 Found 1867 hits with Last Name = 'schmidt' and Initial = 'w'
Found 1867 hits with Last Name = 'schmidt' and Initial = 'w' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50595424
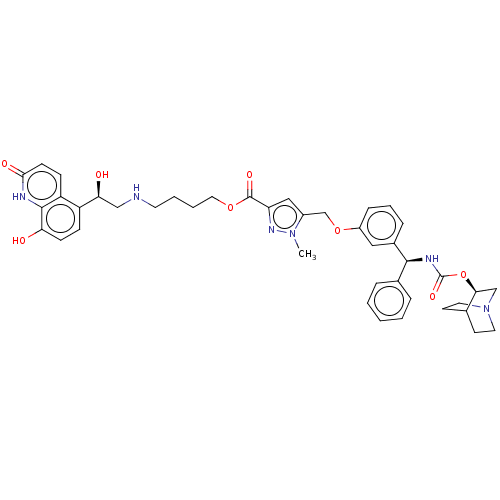
(CHEMBL5172865)Show SMILES Cn1nc(cc1COc1cccc(c1)[C@@H](NC(=O)O[C@H]1CN2CCC1CC2)c1ccccc1)C(=O)OCCCCNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:42.47,wD:14.16,19.20,(.73,4.28,;.18,2.84,;-1.31,2.44,;-1.39,.91,;.04,.35,;1.01,1.55,;2.55,1.47,;3.25,.1,;4.79,.01,;5.49,-1.36,;7.02,-1.44,;7.86,-.15,;7.16,1.22,;5.63,1.3,;8,2.51,;9.54,2.43,;10.24,1.06,;9.4,-.23,;11.78,.98,;12.48,-.4,;11.64,-1.69,;12.33,-3.06,;12.41,-1.64,;13.94,-1.9,;14.01,-.48,;14.71,-1.85,;13.87,-3.14,;7.31,3.89,;5.77,3.97,;5.07,5.34,;5.91,6.63,;7.45,6.55,;8.15,5.18,;-2.69,.07,;-2.61,-1.47,;-4.06,.77,;-5.35,-.07,;-6.72,.63,;-8.01,-.2,;-9.38,.5,;-10.68,-.34,;-12.05,.36,;-13.34,-.48,;-14.71,.22,;-13.26,-2.02,;-14.55,-2.86,;-14.47,-4.39,;-13.1,-5.09,;-13.02,-6.63,;-11.81,-4.26,;-10.44,-4.96,;-9.15,-4.12,;-7.77,-4.82,;-9.23,-2.58,;-10.6,-1.88,;-11.89,-2.72,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569290
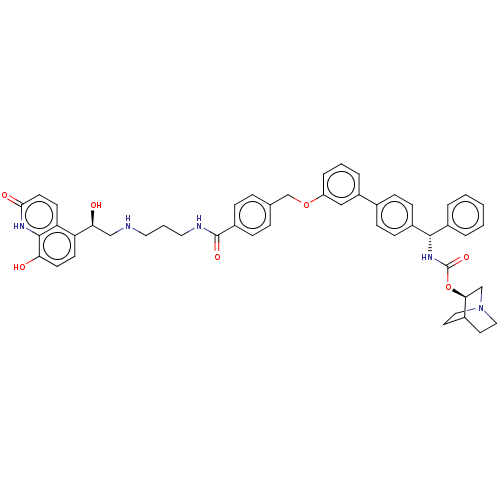
(CHEMBL4871517)Show SMILES O[C@@H](CNCCCNC(=O)c1ccc(COc2cccc(c2)-c2ccc(cc2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:33.34,1.0,wD:28.30,(31.17,-6.07,;32.5,-6.84,;33.84,-6.06,;35.17,-6.83,;36.5,-6.05,;37.84,-6.82,;39.17,-6.05,;40.51,-6.81,;41.84,-6.04,;41.83,-4.5,;43.17,-6.8,;43.18,-8.34,;44.51,-9.11,;45.84,-8.33,;47.18,-9.1,;48.51,-8.32,;49.85,-9.08,;49.85,-10.62,;51.19,-11.38,;52.52,-10.61,;52.51,-9.06,;51.17,-8.3,;53.84,-8.28,;55.17,-9.04,;56.51,-8.27,;56.5,-6.72,;55.16,-5.96,;53.84,-6.74,;57.83,-5.94,;59.17,-6.7,;60.5,-5.92,;60.49,-4.38,;61.84,-6.69,;63.17,-5.91,;63.15,-4.38,;64.49,-3.61,;65.83,-4.37,;65.83,-5.91,;64.5,-6.68,;65.2,-5.33,;63.71,-4.93,;57.82,-4.4,;59.15,-3.63,;59.15,-2.09,;57.81,-1.33,;56.47,-2.11,;56.49,-3.65,;45.83,-6.78,;44.5,-6.02,;32.51,-8.38,;31.18,-9.15,;31.18,-10.69,;32.51,-11.46,;32.51,-13,;33.84,-10.69,;35.17,-11.46,;36.51,-10.69,;37.85,-11.46,;36.52,-9.14,;35.18,-8.36,;33.84,-9.14,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 125I-cyanopindolol from human adrenergic beta2 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569294
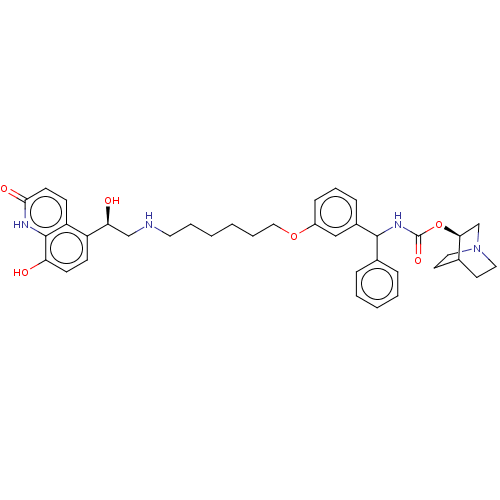
(CHEMBL4863525)Show SMILES O[C@@H](CNCCCCCCOc1cccc(c1)C(NC(=O)O[C@H]1CN2CCC1CC2)c1ccccc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:22.22,1.0,(-.19,-37.45,;1.15,-38.22,;2.48,-37.45,;3.82,-38.21,;5.15,-37.44,;6.49,-38.2,;7.82,-37.43,;9.15,-38.2,;10.48,-37.42,;11.82,-38.19,;13.15,-37.41,;14.49,-38.19,;14.48,-39.72,;15.82,-40.49,;17.15,-39.72,;17.14,-38.17,;15.8,-37.41,;18.47,-37.38,;19.81,-38.15,;21.14,-37.37,;21.13,-35.83,;22.48,-38.13,;23.81,-37.35,;23.79,-35.82,;25.13,-35.05,;26.47,-35.82,;26.47,-37.36,;25.14,-38.13,;25.84,-36.77,;24.35,-36.38,;18.46,-35.85,;19.79,-35.08,;19.79,-33.54,;18.45,-32.77,;17.12,-33.56,;17.13,-35.09,;1.16,-39.76,;-.18,-40.53,;-.18,-42.07,;1.16,-42.85,;1.16,-44.39,;2.49,-42.07,;3.82,-42.84,;5.16,-42.07,;6.49,-42.85,;5.16,-40.53,;3.82,-39.75,;2.49,-40.52,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 125I-cyanopindolol from human adrenergic beta2 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50595404

(CHEMBL5184455)Show SMILES O[C@@H](CNCCCCOC(=O)c1ccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:23.24,28.28,(-14.67,-1.16,;-13.33,-1.93,;-12,-1.16,;-10.67,-1.93,;-9.33,-1.16,;-8,-1.93,;-6.66,-1.16,;-5.33,-1.93,;-4,-1.16,;-2.66,-1.93,;-2.66,-3.47,;-1.33,-1.16,;.01,-1.93,;1.34,-1.16,;1.34,.38,;2.67,1.15,;4.01,.38,;5.34,1.15,;6.67,.39,;8,1.16,;8,2.69,;6.67,3.46,;5.34,2.69,;6.67,5,;8.01,5.77,;9.34,5,;9.34,3.46,;10.67,5.77,;12.01,5,;12.01,3.46,;13.34,2.69,;12.63,3.93,;14.05,4.54,;13.34,5.77,;14.67,5,;14.67,3.46,;5.34,5.77,;5.34,7.31,;4,8.08,;2.67,7.31,;2.67,5.77,;4,5,;-0,1.15,;-1.33,.38,;-13.33,-3.47,;-14.67,-4.24,;-14.67,-5.78,;-13.34,-6.54,;-13.34,-8.08,;-12,-5.77,;-10.67,-6.55,;-9.34,-5.77,;-8.01,-6.54,;-9.34,-4.24,;-10.67,-3.47,;-12,-4.24,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50059585
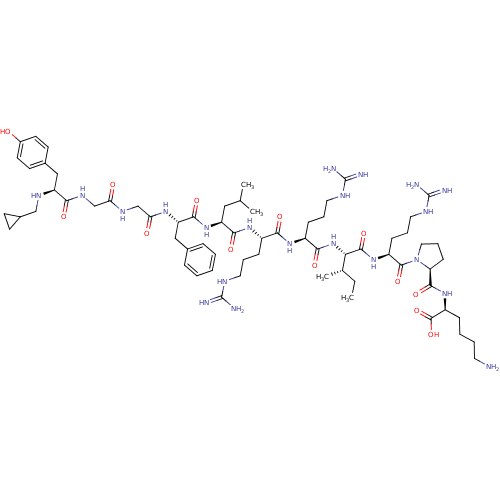
