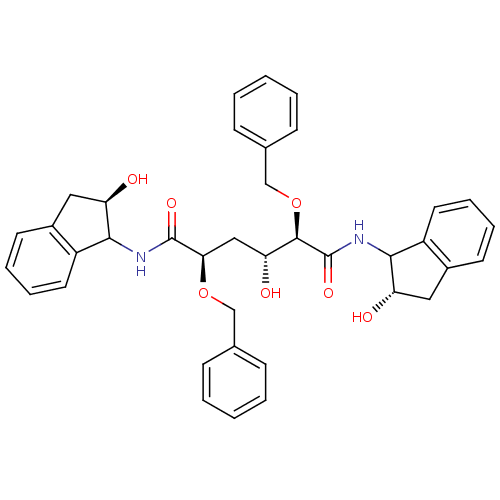 (2,5-Bis-benzyloxy-3-hydroxy-hexanedioic acid bis-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | 36 | n/a | n/a | 6.66E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50265648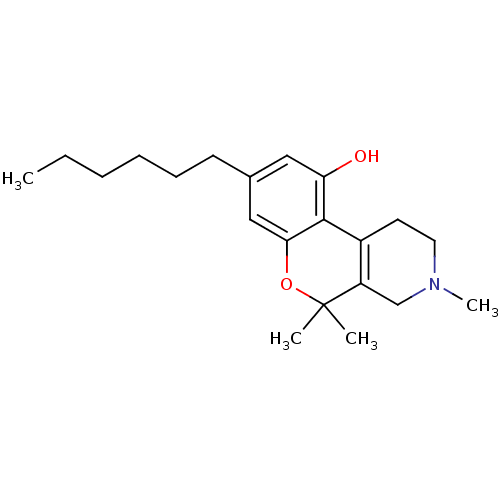 (8-hexyl-3,5,5-trimethyl-2,3,4,5-tetrahydro-1H-chro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor | J Med Chem 52: 369-78 (2009) Article DOI: 10.1021/jm801044g BindingDB Entry DOI: 10.7270/Q2VQ33M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121496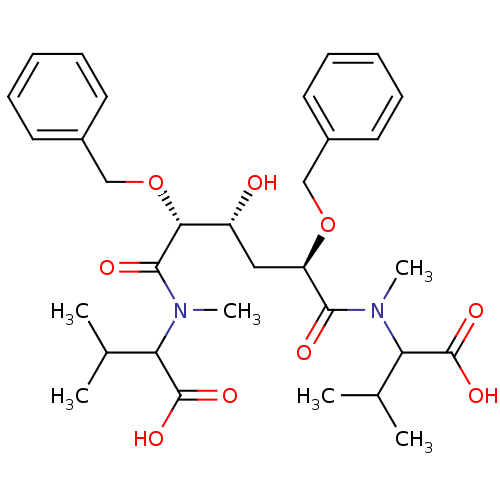 (2-({2,5-Bis-benzyloxy-5-[(1-carboxy-2-methyl-propy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | 102 | n/a | n/a | 3.04E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50265687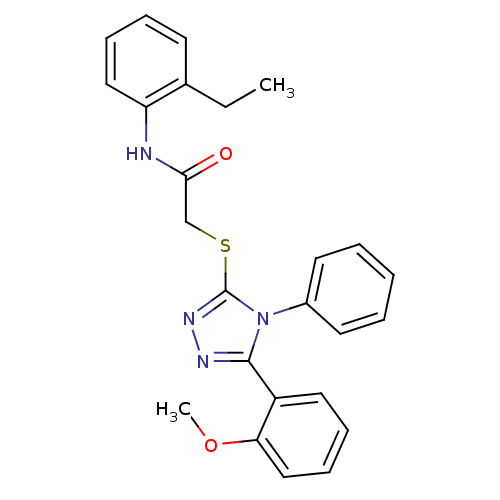 (CHEMBL496781 | N-(2-ethylphenyl)-2-(5-(2-methoxyph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor | J Med Chem 52: 369-78 (2009) Article DOI: 10.1021/jm801044g BindingDB Entry DOI: 10.7270/Q2VQ33M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50066919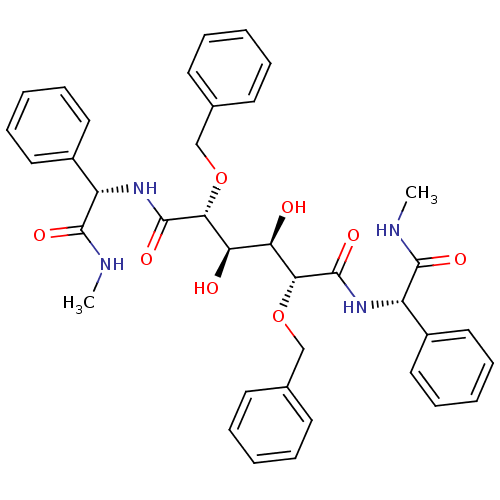 ((2R,3R,4R,5R)-2,5-Bis-benzyloxy-3,4-dihydroxy-hexa...