

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM50012951 (4-Cyclohexyl-2-hydroxy-3-[3-(1H-imidazol-4-yl)-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Tested for inhibition of renin from human | J Med Chem 33: 2707-14 (1990) BindingDB Entry DOI: 10.7270/Q2N29XJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012951 (4-Cyclohexyl-2-hydroxy-3-[3-(1H-imidazol-4-yl)-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Tested for inhibition of human plasma renin | J Med Chem 33: 2707-14 (1990) BindingDB Entry DOI: 10.7270/Q2N29XJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012950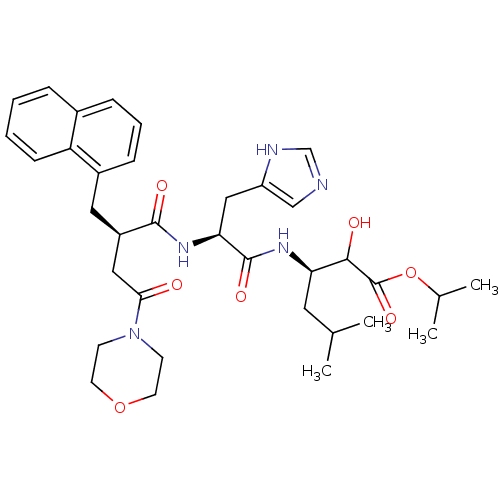 (2-Hydroxy-3-[3-(1H-imidazol-4-yl)-2-(4-morpholin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Tested for inhibition of human plasma renin | J Med Chem 33: 2707-14 (1990) BindingDB Entry DOI: 10.7270/Q2N29XJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012950 (2-Hydroxy-3-[3-(1H-imidazol-4-yl)-2-(4-morpholin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Tested for inhibition of renin from human | J Med Chem 33: 2707-14 (1990) BindingDB Entry DOI: 10.7270/Q2N29XJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50346122 (2-(4-(2-(4-bromo-5-methylthiophen-2-yl)-5-ethyl-6-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates | Bioorg Med Chem Lett 19: 3174-6 (2009) Article DOI: 10.1016/j.bmcl.2009.04.121 BindingDB Entry DOI: 10.7270/Q26110NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50128065 (CHEMBL304858 | CHEMBL324162 | N-allyl-6-(4-(4-brom...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Inhibition of human C-terminal His6-tagged OSC expressed in Pichia pastoris GS115 using 2,3-oxidosqualene as substrate after 90 mins by 1H NMR method | J Med Chem 61: 5047-5053 (2018) Article DOI: 10.1021/acs.jmedchem.8b00484 BindingDB Entry DOI: 10.7270/Q2NK3HN7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022531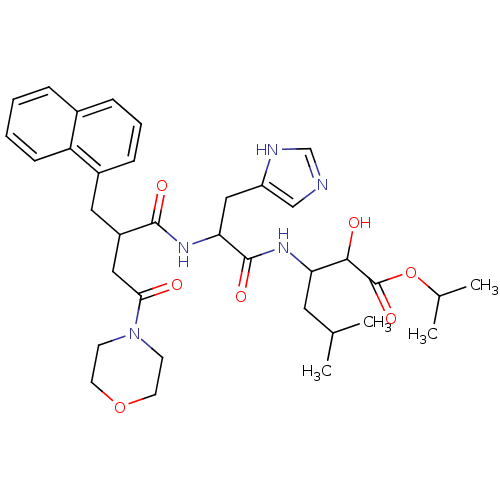 ((+)2-Hydroxy-3-[3-(1H-imidazol-4-yl)-2-(4-morpholi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration was measured against plasma renin from human | J Med Chem 31: 701-4 (1988) BindingDB Entry DOI: 