(CHEMBL442323 | N-CPM[D-Pro-10]Dyn A-(1-11))Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NCC1CC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(O)=O Show InChI InChI=1S/C67H109N21O13/c1-5-40(4)55(62(98)84-47(20-13-31-77-67(73)74)63(99)88-32-14-21-52(88)61(97)85-48(64(100)101)17-9-10-28-68)87-58(94)46(19-12-30-76-66(71)72)82-57(93)45(18-11-29-75-65(69)70)83-59(95)50(33-39(2)3)86-60(96)51(35-41-15-7-6-8-16-41)81-54(91)38-79-53(90)37-80-56(92)49(78-36-43-22-23-43)34-42-24-26-44(89)27-25-42/h6-8,15-16,24-27,39-40,43,45-52,55,78,89H,5,9-14,17-23,28-38,68H2,1-4H3,(H,79,90)(H,80,92)(H,81,91)(H,82,93)(H,83,95)(H,84,98)(H,85,97)(H,86,96)(H,87,94)(H,100,101)(H4,69,70,75)(H4,71,72,76)(H4,73,74,77)/t40-,45-,46-,47-,48-,49-,50-,51-,52-,55-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Affinity against Opioid receptor kappa 1 was determined by measuring the inhibition of [3H]-bremazocine binding to guinea pig cerebellar membranes |
J Med Chem 40: 2733-9 (1997)
Article DOI: 10.1021/jm960747t
BindingDB Entry DOI: 10.7270/Q2D50M2D |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50595426
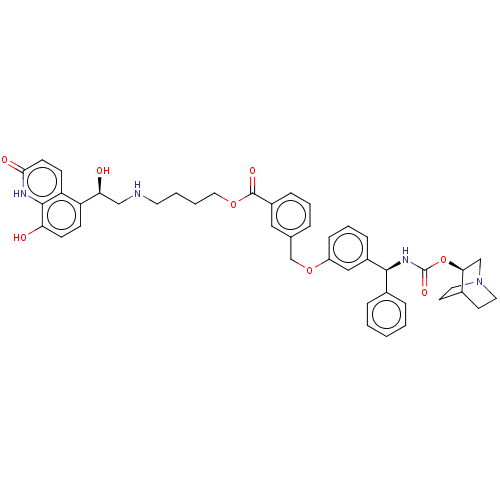
(CHEMBL5193853)Show SMILES O[C@@H](CNCCCCOC(=O)c1cccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)c1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:24.25,29.29,(-13.81,.82,;-12.39,.23,;-11.16,1.16,;-9.74,.56,;-8.52,1.49,;-7.1,.89,;-5.87,1.82,;-4.45,1.23,;-3.22,2.16,;-1.8,1.56,;-1.61,.03,;-.58,2.49,;-.77,4.02,;.46,4.95,;1.88,4.35,;2.07,2.82,;3.49,2.22,;3.68,.7,;5.1,.1,;5.29,-1.43,;6.71,-2.03,;7.94,-1.1,;7.75,.43,;6.33,1.03,;8.98,1.36,;10.4,.76,;10.59,-.76,;9.36,-1.69,;12.01,-1.36,;12.2,-2.89,;10.97,-3.82,;11.16,-5.35,;11.72,-4.04,;13.07,-4.8,;13.62,-3.49,;13.81,-5.02,;12.58,-5.95,;8.78,2.89,;7.36,3.49,;7.17,5.02,;8.4,5.95,;9.82,5.35,;10.01,3.82,;.84,1.89,;-12.39,-1.31,;-13.73,-2.09,;-13.72,-3.63,;-12.39,-4.39,;-12.39,-5.93,;-11.06,-3.62,;-9.72,-4.39,;-8.4,-3.62,;-7.06,-4.39,;-8.4,-2.09,;-9.73,-1.32,;-11.06,-2.08,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50595423
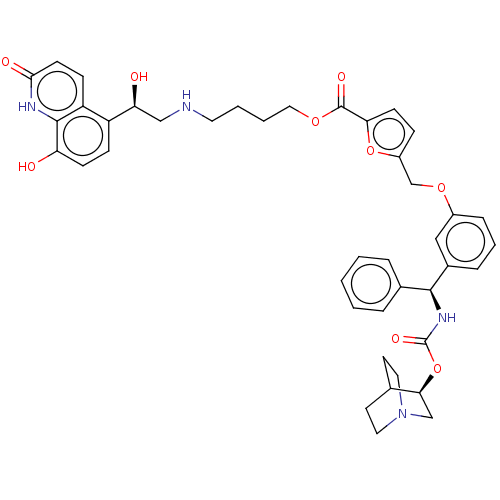
(CHEMBL5190387)Show SMILES O[C@@H](CNCCCCOC(=O)c1ccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)o1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:23.24,28.28,(-14.71,.22,;-13.34,-.48,;-12.05,.36,;-10.68,-.34,;-9.38,.5,;-8.01,-.2,;-6.72,.63,;-5.35,-.07,;-4.06,.77,;-2.69,.07,;-2.61,-1.47,;-1.39,.91,;-1.31,2.44,;.18,2.84,;1.01,1.55,;2.55,1.47,;3.25,.1,;4.79,.01,;5.49,-1.36,;7.02,-1.44,;7.86,-.15,;7.16,1.22,;5.63,1.3,;8,2.51,;9.54,2.43,;10.24,1.06,;9.4,-.23,;11.78,.98,;12.48,-.4,;11.64,-1.69,;12.33,-3.06,;12.41,-1.64,;13.94,-1.9,;14.01,-.48,;14.71,-1.85,;13.87,-3.14,;7.31,3.89,;5.77,3.97,;5.07,5.34,;5.91,6.63,;7.45,6.55,;8.15,5.18,;.04,.35,;-13.26,-2.02,;-14.55,-2.86,;-14.47,-4.39,;-13.1,-5.09,;-13.02,-6.63,;-11.81,-4.26,;-10.44,-4.96,;-9.15,-4.12,;-7.77,-4.82,;-9.23,-2.58,;-10.6,-1.88,;-11.89,-2.72,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50595427
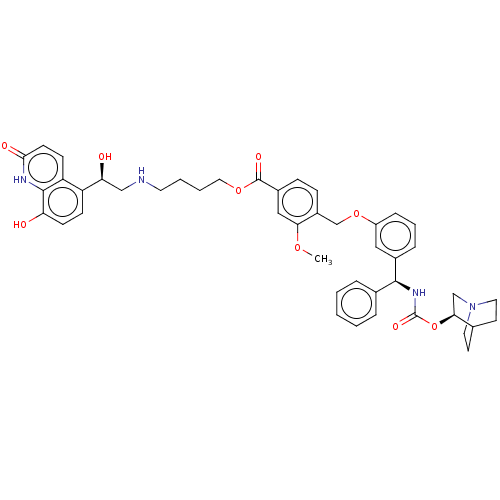
(CHEMBL5200887)Show SMILES COc1cc(ccc1COc1cccc(c1)[C@@H](NC(=O)O[C@H]1CN2CCC1CC2)c1ccccc1)C(=O)OCCCCNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:44.49,wD:16.18,21.22,(3.34,-1.15,;3.34,.39,;2,1.16,;.67,.39,;-.67,1.15,;-.67,2.69,;.67,3.47,;2,2.7,;3.33,3.47,;4.67,2.7,;6,3.47,;6,5.01,;7.33,5.78,;8.67,5.02,;8.67,3.48,;7.33,2.7,;10,2.71,;10,1.17,;8.67,.4,;7.34,1.16,;8.67,-1.14,;7.34,-1.92,;6.01,-1.15,;4.67,-1.92,;6.1,-1.92,;5.92,-3.46,;7.34,-3.46,;6.01,-4.23,;4.68,-3.46,;11.33,3.48,;11.33,5.02,;12.67,5.79,;14,5.02,;14,3.48,;12.67,2.71,;-2,.38,;-2,-1.16,;-3.33,1.15,;-4.67,.38,;-6,1.15,;-7.33,.38,;-8.67,1.14,;-10,.37,;-11.33,1.14,;-12.67,.37,;-14,1.14,;-12.67,-1.17,;-14,-1.94,;-14,-3.48,;-12.66,-4.25,;-12.66,-5.79,;-11.33,-3.48,;-9.99,-4.25,;-8.66,-3.48,;-7.33,-4.24,;-8.66,-1.94,;-10,-1.17,;-11.33,-1.94,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569293
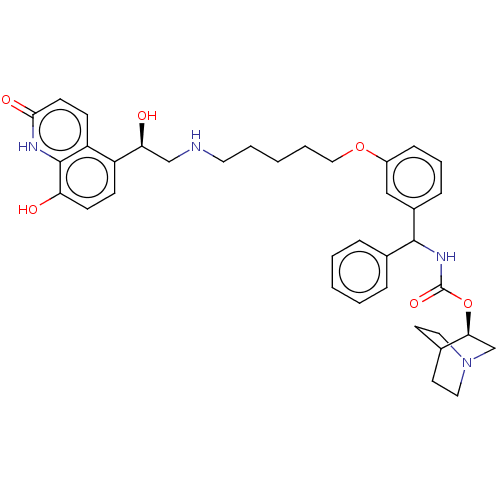
(CHEMBL4874819)Show SMILES O[C@@H](CNCCCCCOc1cccc(c1)C(NC(=O)O[C@H]1CN2CCC1CC2)c1ccccc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:21.21,(24.25,-39.74,;25.59,-40.51,;26.92,-39.73,;28.26,-40.5,;29.59,-39.72,;30.92,-40.49,;32.25,-39.72,;33.59,-40.48,;34.92,-39.71,;36.26,-40.47,;37.6,-39.7,;37.59,-38.17,;38.91,-37.39,;40.26,-38.15,;40.26,-39.7,;38.93,-40.47,;41.6,-40.46,;42.93,-39.69,;44.26,-40.45,;44.27,-42,;45.59,-39.67,;46.93,-40.44,;46.92,-41.99,;48.25,-42.75,;49.59,-41.98,;49.59,-40.44,;48.25,-39.67,;49.01,-41,;47.47,-41.41,;41.6,-42,;40.26,-42.77,;40.27,-44.31,;41.6,-45.07,;42.94,-44.29,;42.93,-42.76,;25.59,-42.05,;24.26,-42.82,;24.26,-44.36,;25.6,-45.13,;25.6,-46.67,;26.93,-44.36,;28.26,-45.13,;29.6,-44.36,;30.93,-45.13,;29.6,-42.81,;28.26,-42.03,;26.93,-42.81,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 125I-cyanopindolol from human adrenergic beta2 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50059583