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV-1 protease expressed in E. coli in sensitive fluorometric assay | J Med Chem 41: 3782-92 (1998) Article DOI: 10.1021/jm970777b BindingDB Entry DOI: 10.7270/Q2PK0F93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50066919 ((2R,3R,4R,5R)-2,5-Bis-benzyloxy-3,4-dihydroxy-hexa...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.30 | n/a | n/a | 27.4 | n/a | n/a | 2.05E+8 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50366324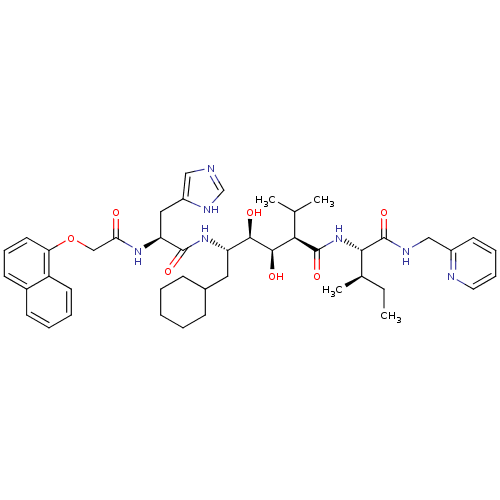 (CHEMBL1790545 | U-75875) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80 | n/a | n/a | 0.484 | n/a | n/a | 6.76E+6 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM346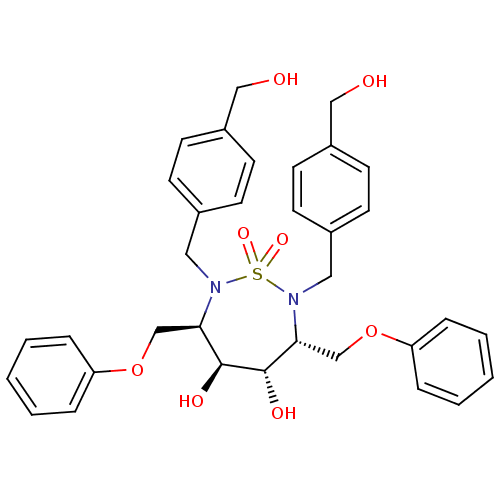 ((3R,4S,5S,6R)-4,5-dihydroxy-2,7-bis({[4-(hydroxyme...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10 | n/a | n/a | 39.8 | n/a | n/a | 6.87E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50180036 ((6aR,10aR)-3-(1,1-dimethylbutyl)-6a,7,10,10a-tetra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor | J Med Chem 52: 369-78 (2009) Article DOI: 10.1021/jm801044g BindingDB Entry DOI: 10.7270/Q2VQ33M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM342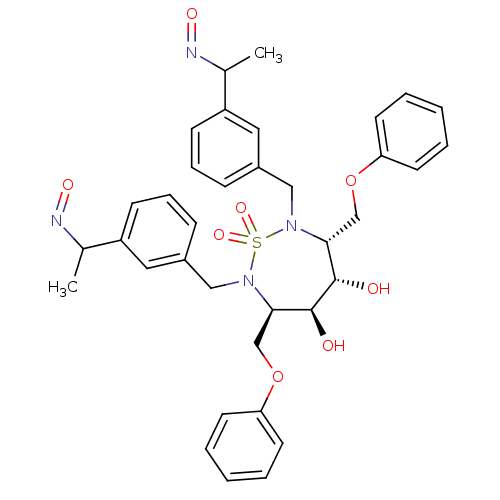 ((3R,4S,5S,6R)-4,5-dihydroxy-2,7-bis({3-[(1E)-1-(hy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.60 | n/a | n/a | 169 | n/a | n/a | 5.12E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50265747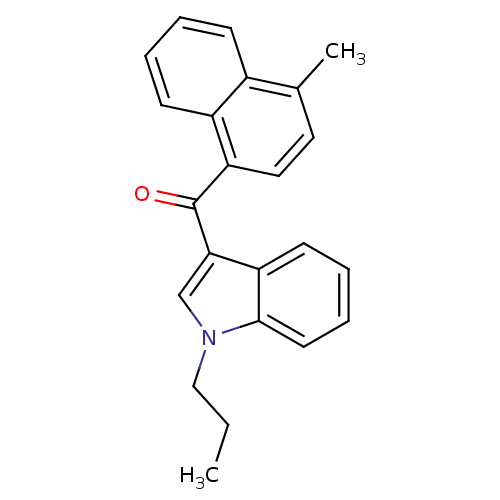 ((4-methylnaphthalen-1-yl)(1-propyl-1H-indol-3-yl)m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor | J Med Chem 52: 369-78 (2009) Article DOI: 10.1021/jm801044g BindingDB Entry DOI: 10.7270/Q2VQ33M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM326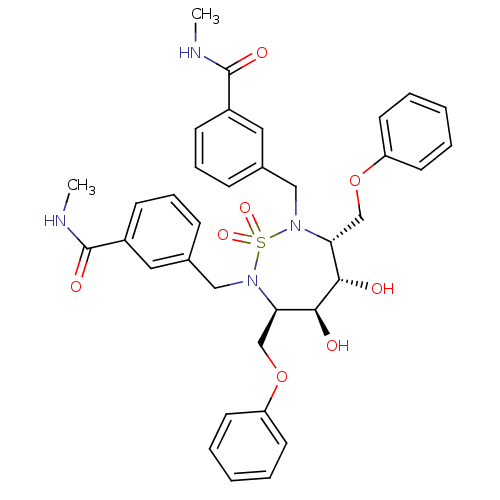 ((3R,4S,5S,6R)-2,7-Bis[3-(N-methylcarbamoyl)benzyl]...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.20 | n/a | n/a | 675 | n/a | n/a | 4.99E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50265647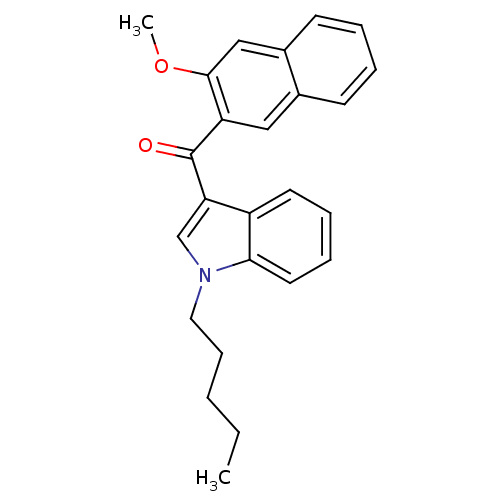 ((3-methoxynaphthalen-2-yl)(1-pentyl-1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor | J Med Chem 52: 369-78 (2009) Article DOI: 10.1021/jm801044g BindingDB Entry DOI: 10.7270/Q2VQ33M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50222797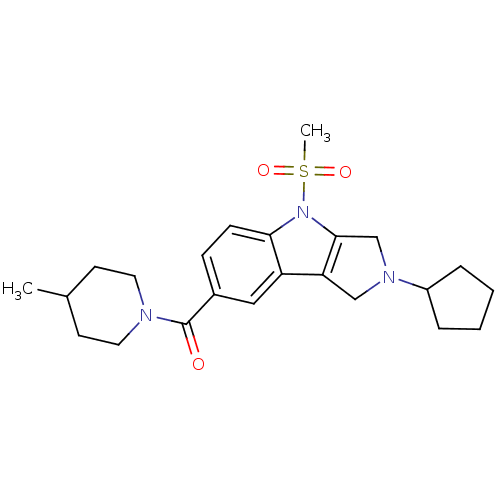 ((2-cyclopentyl-4-(methylsulfonyl)-1,2,3,4-tetrahyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor | J Med Chem 52: 369-78 (2009) Article DOI: 10.1021/jm801044g BindingDB Entry DOI: 10.7270/Q2VQ33M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM335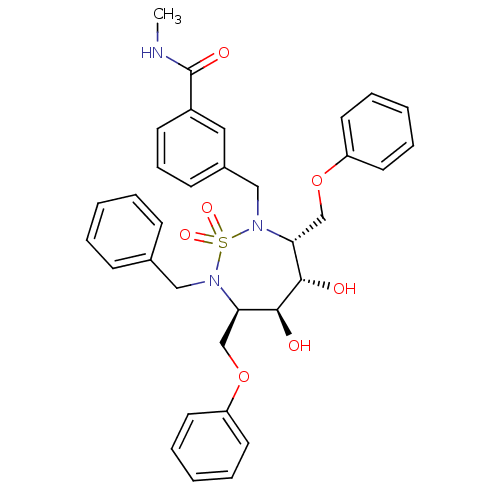 ((3R,4S,5S,6R)-3,6-Bis(phenoxymethyl)-4,5-dihydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB PubMed | 10.1 | n/a | n/a | 577 | n/a | n/a | 1.88E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50222793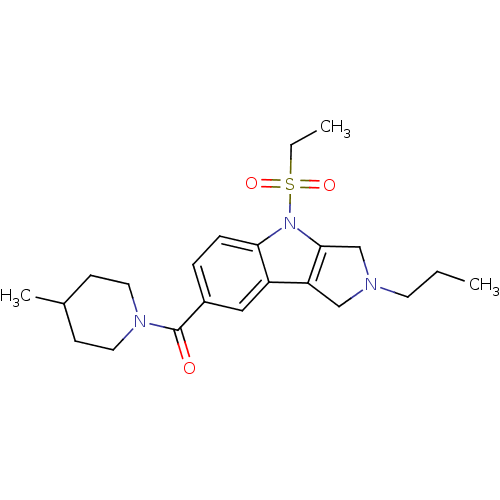 ((4-(ethylsulfonyl)-2-propyl-1,2,3,4-tetrahydropyrr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor | J Med Chem 52: 369-78 (2009) Article DOI: 10.1021/jm801044g BindingDB Entry DOI: 10.7270/Q2VQ33M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50234418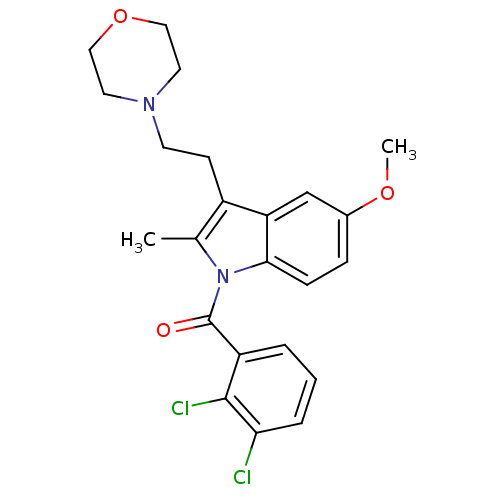 ((2,3-Dichloro-phenyl)-[5-methoxy-2-methyl-3-(2-mor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor | J Med Chem 52: 369-78 (2009) Article DOI: 10.1021/jm801044g BindingDB Entry DOI: 10.7270/Q2VQ33M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50240608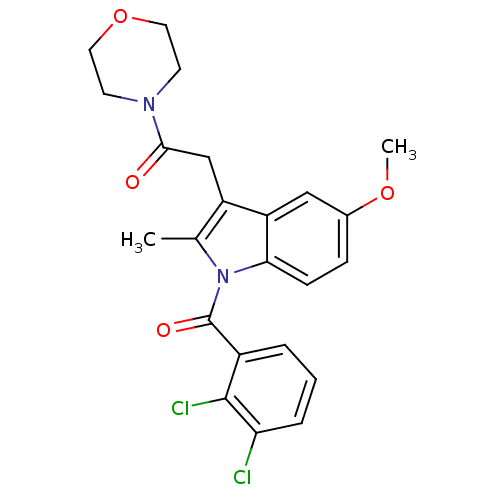 (2-(1-(2,3-dichlorobenzoyl)-5-methoxy-2-methyl-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor | J Med Chem 52: 369-78 (2009) Article DOI: 10.1021/jm801044g BindingDB Entry DOI: 10.7270/Q2VQ33M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21283 (3-[(2-iodo-5-nitrophenyl)carbonyl]-1-[(1-methylpip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 15.