10.7270/Q26H4GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM912 ((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Tested for inhibition of Cathepsin D from bovine | J Med Chem 33: 2707-14 (1990) BindingDB Entry DOI: 10.7270/Q2N29XJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022525 (2-Hydroxy-3-[3-(1H-imidazol-4-yl)-2-(4-morpholin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against human renin | J Med Chem 31: 701-4 (1988) BindingDB Entry DOI: 10.7270/Q26H4GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50346121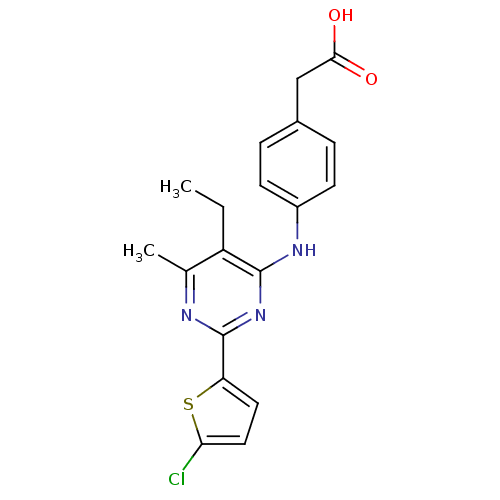 (2-(4-(2-(5-chlorothiophen-2-yl)-5-ethyl-6-methylpy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates | Bioorg Med Chem Lett 19: 3174-6 (2009) Article DOI: 10.1016/j.bmcl.2009.04.121 BindingDB Entry DOI: 10.7270/Q26110NQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50346123 (2-(4-(5-ethyl-2-(5-fluorothiophen-2-yl)-6-methylpy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates | Bioorg Med Chem Lett 19: 3174-6 (2009) Article DOI: 10.1016/j.bmcl.2009.04.121 BindingDB Entry DOI: 10.7270/Q26110NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50456340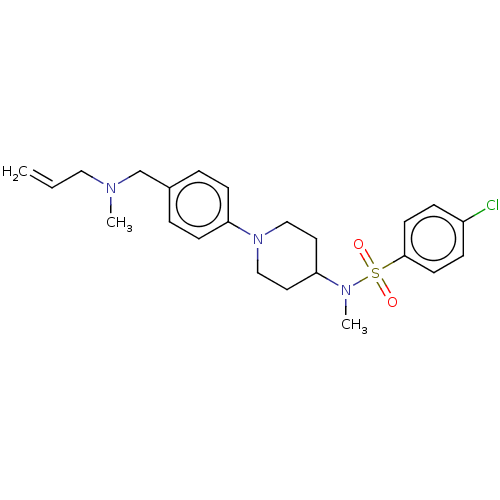 (CHEMBL4207987) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Inhibition of human C-terminal His6-tagged OSC expressed in Pichia pastoris GS115 using 2,3-oxidosqualene as substrate after 90 mins by 1H NMR method | J Med Chem 61: 5047-5053 (2018) Article DOI: 10.1021/acs.jmedchem.8b00484 BindingDB Entry DOI: 10.7270/Q2NK3HN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM912 ((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Tested for inhibition of pepsin from porcine | J Med Chem 33: 2707-14 (1990) BindingDB Entry DOI: 10.7270/Q2N29XJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50346120 (2-(4-(2-(5-bromothiophen-2-yl)-5-ethyl-6-methylpyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates | Bioorg Med Chem Lett 19: 3174-6 (2009) Article DOI: 10.1016/j.bmcl.2009.04.121 BindingDB Entry DOI: 10.7270/Q26110NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022526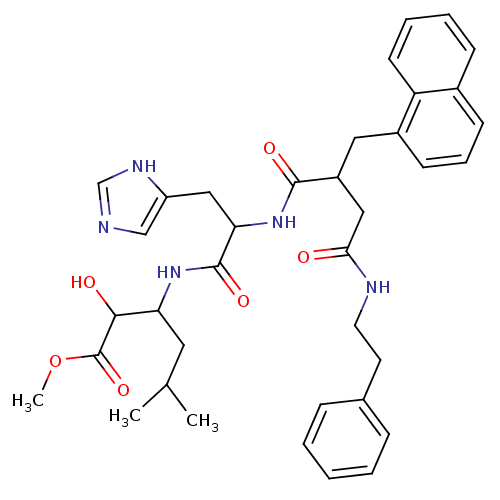 ((-)2-Hydroxy-3-{3-(1H-imidazol-4-yl)-2-[3-naphthal...