(CHEMBL268818 | N-benzyl[D-Pro-10]Dyn A-(1-11) | Ph...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NCc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(O)=O Show InChI InChI=1S/C70H109N21O13/c1-5-43(4)58(65(101)87-50(25-16-34-80-70(76)77)66(102)91-35-17-26-55(91)64(100)88-51(67(103)104)22-12-13-31-71)90-61(97)49(24-15-33-79-69(74)75)85-60(96)48(23-14-32-78-68(72)73)86-62(98)53(36-42(2)3)89-63(99)54(38-44-18-8-6-9-19-44)84-57(94)41-82-56(93)40-83-59(95)52(37-45-27-29-47(92)30-28-45)81-39-46-20-10-7-11-21-46/h6-11,18-21,27-30,42-43,48-55,58,81,92H,5,12-17,22-26,31-41,71H2,1-4H3,(H,82,93)(H,83,95)(H,84,94)(H,85,96)(H,86,98)(H,87,101)(H,88,100)(H,89,99)(H,90,97)(H,103,104)(H4,72,73,78)(H4,74,75,79)(H4,76,77,80)/t43-,48-,49-,50-,51-,52-,53-,54-,55-,58-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Affinity against Opioid receptor kappa 1 was determined by measuring the inhibition of [3H]-bremazocine binding to guinea pig cerebellar membranes |
J Med Chem 40: 2733-9 (1997)
Article DOI: 10.1021/jm960747t
BindingDB Entry DOI: 10.7270/Q2D50M2D |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50002346
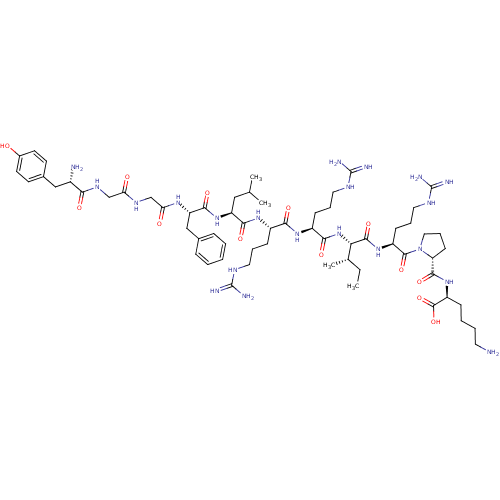
(CHEMBL384584 | [D-pro10]Dynorphin A(1-11))Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCCN)C(O)=O Show InChI InChI=1S/C63H103N21O13/c1-5-37(4)51(58(94)80-44(20-13-29-74-63(70)71)59(95)84-30-14-21-48(84)57(93)81-45(60(96)97)17-9-10-26-64)83-54(90)43(19-12-28-73-62(68)69)78-53(89)42(18-11-27-72-61(66)67)79-55(91)46(31-36(2)3)82-56(92)47(33-38-15-7-6-8-16-38)77-50(87)35-75-49(86)34-76-52(88)41(65)32-39-22-24-40(85)25-23-39/h6-8,15-16,22-25,36-37,41-48,51,85H,5,9-14,17-21,26-35,64-65H2,1-4H3,(H,75,86)(H,76,88)(H,77,87)(H,78,89)(H,79,91)(H,80,94)(H,81,93)(H,82,92)(H,83,90)(H,96,97)(H4,66,67,72)(H4,68,69,73)(H4,70,71,74)/t37-,41-,42-,43-,44-,45-,46-,47-,48+,51-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Affinity against Opioid receptor kappa 1 was determined by measuring the inhibition of [3H]-bremazocine binding to guinea pig cerebellar membranes |
J Med Chem 40: 2733-9 (1997)
Article DOI: 10.1021/jm960747t
BindingDB Entry DOI: 10.7270/Q2D50M2D |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50595403
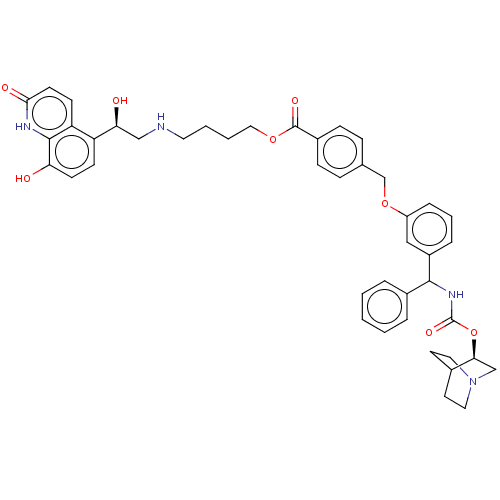
(CHEMBL5208201)Show SMILES O[C@@H](CNCCCCOC(=O)c1ccc(COc2cccc(c2)C(NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:28.28,(-14.67,-1.16,;-13.33,-1.93,;-12,-1.16,;-10.66,-1.93,;-9.33,-1.16,;-8,-1.93,;-6.66,-1.16,;-5.33,-1.93,;-3.99,-1.16,;-2.66,-1.93,;-2.66,-3.47,;-1.33,-1.16,;.01,-1.93,;1.34,-1.16,;1.34,.38,;2.67,1.15,;4.01,.38,;5.34,1.15,;6.67,.39,;8,1.16,;8,2.69,;6.67,3.46,;5.34,2.69,;6.67,5,;8,5.77,;9.34,5,;9.34,3.46,;10.67,5.77,;12.01,5,;12.01,3.46,;13.34,2.69,;14.67,3.46,;14.67,5,;13.34,5.77,;14.05,4.54,;12.63,3.93,;5.34,5.77,;5.34,7.31,;4,8.08,;2.67,7.31,;2.67,5.77,;4,5,;-0,1.15,;-1.33,.38,;-13.33,-3.47,;-14.67,-4.24,;-14.67,-5.78,;-13.33,-6.54,;-13.33,-8.08,;-12,-5.77,;-10.67,-6.54,;-9.34,-5.77,;-8.01,-6.54,;-9.34,-4.24,;-10.67,-3.47,;-12,-4.24,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569292
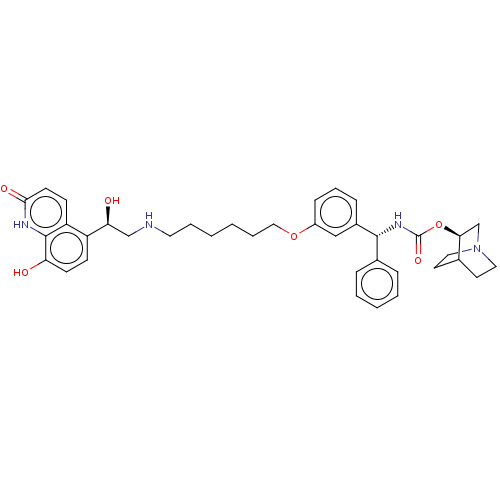
(CHEMBL4857743)Show SMILES O[C@@H](CNCCCCCCOc1cccc(c1)[C@H](NC(=O)O[C@H]1CN2CCC1CC2)c1ccccc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:17.18,22.22,1.0,(52.87,-39.55,;54.2,-40.32,;55.53,-39.55,;56.87,-40.31,;58.2,-39.54,;59.54,-40.3,;60.87,-39.53,;62.21,-40.3,;63.54,-39.52,;64.87,-40.29,;66.2,-39.51,;67.54,-40.29,;67.53,-41.82,;68.87,-42.59,;70.2,-41.82,;70.19,-40.27,;68.86,-39.51,;71.53,-39.48,;72.86,-40.25,;74.19,-39.47,;74.19,-37.93,;75.53,-40.23,;76.86,-39.45,;76.85,-37.93,;78.19,-37.15,;79.52,-37.92,;79.52,-39.46,;78.2,-40.23,;78.89,-38.87,;77.41,-38.48,;71.52,-37.95,;72.85,-37.18,;72.84,-35.64,;71.5,-34.87,;70.17,-35.66,;70.18,-37.19,;54.21,-41.86,;52.88,-42.63,;52.88,-44.17,;54.21,-44.95,;54.21,-46.49,;55.54,-44.17,;56.87,-44.94,;58.21,-44.17,;59.55,-44.95,;58.22,-42.63,;56.88,-41.85,;55.54,-42.62,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 125I-cyanopindolol from human adrenergic beta2 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50059586
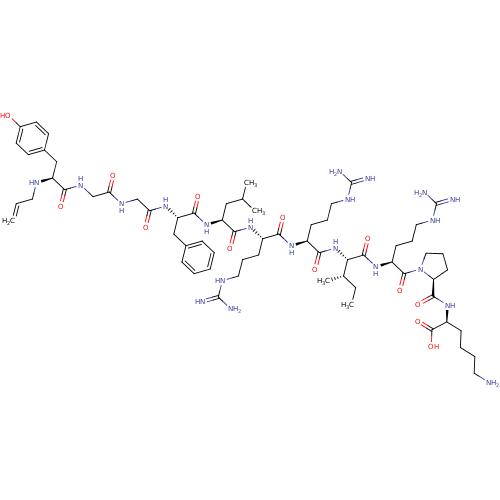
(CHEMBL406338 | N-allyl[D-Pro-10]Dyn A-(1-11))Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NCC=C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(O)=O Show InChI InChI=1S/C66H107N21O13/c1-6-29-74-48(35-42-24-26-43(88)27-25-42)55(91)79-37-52(89)78-38-53(90)80-50(36-41-17-9-8-10-18-41)59(95)85-49(34-39(3)4)58(94)82-44(20-13-30-75-64(68)69)56(92)81-45(21-14-31-76-65(70)71)57(93)86-54(40(5)7-2)61(97)83-46(22-15-32-77-66(72)73)62(98)87-33-16-23-51(87)60(96)84-47(63(99)100)19-11-12-28-67/h6,8-10,17-18,24-27,39-40,44-51,54,74,88H,1,7,11-16,19-23,28-38,67H2,2-5H3,(H,78,89)(H,79,91)(H,80,90)(H,81,92)(H,82,94)(H,83,97)(H,84,96)(H,85,95)(H,86,93)(H,99,100)(H4,68,69,75)(H4,70,71,76)(H4,72,73,77)/t40-,44-,45-,46-,47-,48-,49-,50-,51-,54-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Affinity against Opioid receptor kappa 1 was determined by measuring the inhibition of [3H]-bremazocine binding to guinea pig cerebellar membranes |