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor | J Med Chem 52: 369-78 (2009) Article DOI: 10.1021/jm801044g BindingDB Entry DOI: 10.7270/Q2VQ33M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50265755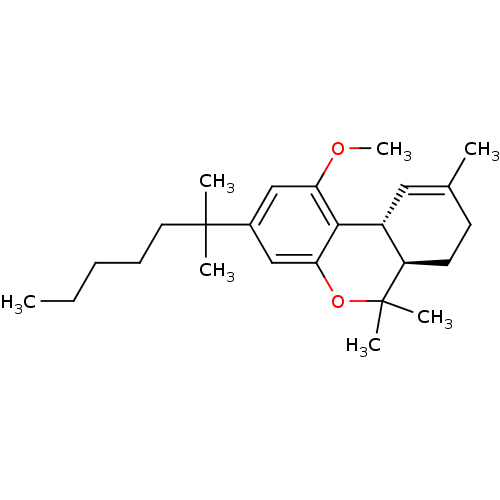 ((6aR,10aR)-1-methoxy-6,6,9-trimethyl-3-(2-methylhe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor | J Med Chem 52: 369-78 (2009) Article DOI: 10.1021/jm801044g BindingDB Entry DOI: 10.7270/Q2VQ33M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121498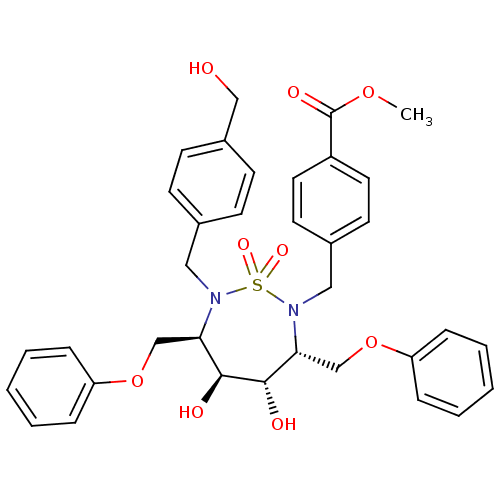 (4-[4,5-Dihydroxy-7-(4-hydroxymethyl-benzyl)-1,1-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB PubMed | 19 | n/a | n/a | 343 | n/a | n/a | 2.21E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1163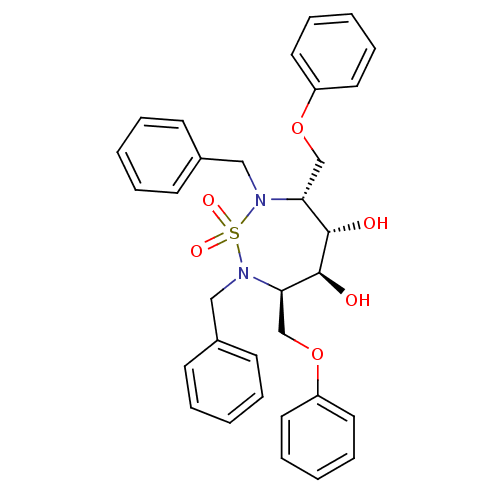 ((3R,4S,5S,6R)-2,7-Dibenzyl-3,6-bis(phenoxymethyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PubMed | 19.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50240605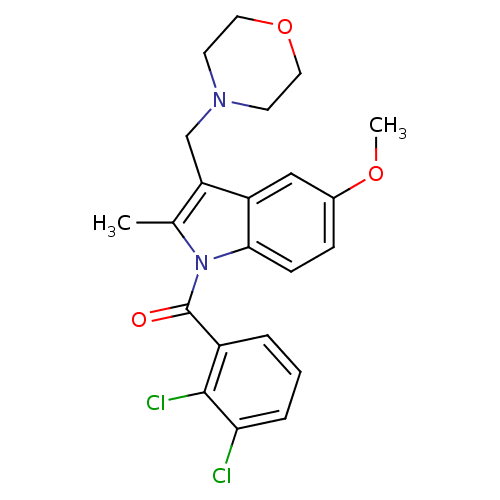 ((2,3-Dichloro-phenyl)-(5-methoxy-2-methyl-3-morpho...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor | J Med Chem 52: 369-78 (2009) Article DOI: 10.1021/jm801044g BindingDB Entry DOI: 10.7270/Q2VQ33M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1382 total ) | Next | Last >> |