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration was measured against plasma renin from dog | J Med Chem 31: 701-4 (1988) BindingDB Entry DOI: 10.7270/Q26H4GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022529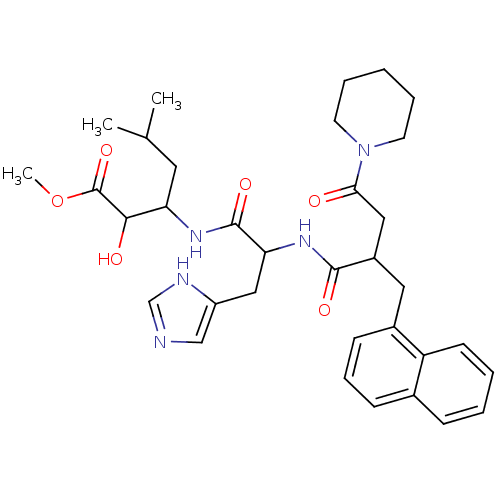 (2-Hydroxy-3-[3-(1H-imidazol-4-yl)-2-(2-naphthalen-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration was measured against plasma renin from dog | J Med Chem 31: 701-4 (1988) BindingDB Entry DOI: 10.7270/Q26H4GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022530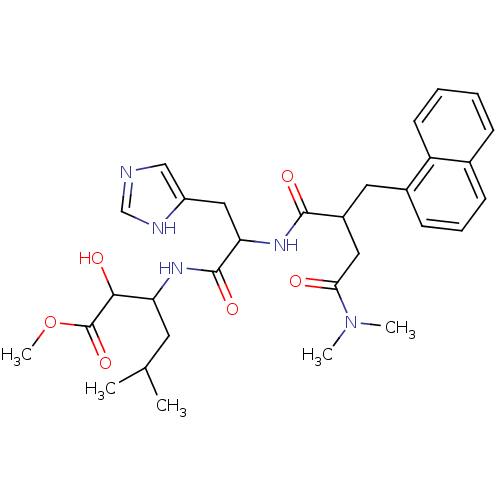 (3-[2-(3-Dimethylcarbamoyl-2-naphthalen-1-ylmethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration was measured against plasma renin from human | J Med Chem 31: 701-4 (1988) BindingDB Entry DOI: 10.7270/Q26H4GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022530 (3-[2-(3-Dimethylcarbamoyl-2-naphthalen-1-ylmethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration was measured against plasma renin from dog | J Med Chem 31: 701-4 (1988) BindingDB Entry DOI: 10.7270/Q26H4GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022531 ((+)2-Hydroxy-3-[3-(1H-imidazol-4-yl)-2-(4-morpholi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration was measured against plasma renin from dog | J Med Chem 31: 701-4 (1988) BindingDB Entry DOI: 10.7270/Q26H4GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50346088 ((1r,4r)-4-cyano-4-(3-(cyclopentyloxy)-4-methoxyphe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human PDE4D3 incubated for 10 mins using cAMP and [3H]cAMP substrates | Bioorg Med Chem Lett 19: 3174-6 (2009) Article DOI: 10.1016/j.bmcl.2009.04.121 BindingDB Entry DOI: 10.7270/Q26110NQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50346119 (2-(4-(5-ethyl-6-methyl-2-(5-(methylthio)thiophen-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates | Bioorg Med Chem Lett 19: 3174-6 (2009) Article DOI: 10.1016/j.bmcl.2009.04.121 BindingDB Entry DOI: 10.7270/Q26110NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50346100 (4-(5-allyl-6-ethyl-2-phenylpyrimidin-4-ylamino)ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates | Bioorg Med Chem Lett 19: 3174-6 (2009) Article DOI: 10.1016/j.bmcl.2009.04.121 BindingDB Entry DOI: 10.7270/Q26110NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50346088 ((1r,4r)-4-cyano-4-(3-(cyclopentyloxy)-4-methoxyphe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates | Bioorg Med Chem Lett 19: 3174-6 (2009) Article DOI: 10.1016/j.bmcl.2009.04.121 BindingDB Entry DOI: 10.7270/Q26110NQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022529 (2-Hydroxy-3-[3-(1H-imidazol-4-yl)-2-(2-naphthalen-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration was measured against plasma renin from human | J Med Chem 31: 701-4 (1988) BindingDB Entry DOI: 10.7270/Q26H4GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022525 (2-Hydroxy-3-[3-(1H-imidazol-4-yl)-2-(4-morpholin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against human renin | J Med Chem 31: 701-4 (1988) BindingDB Entry DOI: 10.7270/Q26H4GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022530 (3-[2-(3-Dimethylcarbamoyl-2-naphthalen-1-ylmethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration was measured against plasma renin from human | J Med Chem 31: 701-4 (1988) BindingDB Entry DOI: 10.7270/Q26H4GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022527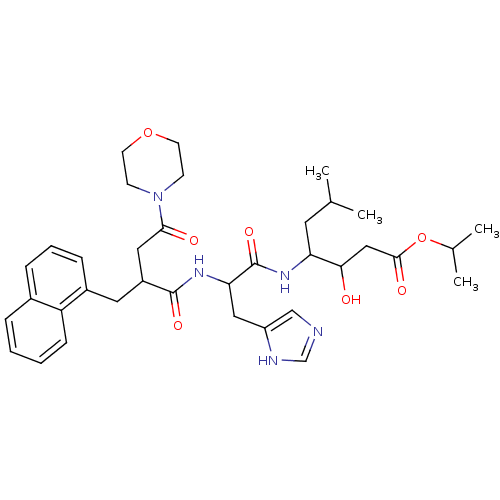 (3-Hydroxy-4-[3-(1H-imidazol-4-yl)-2-(4-morpholin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration was measured against plasma renin from dog | J Med Chem 31: 701-4 (1988) BindingDB Entry DOI: 10.7270/Q26H4GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022527 (3-Hydroxy-4-[3-(1H-imidazol-4-yl)-2-(4-morpholin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration was measured against plasma renin from human | J Med Chem 31: 701-4 (1988) BindingDB Entry DOI: 10.7270/Q26H4GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50346104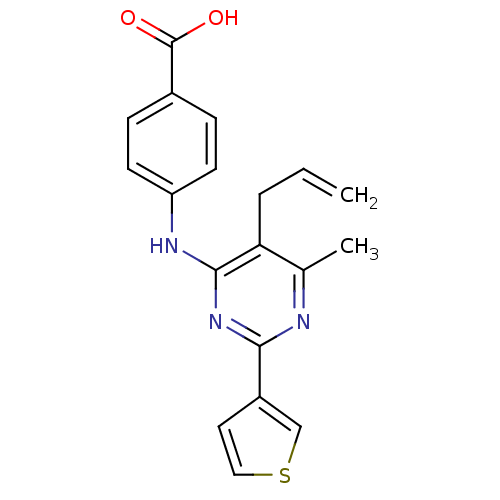 (4-(5-allyl-6-methyl-2-(thiophen-3-yl)pyrimidin-4-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates | Bioorg Med Chem Lett 19: 3174-6 (2009) Article DOI: 10.1016/j.bmcl.2009.04.121 BindingDB Entry DOI: 