J Med Chem 40: 2733-9 (1997)
Article DOI: 10.1021/jm960747t
BindingDB Entry DOI: 10.7270/Q2D50M2D |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409005
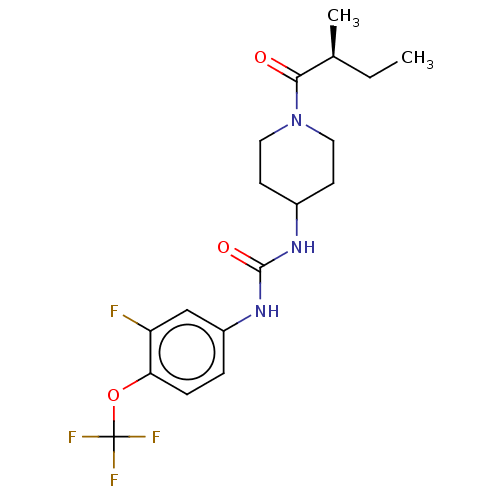
(US10377744, Compound No. 26 | US11123311, Compound...)Show SMILES CC[C@H](C)C(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)c(F)c1 |r| Show InChI InChI=1S/C18H23F4N3O3/c1-3-11(2)16(26)25-8-6-12(7-9-25)23-17(27)24-13-4-5-15(14(19)10-13)28-18(20,21)22/h4-5,10-12H,3,6-9H2,1-2H3,(H2,23,24,27)/t11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to purified recombinant human sEH by FRET-displacement assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01886
BindingDB Entry DOI: 10.7270/Q22V2KSZ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50595425
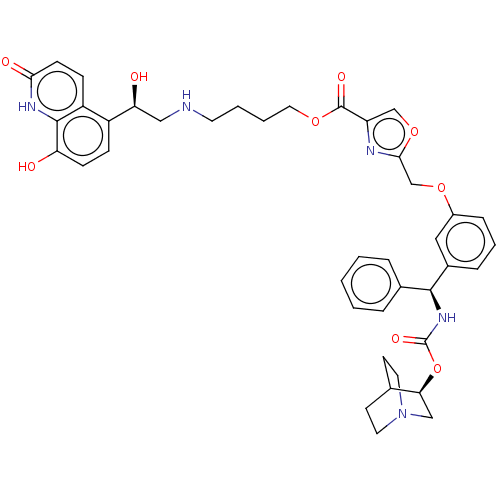
(CHEMBL5208957)Show SMILES O[C@@H](CNCCCCOC(=O)c1coc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)n1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:23.24,28.28,(-14.71,.22,;-13.34,-.48,;-12.05,.36,;-10.68,-.34,;-9.38,.5,;-8.01,-.2,;-6.72,.63,;-5.35,-.07,;-4.06,.77,;-2.69,.07,;-2.61,-1.47,;-1.39,.91,;-1.31,2.44,;.18,2.84,;1.01,1.55,;2.55,1.47,;3.25,.1,;4.79,.01,;5.49,-1.36,;7.02,-1.44,;7.86,-.15,;7.16,1.22,;5.63,1.3,;8,2.51,;9.54,2.43,;10.24,1.06,;9.4,-.23,;11.78,.98,;12.48,-.4,;11.64,-1.69,;12.33,-3.06,;12.41,-1.64,;13.94,-1.9,;14.01,-.48,;14.71,-1.85,;13.87,-3.14,;7.31,3.89,;5.77,3.97,;5.07,5.34,;5.91,6.63,;7.45,6.55,;8.15,5.18,;.04,.35,;-13.26,-2.02,;-14.55,-2.86,;-14.47,-4.39,;-13.1,-5.09,;-13.02,-6.63,;-11.81,-4.26,;-10.44,-4.96,;-9.15,-4.12,;-7.77,-4.82,;-9.23,-2.58,;-10.6,-1.88,;-11.89,-2.72,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569299
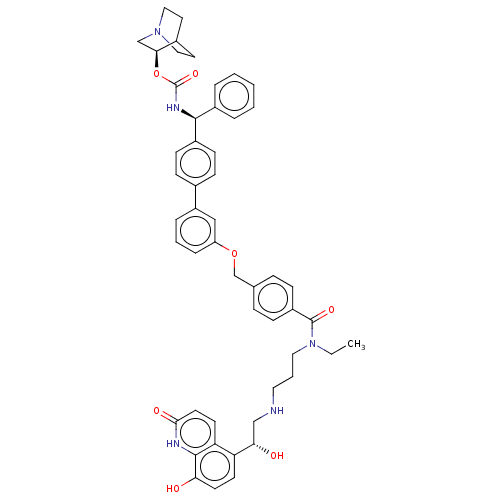
(CHEMBL4866806)Show SMILES CCN(CCCNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12)C(=O)c1ccc(COc2cccc(c2)-c2ccc(cc2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1 |r,wU:47.50,8.8,wD:42.46,(11.54,-25.97,;12.87,-25.19,;12.87,-23.65,;11.53,-22.89,;10.2,-23.66,;8.87,-22.89,;7.54,-23.67,;6.2,-22.9,;4.87,-23.68,;3.53,-22.91,;4.87,-25.22,;3.54,-25.99,;3.54,-27.53,;4.88,-28.3,;4.88,-29.84,;6.21,-27.53,;7.54,-28.3,;8.88,-27.53,;10.21,-28.3,;8.88,-25.98,;7.54,-25.2,;6.21,-25.98,;14.2,-22.88,;14.2,-21.34,;15.54,-23.64,;15.54,-25.18,;16.87,-25.94,;18.21,-25.17,;19.54,-25.94,;20.87,-25.16,;22.21,-25.92,;22.22,-27.46,;23.55,-28.22,;24.88,-27.45,;24.87,-25.9,;23.53,-25.14,;26.2,-25.12,;27.54,-25.88,;28.87,-25.11,;28.86,-23.56,;27.53,-22.8,;26.21,-23.58,;30.19,-22.78,;31.53,-23.54,;32.86,-22.76,;32.85,-21.22,;34.2,-23.52,;35.53,-22.75,;35.52,-21.22,;36.85,-20.44,;38.19,-21.21,;38.19,-22.75,;36.87,-23.52,;37.56,-22.17,;36.07,-21.77,;30.19,-21.24,;31.52,-20.47,;31.51,-18.93,;30.17,-18.17,;28.84,-18.95,;28.85,-20.49,;18.2,-23.62,;16.86,-22.86,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 125I-cyanopindolol from human adrenergic beta2 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50595405
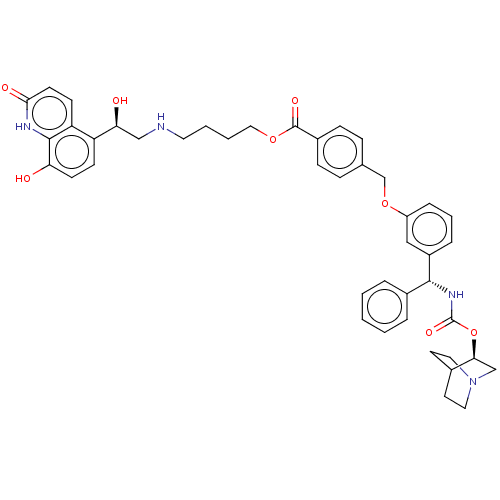
(CHEMBL5184598)Show SMILES O[C@@H](CNCCCCOC(=O)c1ccc(COc2cccc(c2)[C@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,23.24,wD:28.28,(-14.67,-1.16,;-13.33,-1.93,;-12,-1.16,;-10.67,-1.93,;-9.33,-1.16,;-8,-1.93,;-6.66,-1.16,;-5.33,-1.93,;-4,-1.16,;-2.66,-1.93,;-2.66,-3.47,;-1.33,-1.16,;.01,-1.93,;1.34,-1.16,;1.34,.38,;2.67,1.15,;4.01,.38,;5.34,1.15,;6.67,.39,;8,1.16,;8,2.69,;6.67,3.46,;5.34,2.69,;6.67,5,;8,5.77,;9.34,5,;9.34,3.46,;10.67,5.77,;12.01,5,;12.01,3.46,;13.34,2.69,;12.63,3.93,;14.05,4.54,;13.34,5.77,;14.67,5,;14.67,3.46,;5.34,5.77,;5.34,7.31,;4,8.08,;2.67,7.31,;2.67,5.77,;4,5,;-0,1.15,;-1.33,.38,;-13.33,-3.47,;-14.67,-4.24,;-14.67,-5.78,;-13.34,-6.54,;-13.34,-8.08,;-12,-5.77,;-10.67,-6.54,;-9.34,-5.77,;-8.01,-6.54,;-9.34,-4.24,;-10.67,-3.47,;-12,-4.24,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50595423

(CHEMBL5190387)Show SMILES O[C@@H](CNCCCCOC(=O)c1ccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)o1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:23.24,28.28,(-14.71,.22,;-13.34,-.48,;-12.05,.36,;-10.68,-.34,;-9.38,.5,;-8.01,-.2,;-6.72,.63,;-5.35,-.07,;-4.06,.77,;-2.69,.07,;-2.61,-1.47,;-1.39,.91,;-1.31,2.44,;.18,2.84,;1.01,1.55,;2.55,1.47,;3.25,.1,;4.79,.01,;5.49,-1.36,;7.02,-1.44,;7.86,-.15,;7.16,1.22,;5.63,1.3,;8,2.51,;9.54,2.43,;10.24,1.06,;9.4,-.23,;11.78,.98,;12.48,-.4,;11.64,-1.69,;12.33,-3.06,;12.41,-1.64,;13.94,-1.9,;14.01,-.48,;14.71,-1.85,;13.87,-3.14,;7.31,3.89,;5.77,3.97,;5.07,5.34,;5.91,6.63,;7.45,6.55,;8.15,5.18,;.04,.35,;-13.26,-2.02,;-14.55,-2.86,;-14.47,-4.39,;-13.1,-5.09,;-13.02,-6.63,;-11.81,-4.26,;-10.44,-4.96,;-9.15,-4.12,;-7.77,-4.82,;-9.23,-2.58,;-10.6,-1.88,;-11.89,-2.72,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50595424

(CHEMBL5172865)Show SMILES Cn1nc(cc1COc1cccc(c1)[C@@H](NC(=O)O[C@H]1CN2CCC1CC2)c1ccccc1)C(=O)OCCCCNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:42.47,wD:14.16,19.20,(.73,4.28,;.18,2.84,;-1.31,2.44,;-1.39,.91,;.04,.35,;1.01,1.55,;2.55,1.47,;3.25,.1,;4.79,.01,;5.49,-1.36,;7.02,-1.44,;7.86,-.15,;7.16,1.22,;5.63,1.3,;8,2.51,;9.54,2.43,;10.24,1.06,;9.4,-.23,;11.78,.98,;12.48,-.4,;11.64,-1.69,;12.33,-3.06,;12.41,-1.64,;13.94,-1.9,;14.01,-.48,;14.71,-1.85,;13.87,-3.14,;7.31,3.89,;5.77,3.97,;5.07,5.34,;5.91,6.63,;7.45,6.55,;8.15,5.18,;-2.69,.07,;-2.61,-1.47,;-4.06,.77,;-5.35,-.07,;-6.72,.63,;-8.01,-.2,;-9.38,.5,;-10.68,-.34,;-12.05,.36,;-13.34,-.48,;-14.71,.22,;-13.26,-2.02,;-14.55,-2.86,;-14.47,-4.39,;-13.1,-5.09,;-13.02,-6.63,;-11.81,-4.26,;-10.44,-4.96,;-9.15,-4.12,;-7.77,-4.82,;-9.23,-2.58,;-10.6,-1.88,;-11.89,-2.72,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50595426

(CHEMBL5193853)Show SMILES O[C@@H](CNCCCCOC(=O)c1cccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)c1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:24.25,29.29,(-13.81,.82,;-12.39,.23,;-11.16,1.16,;-9.74,.56,;-8.52,1.49,;-7.1,.89,;-5.87,1.82,;-4.45,1.23,;-3.22,2.16,;-1.8,1.56,;-1.61,.03,;-.58,2.49,;-.77,4.02,;.46,4.95,;1.88,4.35,;2.07,2.82,;3.49,2.22,;3.68,.7,;5.1,.1,;5.29,-1.43,;6.71,-2.03,;7.94,-1.1,;7.75,.43,;6.33,1.03,;8.98,1.36,;10.4,.76,;10.59,-.76,;9.36,-1.69,;12.01,-1.36,;12.2,-2.89,;10.97,-3.82,;11.16,-5.35,;11.72,-4.04,;13.07,-4.8,;13.62,-3.49,;13.81,-5.02,;12.58,-5.95,;8.78,2.89,;7.36,3.49,;7.17,5.02,;8.4,5.95,;9.82,5.35,;10.01,3.82,;.84,1.89,;-12.39,-1.31,;-13.73,-2.09,;-13.72,-3.63,;-12.39,-4.39,;-12.39,-5.93,;-11.06,-3.62,;-9.72,-4.39,;-8.4,-3.62,;-7.06,-4.39,;-8.4,-2.09,;-9.73,-1.32,;-11.06,-2.08,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569300
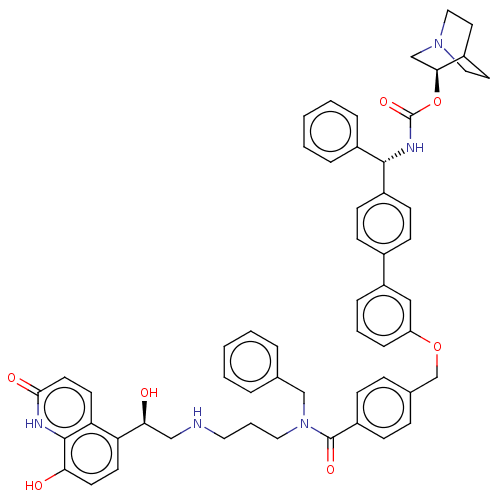
(CHEMBL4871702)Show SMILES O[C@@H](CNCCCN(Cc1ccccc1)C(=O)c1ccc(COc2cccc(c2)-c2ccc(cc2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:40.42,1.0,wD:35.38,(46.1,-20.06,;47.43,-20.83,;48.76,-20.05,;50.1,-20.82,;51.43,-20.05,;52.77,-20.81,;54.1,-20.04,;55.44,-20.81,;55.44,-22.35,;56.53,-23.43,;56.13,-24.91,;57.21,-25.99,;58.7,-25.59,;59.09,-24.1,;58,-23.03,;56.77,-20.03,;56.76,-18.49,;58.11,-20.8,;58.11,-22.33,;59.44,-23.1,;60.78,-22.33,;62.11,-23.09,;63.44,-22.31,;64.78,-23.08,;64.79,-24.61,;66.12,-25.38,;67.45,-24.6,;67.44,-23.05,;66.11,-22.3,;68.78,-22.27,;70.11,-23.04,;71.44,-22.26,;71.43,-20.71,;70.1,-19.96,;68.78,-20.73,;72.77,-19.93,;74.11,-20.69,;75.44,-19.91,;75.43,-18.37,;76.77,-20.68,;78.1,-19.9,;78.09,-18.37,;79.43,-17.6,;80.77,-18.36,;80.77,-19.9,;79.44,-20.68,;80.14,-19.32,;78.65,-18.92,;72.76,-18.39,;74.09,-17.62,;74.08,-16.08,;72.75,-15.32,;71.41,-16.1,;71.42,-17.64,;60.77,-20.78,;59.43,-20.02,;47.44,-22.37,;46.11,-23.14,;46.1,-24.69,;47.44,-25.46,;47.44,-27,;48.77,-24.68,;50.1,-25.45,;51.44,-24.68,;52.78,-25.46,;51.45,-23.14,;50.11,-22.36,;48.77,-23.13,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 125I-cyanopindolol from human adrenergic beta2 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569295
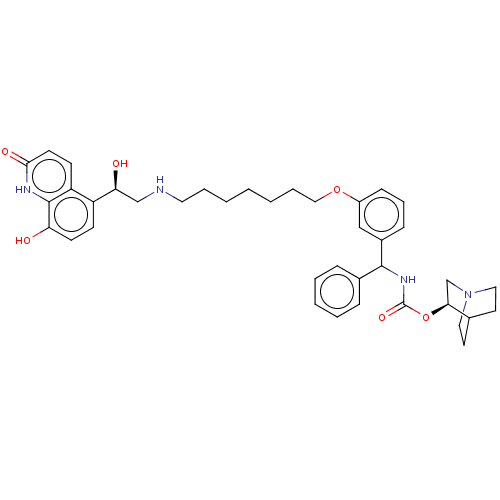
(CHEMBL4854418)Show SMILES O[C@@H](CNCCCCCCCOc1cccc(c1)C(NC(=O)O[C@H]1CN2CCC1CC2)c1ccccc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:23.23,(39.22,-23.81,;40.55,-24.58,;41.88,-23.8,;43.22,-24.57,;44.55,-23.79,;45.89,-24.56,;47.22,-23.79,;48.55,-24.55,;49.89,-23.78,;51.22,-24.54,;52.55,-23.77,;53.89,-24.54,;55.22,-23.76,;55.21,-22.23,;56.54,-21.45,;57.88,-22.22,;57.88,-23.76,;56.55,-24.53,;59.22,-24.53,;60.55,-23.75,;61.89,-24.51,;61.89,-26.06,;63.22,-23.74,;64.55,-24.5,;64.55,-26.05,;65.88,-26.81,;67.21,-26.05,;67.21,-24.5,;65.87,-23.73,;66.63,-25.06,;65.1,-25.47,;59.22,-26.07,;57.89,-26.83,;57.89,-28.37,;59.23,-29.14,;60.56,-28.36,;60.55,-26.82,;40.56,-26.12,;39.23,-26.89,;39.23,-28.43,;40.56,-29.2,;40.56,-30.74,;41.89,-28.43,;43.22,-29.2,;44.56,-28.43,;45.89,-29.2,;44.56,-26.88,;43.23,-26.1,;41.89,-26.88,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 125I-cyanopindolol from human adrenergic beta2 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50569290