10.7270/Q26110NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50346112 (4-(5-allyl-2-(5-methoxythiophen-2-yl)-6-methylpyri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates | Bioorg Med Chem Lett 19: 3174-6 (2009) Article DOI: 10.1016/j.bmcl.2009.04.121 BindingDB Entry DOI: 10.7270/Q26110NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022526 ((-)2-Hydroxy-3-{3-(1H-imidazol-4-yl)-2-[3-naphthal...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against human renin | J Med Chem 31: 701-4 (1988) BindingDB Entry DOI: 10.7270/Q26H4GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50346100 (4-(5-allyl-6-ethyl-2-phenylpyrimidin-4-ylamino)ben...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human PDE4D3 incubated for 10 mins using cAMP and [3H]cAMP substrates | Bioorg Med Chem Lett 19: 3174-6 (2009) Article DOI: 10.1016/j.bmcl.2009.04.121 BindingDB Entry DOI: 10.7270/Q26110NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022526 ((-)2-Hydroxy-3-{3-(1H-imidazol-4-yl)-2-[3-naphthal...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration was measured against plasma renin from human | J Med Chem 31: 701-4 (1988) BindingDB Entry DOI: 10.7270/Q26H4GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012952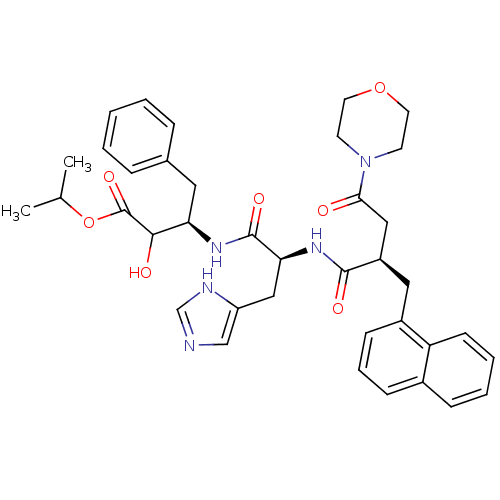 (2-Hydroxy-3-[3-(1H-imidazol-4-yl)-2-(4-morpholin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Tested for inhibition of renin from human | J Med Chem 33: 2707-14 (1990) BindingDB Entry DOI: 10.7270/Q2N29XJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50346103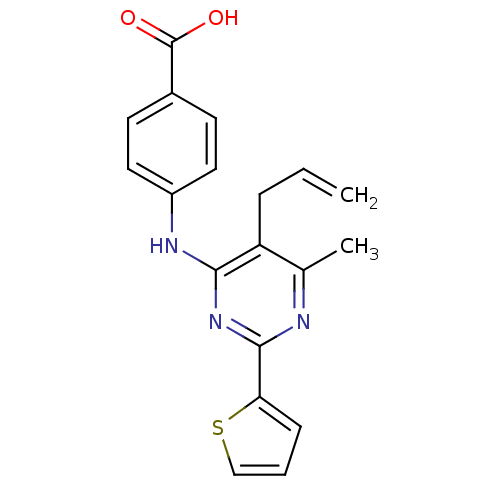 (4-(5-allyl-6-methyl-2-(thiophen-2-yl)pyrimidin-4-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates | Bioorg Med Chem Lett 19: 3174-6 (2009) Article DOI: 10.1016/j.bmcl.2009.04.121 BindingDB Entry DOI: 10.7270/Q26110NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50346096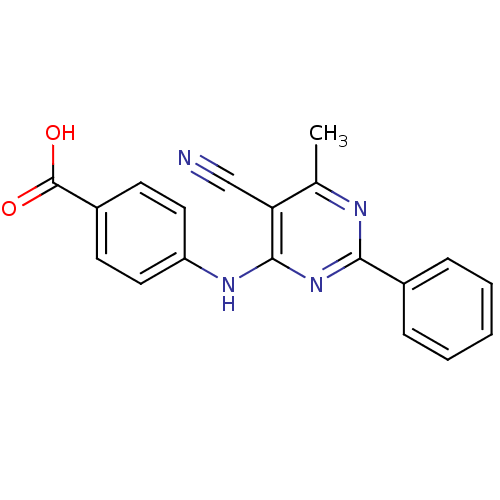 (4-(5-cyano-6-methyl-2-phenylpyrimidin-4-ylamino)be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates | Bioorg Med Chem Lett 19: 3174-6 (2009) Article DOI: 10.1016/j.bmcl.2009.04.121 BindingDB Entry DOI: 