(CHEMBL4871517)Show SMILES O[C@@H](CNCCCNC(=O)c1ccc(COc2cccc(c2)-c2ccc(cc2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:33.34,1.0,wD:28.30,(31.17,-6.07,;32.5,-6.84,;33.84,-6.06,;35.17,-6.83,;36.5,-6.05,;37.84,-6.82,;39.17,-6.05,;40.51,-6.81,;41.84,-6.04,;41.83,-4.5,;43.17,-6.8,;43.18,-8.34,;44.51,-9.11,;45.84,-8.33,;47.18,-9.1,;48.51,-8.32,;49.85,-9.08,;49.85,-10.62,;51.19,-11.38,;52.52,-10.61,;52.51,-9.06,;51.17,-8.3,;53.84,-8.28,;55.17,-9.04,;56.51,-8.27,;56.5,-6.72,;55.16,-5.96,;53.84,-6.74,;57.83,-5.94,;59.17,-6.7,;60.5,-5.92,;60.49,-4.38,;61.84,-6.69,;63.17,-5.91,;63.15,-4.38,;64.49,-3.61,;65.83,-4.37,;65.83,-5.91,;64.5,-6.68,;65.2,-5.33,;63.71,-4.93,;57.82,-4.4,;59.15,-3.63,;59.15,-2.09,;57.81,-1.33,;56.47,-2.11,;56.49,-3.65,;45.83,-6.78,;44.5,-6.02,;32.51,-8.38,;31.18,-9.15,;31.18,-10.69,;32.51,-11.46,;32.51,-13,;33.84,-10.69,;35.17,-11.46,;36.51,-10.69,;37.85,-11.46,;36.52,-9.14,;35.18,-8.36,;33.84,-9.14,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methylscopolamine from human muscarinic M3 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM21392
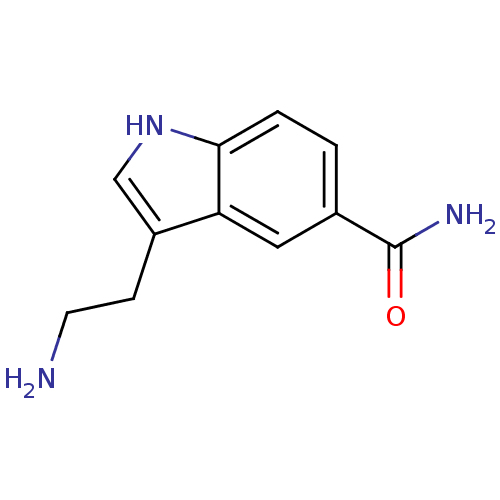
(3-(2-aminoethyl)-1H-indole-5-carboxamide | 5-CT | ...)Show InChI InChI=1S/C11H13N3O/c12-4-3-8-6-14-10-2-1-7(11(13)15)5-9(8)10/h1-2,5-6,14H,3-4,12H2,(H2,13,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of Groningen
Curated by ChEMBL
| Assay Description
Binding affinity at rat 5-hydroxytryptamine 7 receptor. |
J Med Chem 46: 5365-74 (2003)
Article DOI: 10.1021/jm030826m
BindingDB Entry DOI: 10.7270/Q2K93B8V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50569301
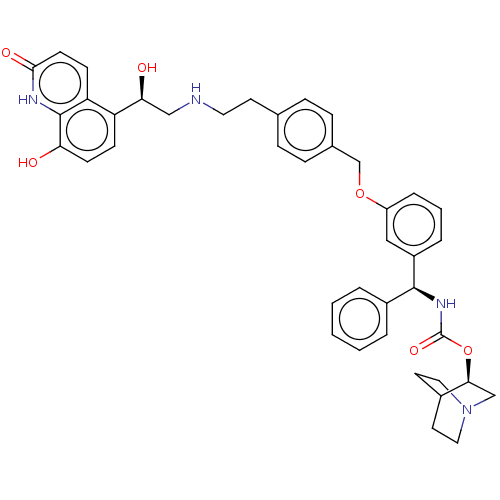
(CHEMBL4854091)Show SMILES O[C@@H](CNCCc1ccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,18.19,wD:23.23,(1.74,-37.97,;3.08,-38.73,;4.41,-37.96,;5.75,-38.72,;7.08,-37.95,;8.41,-38.72,;9.75,-37.94,;11.08,-38.71,;12.41,-37.94,;12.4,-36.4,;13.74,-35.62,;15.07,-36.39,;16.4,-35.61,;16.39,-34.08,;17.71,-33.3,;19.06,-34.06,;19.06,-35.6,;17.73,-36.38,;20.4,-36.37,;21.73,-35.59,;23.07,-36.36,;23.07,-37.9,;24.4,-35.58,;25.74,-36.35,;25.73,-37.9,;27.06,-38.66,;28.39,-37.89,;28.39,-36.35,;27.05,-35.58,;27.81,-36.91,;26.28,-37.32,;20.41,-37.91,;19.08,-38.68,;19.08,-40.22,;20.42,-40.98,;21.76,-40.2,;21.74,-38.66,;11.06,-35.63,;9.73,-36.41,;3.08,-40.27,;1.76,-41.04,;1.75,-42.59,;3.09,-43.36,;3.09,-44.9,;4.42,-42.58,;5.75,-43.35,;7.09,-42.59,;8.42,-43.36,;7.09,-41.04,;5.75,-40.26,;4.42,-41.04,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50569301

(CHEMBL4854091)Show SMILES O[C@@H](CNCCc1ccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,18.19,wD:23.23,(1.74,-37.97,;3.08,-38.73,;4.41,-37.96,;5.75,-38.72,;7.08,-37.95,;8.41,-38.72,;9.75,-37.94,;11.08,-38.71,;12.41,-37.94,;12.4,-36.4,;13.74,-35.62,;15.07,-36.39,;16.4,-35.61,;16.39,-34.08,;17.71,-33.3,;19.06,-34.06,;19.06,-35.6,;17.73,-36.38,;20.4,-36.37,;21.73,-35.59,;23.07,-36.36,;23.07,-37.9,;24.4,-35.58,;25.74,-36.35,;25.73,-37.9,;27.06,-38.66,;28.39,-37.89,;28.39,-36.35,;27.05,-35.58,;27.81,-36.91,;26.28,-37.32,;20.41,-37.91,;19.08,-38.68,;19.08,-40.22,;20.42,-40.98,;21.76,-40.2,;21.74,-38.66,;11.06,-35.63,;9.73,-36.41,;3.08,-40.27,;1.76,-41.04,;1.75,-42.59,;3.09,-43.36,;3.09,-44.9,;4.42,-42.58,;5.75,-43.35,;7.09,-42.59,;8.42,-43.36,;7.09,-41.04,;5.75,-40.26,;4.42,-41.04,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methylscopolamine from human muscarinic M3 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50569300

(CHEMBL4871702)Show SMILES O[C@@H](CNCCCN(Cc1ccccc1)C(=O)c1ccc(COc2cccc(c2)-c2ccc(cc2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:40.42,1.0,wD:35.38,(46.1,-20.06,;47.43,-20.83,;48.76,-20.05,;50.1,-20.82,;51.43,-20.05,;52.77,-20.81,;54.1,-20.04,;55.44,-20.81,;55.44,-22.35,;56.53,-23.43,;56.13,-24.91,;57.21,-25.99,;58.7,-25.59,;59.09,-24.1,;58,-23.03,;56.77,-20.03,;56.76,-18.49,;58.11,-20.8,;58.11,-22.33,;59.44,-23.1,;60.78,-22.33,;62.11,-23.09,;63.44,-22.31,;64.78,-23.08,;64.79,-24.61,;66.12,-25.38,;67.45,-24.6,;67.44,-23.05,;66.11,-22.3,;68.78,-22.27,;70.11,-23.04,;71.44,-22.26,;71.43,-20.71,;70.1,-19.96,;68.78,-20.73,;72.77,-19.93,;74.11,-20.69,;75.44,-19.91,;75.43,-18.37,;76.77,-20.68,;78.1,-19.9,;78.09,-18.37,;79.43,-17.6,;80.77,-18.36,;80.77,-19.9,;79.44,-20.68,;80.14,-19.32,;78.65,-18.92,;72.76,-18.39,;74.09,-17.62,;74.08,-16.08,;72.75,-15.32,;71.41,-16.1,;71.42,-17.64,;60.77,-20.78,;59.43,-20.02,;47.44,-22.37,;46.11,-23.14,;46.1,-24.69,;47.44,-25.46,;47.44,-27,;48.77,-24.68,;50.1,-25.45,;51.44,-24.68,;52.78,-25.46,;51.45,-23.14,;50.11,-22.36,;48.77,-23.13,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methylscopolamine from human muscarinic M3 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50569291
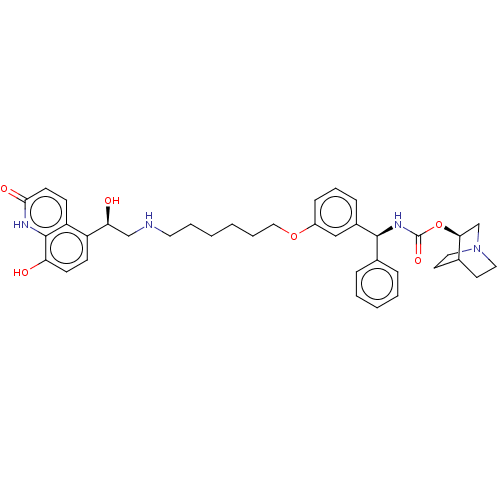
(CHEMBL4847910)Show SMILES O[C@@H](CNCCCCCCOc1cccc(c1)[C@@H](NC(=O)O[C@H]1CN2CCC1CC2)c1ccccc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:22.22,1.0,wD:17.18,(1.78,-6.07,;3.12,-6.84,;4.45,-6.06,;5.79,-6.83,;7.12,-6.05,;8.45,-6.82,;9.78,-6.05,;11.12,-6.81,;12.45,-6.04,;13.79,-6.8,;15.12,-6.03,;16.45,-6.8,;16.45,-8.34,;17.78,-9.11,;19.12,-8.33,;19.11,-6.78,;17.77,-6.02,;20.44,-6,;21.78,-6.76,;23.11,-5.98,;23.1,-4.44,;24.45,-6.75,;25.77,-5.97,;25.76,-4.44,;27.1,-3.67,;28.44,-4.43,;28.44,-5.97,;27.11,-6.75,;27.81,-5.39,;26.32,-4.99,;20.43,-4.46,;21.76,-3.69,;21.76,-2.16,;20.42,-1.39,;19.08,-2.17,;19.1,-3.71,;3.12,-8.38,;1.79,-9.15,;1.79,-10.69,;3.13,-11.46,;3.13,-13,;4.46,-10.69,;5.79,-11.46,;7.13,-10.69,;8.46,-11.46,;7.13,-9.14,;5.79,-8.36,;4.46,-9.14,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methylscopolamine from human muscarinic M3 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50595404

(CHEMBL5184455)Show SMILES O[C@@H](CNCCCCOC(=O)c1ccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:23.24,28.28,(-14.67,-1.16,;-13.33,-1.93,;-12,-1.16,;-10.67,-1.93,;-9.33,-1.16,;-8,-1.93,;-6.66,-1.16,;-5.33,-1.93,;-4,-1.16,;-2.66,-1.93,;-2.66,-3.47,;-1.33,-1.16,;.01,-1.93,;1.34,-1.16,;1.34,.38,;2.67,1.15,;4.01,.38,;5.34,1.15,;6.67,.39,;8,1.16,;8,2.69,;6.67,3.46,;5.34,2.69,;6.67,5,;8.01,5.77,;9.34,5,;9.34,3.46,;10.67,5.77,;12.01,5,;12.01,3.46,;13.34,2.69,;12.63,3.93,;14.05,4.54,;13.34,5.77,;14.67,5,;14.67,3.46,;5.34,5.77,;5.34,7.31,;4,8.08,;2.67,7.31,;2.67,5.77,;4,5,;-0,1.15,;-1.33,.38,;-13.33,-3.47,;-14.67,-4.24,;-14.67,-5.78,;-13.34,-6.54,;-13.34,-8.08,;-12,-5.77,;-10.67,-6.55,;-9.34,-5.77,;-8.01,-6.54,;-9.34,-4.24,;-10.67,-3.47,;-12,-4.24,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409003
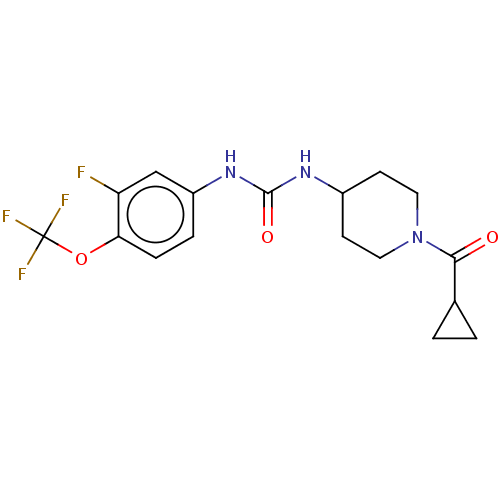
(US10377744, Compound No. 24 | US11123311, Compound...)Show SMILES Fc1cc(NC(=O)NC2CCN(CC2)C(=O)C2CC2)ccc1OC(F)(F)F Show InChI InChI=1S/C17H19F4N3O3/c18-13-9-12(3-4-14(13)27-17(19,20)21)23-16(26)22-11-5-7-24(8-6-11)15(25)10-1-2-10/h3-4,9-11H,1-2,5-8H2,(H2,22,23,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to purified recombinant human sEH by FRET-displacement assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01886
BindingDB Entry DOI: 10.7270/Q22V2KSZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50398598
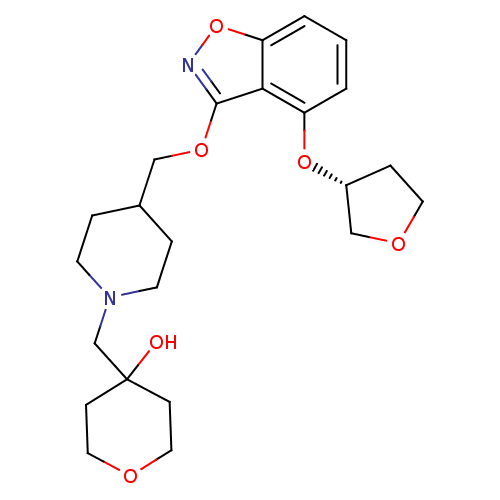
(CHEMBL2152922)Show SMILES OC1(CN2CCC(COc3noc4cccc(O[C@@H]5CCOC5)c34)CC2)CCOCC1 |r| Show InChI InChI=1S/C23H32N2O6/c26-23(7-12-27-13-8-23)16-25-9-4-17(5-10-25)14-29-22-21-19(30-18-6-11-28-15-18)2-1-3-20(21)31-24-22/h1-3,17-18,26H,4-16H2/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting |
J Med Chem 55: 9240-54 (2012)
Article DOI: 10.1021/jm300953p
BindingDB Entry DOI: 10.7270/Q2FQ9XRB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50569296
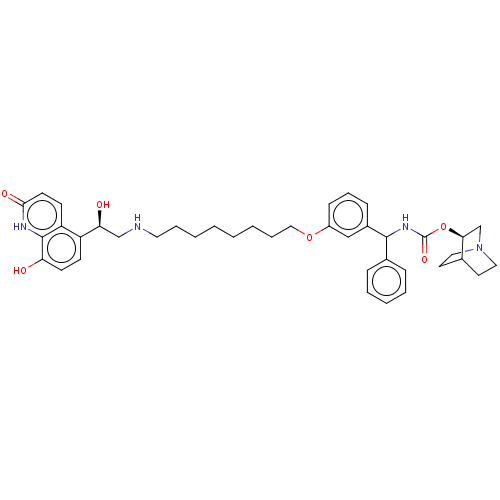
(CHEMBL4852629)Show SMILES O[C@@H](CNCCCCCCCCOc1cccc(c1)C(NC(=O)O[C@H]1CN2CCC1CC2)c1ccccc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,24.24,(3.65,-24.81,;4.98,-25.58,;6.32,-24.81,;7.65,-25.57,;8.98,-24.8,;10.32,-25.56,;11.65,-24.79,;12.99,-25.56,;14.32,-24.78,;15.65,-25.55,;16.99,-24.77,;18.32,-25.54,;19.65,-24.77,;20.99,-25.42,;20.98,-26.95,;22.32,-27.72,;23.65,-26.94,;23.64,-25.4,;22.31,-24.64,;24.98,-24.61,;26.31,-25.37,;27.64,-24.6,;27.63,-23.06,;28.98,-25.36,;30.31,-24.58,;30.3,-23.05,;31.63,-22.28,;32.97,-23.05,;32.97,-24.59,;31.65,-25.36,;32.34,-24,;30.85,-23.61,;24.97,-23.08,;26.3,-22.31,;26.29,-20.77,;24.95,-20,;23.62,-20.79,;23.63,-22.32,;4.99,-27.12,;3.66,-27.89,;3.66,-29.44,;4.99,-30.21,;5,-31.75,;6.32,-29.43,;7.65,-30.2,;9,-29.43,;10.33,-30.21,;9,-27.89,;7.66,-27.11,;6.32,-27.88,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methylscopolamine from human muscarinic M3 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569302
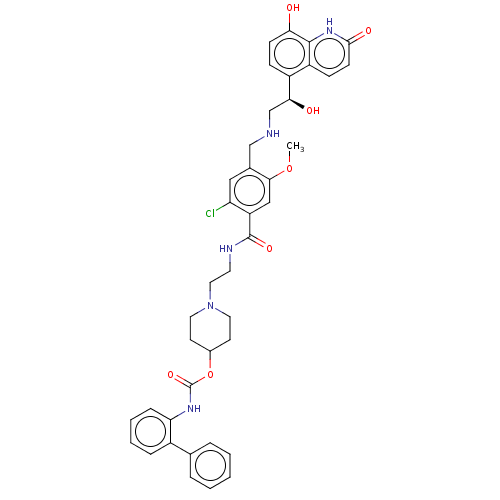
(CHEMBL4878138)Show SMILES COc1cc(C(=O)NCCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)c(Cl)cc1CNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 125I-cyanopindolol from human adrenergic beta2 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50595425