10.7270/Q26110NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50456341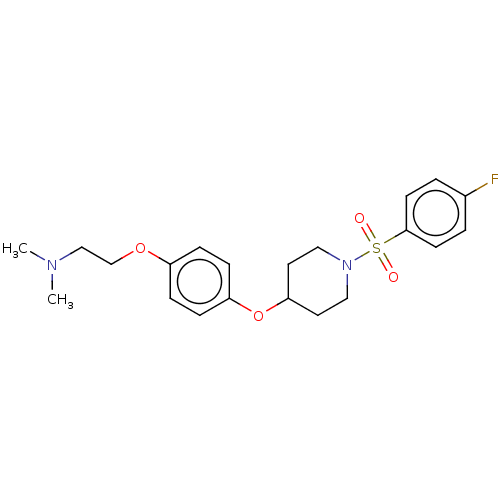 (CHEMBL4212932) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Inhibition of human C-terminal His6-tagged OSC expressed in Pichia pastoris GS115 using 2,3-oxidosqualene as substrate after 90 mins by 1H NMR method | J Med Chem 61: 5047-5053 (2018) Article DOI: 10.1021/acs.jmedchem.8b00484 BindingDB Entry DOI: 10.7270/Q2NK3HN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022524 (2-Hydroxy-3-[3-(1H-imidazol-4-yl)-2-(4-morpholin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against human renin | J Med Chem 31: 701-4 (1988) BindingDB Entry DOI: 10.7270/Q26H4GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022524 (2-Hydroxy-3-[3-(1H-imidazol-4-yl)-2-(4-morpholin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against human renin | J Med Chem 31: 701-4 (1988) BindingDB Entry DOI: 10.7270/Q26H4GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50346091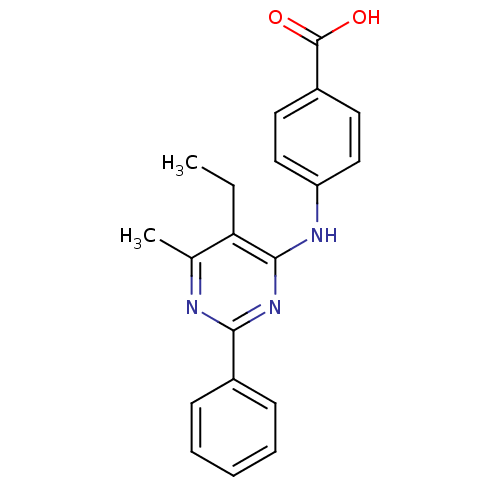 (4-(5-ethyl-6-methyl-2-phenylpyrimidin-4-ylamino)be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates | Bioorg Med Chem Lett 19: 3174-6 (2009) Article DOI: 10.1016/j.bmcl.2009.04.121 BindingDB Entry DOI: 10.7270/Q26110NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022531 ((+)2-Hydroxy-3-[3-(1H-imidazol-4-yl)-2-(4-morpholi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against human renin | J Med Chem 31: 701-4 (1988) BindingDB Entry DOI: 10.7270/Q26H4GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50346111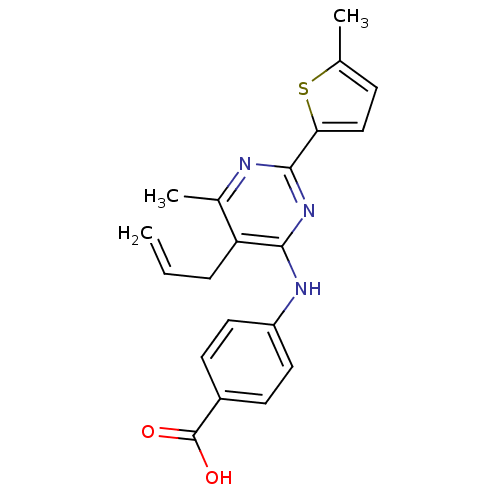 (4-(5-allyl-6-methyl-2-(5-methylthiophen-2-yl)pyrim...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates | Bioorg Med Chem Lett 19: 3174-6 (2009) Article DOI: 10.1016/j.bmcl.2009.04.121 BindingDB Entry DOI: 10.7270/Q26110NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50346089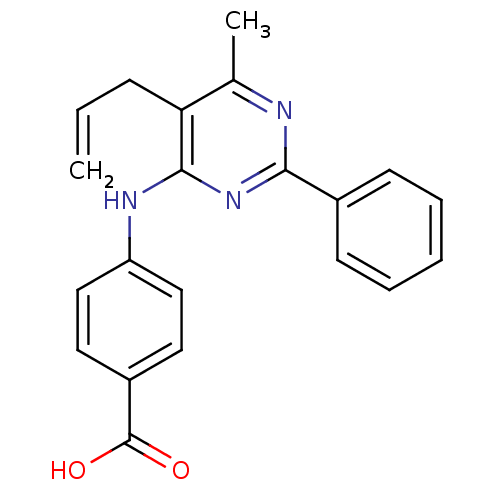 (4-(5-allyl-6-methyl-2-phenylpyrimidin-4-ylamino)be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates | Bioorg Med Chem Lett 19: 3174-6 (2009) Article DOI: 10.1016/j.bmcl.2009.04.121 BindingDB Entry DOI: 10.7270/Q26110NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50346118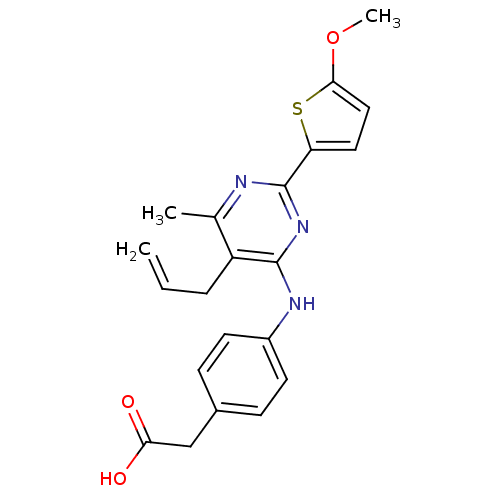 (2-(4-(5-allyl-2-(5-methoxythiophen-2-yl)-6-methylp...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates | Bioorg Med Chem Lett 19: 3174-6 (2009) Article DOI: 10.1016/j.bmcl.2009.04.121 BindingDB Entry DOI: 10.7270/Q26110NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50346109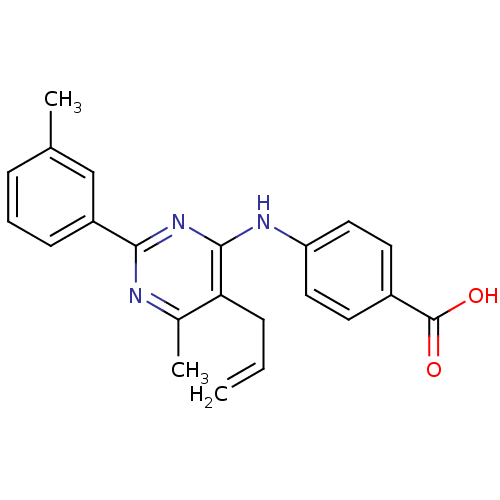 (4-(5-allyl-6-methyl-2-m-tolylpyrimidin-4-ylamino)b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates | Bioorg Med Chem Lett 19: 3174-6 (2009) Article DOI: 10.1016/j.bmcl.2009.04.121 BindingDB Entry DOI: 10.7270/Q26110NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jnatprod.2c00395 BindingDB Entry DOI: 10.7270/Q23N27FK | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50346108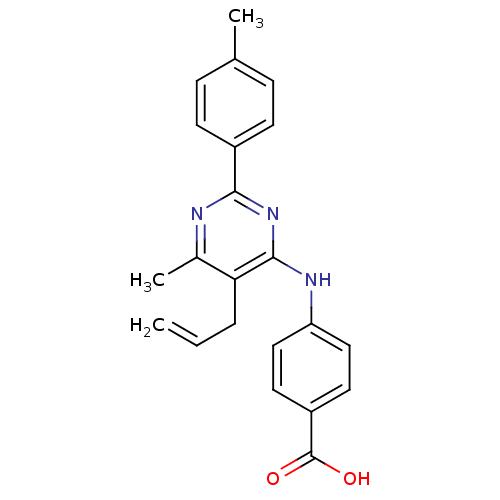 (4-(5-allyl-6-methyl-2-p-tolylpyrimidin-4-ylamino)b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates | Bioorg Med Chem Lett 19: 3174-6 (2009) Article DOI: 10.1016/j.bmcl.2009.04.121 BindingDB Entry DOI: 10.7270/Q26110NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50346110 (4-(5-allyl-2-(4-methoxyphenyl)-6-methylpyrimidin-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates | Bioorg Med Chem Lett 19: 3174-6 (2009) Article DOI: 10.1016/j.bmcl.2009.04.121 BindingDB Entry DOI: 10.7270/Q26110NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50456342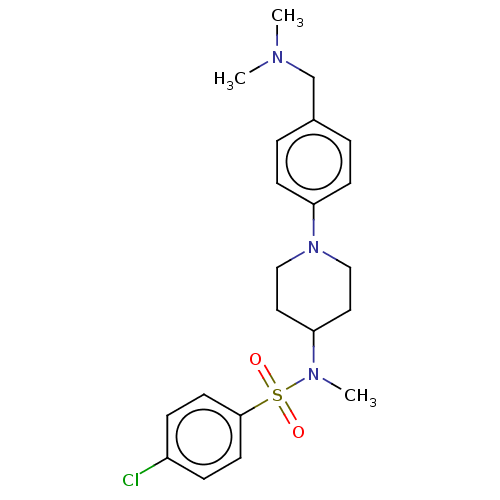 (CHEMBL4210513) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Inhibition of human C-terminal His6-tagged OSC expressed in Pichia pastoris GS115 using 2,3-oxidosqualene as substrate after 90 mins by 1H NMR method | J Med Chem 61: 5047-5053 (2018) Article DOI: 10.1021/acs.jmedchem.8b00484 BindingDB Entry DOI: 10.7270/Q2NK3HN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012952 (2-Hydroxy-3-[3-(1H-imidazol-4-yl)-2-(4-morpholin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Tested for inhibition of human plasma renin | J Med Chem 33: 2707-14 (1990) BindingDB Entry DOI: 10.7270/Q2N29XJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 191 total ) | Next | Last >> |