(CHEMBL5208957)Show SMILES O[C@@H](CNCCCCOC(=O)c1coc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)n1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:23.24,28.28,(-14.71,.22,;-13.34,-.48,;-12.05,.36,;-10.68,-.34,;-9.38,.5,;-8.01,-.2,;-6.72,.63,;-5.35,-.07,;-4.06,.77,;-2.69,.07,;-2.61,-1.47,;-1.39,.91,;-1.31,2.44,;.18,2.84,;1.01,1.55,;2.55,1.47,;3.25,.1,;4.79,.01,;5.49,-1.36,;7.02,-1.44,;7.86,-.15,;7.16,1.22,;5.63,1.3,;8,2.51,;9.54,2.43,;10.24,1.06,;9.4,-.23,;11.78,.98,;12.48,-.4,;11.64,-1.69,;12.33,-3.06,;12.41,-1.64,;13.94,-1.9,;14.01,-.48,;14.71,-1.85,;13.87,-3.14,;7.31,3.89,;5.77,3.97,;5.07,5.34,;5.91,6.63,;7.45,6.55,;8.15,5.18,;.04,.35,;-13.26,-2.02,;-14.55,-2.86,;-14.47,-4.39,;-13.1,-5.09,;-13.02,-6.63,;-11.81,-4.26,;-10.44,-4.96,;-9.15,-4.12,;-7.77,-4.82,;-9.23,-2.58,;-10.6,-1.88,;-11.89,-2.72,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50059581
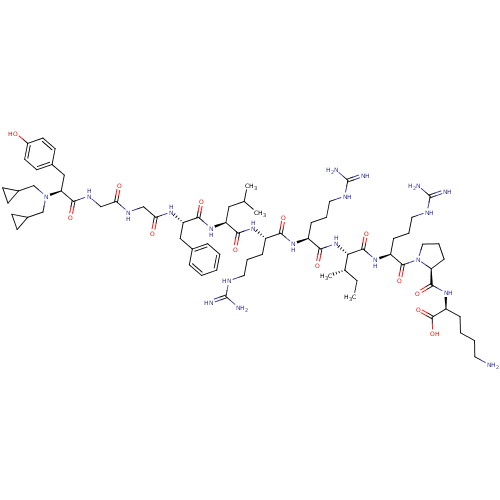
(CHEMBL406893 | N,N-diCPM[D-Pro-10]Dyn A-(1-11))Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)N(CC1CC1)CC1CC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(O)=O Show InChI InChI=1S/C71H115N21O13/c1-5-43(4)59(66(102)87-51(20-13-33-81-71(77)78)67(103)92-34-14-21-55(92)65(101)88-52(68(104)105)17-9-10-30-72)90-61(97)50(19-12-32-80-70(75)76)85-60(96)49(18-11-31-79-69(73)74)86-62(98)53(35-42(2)3)89-63(99)54(36-44-15-7-6-8-16-44)84-58(95)39-82-57(94)38-83-64(100)56(37-45-26-28-48(93)29-27-45)91(40-46-22-23-46)41-47-24-25-47/h6-8,15-16,26-29,42-43,46-47,49-56,59,93H,5,9-14,17-25,30-41,72H2,1-4H3,(H,82,94)(H,83,100)(H,84,95)(H,85,96)(H,86,98)(H,87,102)(H,88,101)(H,89,99)(H,90,97)(H,104,105)(H4,73,74,79)(H4,75,76,80)(H4,77,78,81)/t43-,49-,50-,51-,52-,53-,54-,55-,56-,59-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Affinity against Opioid receptor kappa 1 was determined by measuring the inhibition of [3H]-bremazocine binding to guinea pig cerebellar membranes |
J Med Chem 40: 2733-9 (1997)
Article DOI: 10.1021/jm960747t
BindingDB Entry DOI: 10.7270/Q2D50M2D |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50595427

(CHEMBL5200887)Show SMILES COc1cc(ccc1COc1cccc(c1)[C@@H](NC(=O)O[C@H]1CN2CCC1CC2)c1ccccc1)C(=O)OCCCCNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:44.49,wD:16.18,21.22,(3.34,-1.15,;3.34,.39,;2,1.16,;.67,.39,;-.67,1.15,;-.67,2.69,;.67,3.47,;2,2.7,;3.33,3.47,;4.67,2.7,;6,3.47,;6,5.01,;7.33,5.78,;8.67,5.02,;8.67,3.48,;7.33,2.7,;10,2.71,;10,1.17,;8.67,.4,;7.34,1.16,;8.67,-1.14,;7.34,-1.92,;6.01,-1.15,;4.67,-1.92,;6.1,-1.92,;5.92,-3.46,;7.34,-3.46,;6.01,-4.23,;4.68,-3.46,;11.33,3.48,;11.33,5.02,;12.67,5.79,;14,5.02,;14,3.48,;12.67,2.71,;-2,.38,;-2,-1.16,;-3.33,1.15,;-4.67,.38,;-6,1.15,;-7.33,.38,;-8.67,1.14,;-10,.37,;-11.33,1.14,;-12.67,.37,;-14,1.14,;-12.67,-1.17,;-14,-1.94,;-14,-3.48,;-12.66,-4.25,;-12.66,-5.79,;-11.33,-3.48,;-9.99,-4.25,;-8.66,-3.48,;-7.33,-4.24,;-8.66,-1.94,;-10,-1.17,;-11.33,-1.94,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569297
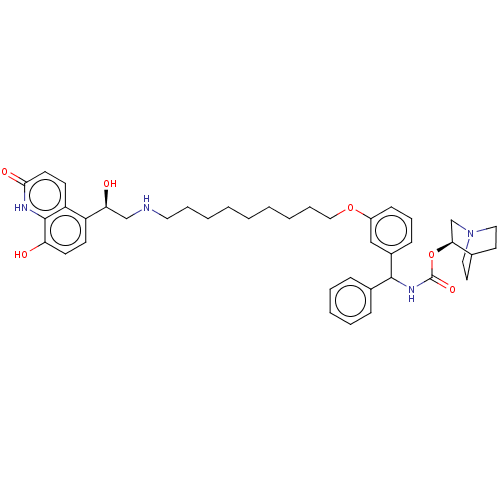
(CHEMBL4846536)Show SMILES O[C@@H](CNCCCCCCCCCOc1cccc(c1)C(NC(=O)O[C@H]1CN2CCC1CC2)c1ccccc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:25.25,(48.77,-6.75,;50.1,-7.51,;51.43,-6.74,;52.77,-7.5,;54.1,-6.73,;55.44,-7.5,;56.77,-6.72,;58.11,-7.49,;59.44,-6.71,;60.77,-7.48,;62.1,-6.71,;63.44,-7.47,;64.77,-6.7,;66.11,-7.46,;67.44,-6.69,;67.43,-5.16,;68.75,-4.38,;70.1,-5.15,;70.1,-6.69,;68.77,-7.46,;71.44,-7.45,;72.77,-6.68,;74.11,-7.44,;74.11,-8.99,;75.44,-6.67,;76.77,-7.43,;76.77,-8.98,;78.1,-9.74,;79.43,-8.97,;79.43,-7.43,;78.09,-6.66,;78.85,-7.99,;77.32,-8.4,;71.44,-8.99,;70.11,-9.76,;70.11,-11.3,;71.45,-12.07,;72.78,-11.28,;72.77,-9.75,;50.11,-9.05,;48.78,-9.82,;48.78,-11.37,;50.11,-12.14,;50.11,-13.68,;51.44,-11.36,;52.77,-12.13,;54.11,-11.37,;55.44,-12.14,;54.12,-9.82,;52.78,-9.04,;51.44,-9.82,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 125I-cyanopindolol from human adrenergic beta2 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569296

(CHEMBL4852629)Show SMILES O[C@@H](CNCCCCCCCCOc1cccc(c1)C(NC(=O)O[C@H]1CN2CCC1CC2)c1ccccc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,24.24,(3.65,-24.81,;4.98,-25.58,;6.32,-24.81,;7.65,-25.57,;8.98,-24.8,;10.32,-25.56,;11.65,-24.79,;12.99,-25.56,;14.32,-24.78,;15.65,-25.55,;16.99,-24.77,;18.32,-25.54,;19.65,-24.77,;20.99,-25.42,;20.98,-26.95,;22.32,-27.72,;23.65,-26.94,;23.64,-25.4,;22.31,-24.64,;24.98,-24.61,;26.31,-25.37,;27.64,-24.6,;27.63,-23.06,;28.98,-25.36,;30.31,-24.58,;30.3,-23.05,;31.63,-22.28,;32.97,-23.05,;32.97,-24.59,;31.65,-25.36,;32.34,-24,;30.85,-23.61,;24.97,-23.08,;26.3,-22.31,;26.29,-20.77,;24.95,-20,;23.62,-20.79,;23.63,-22.32,;4.99,-27.12,;3.66,-27.89,;3.66,-29.44,;4.99,-30.21,;5,-31.75,;6.32,-29.43,;7.65,-30.2,;9,-29.43,;10.33,-30.21,;9,-27.89,;7.66,-27.11,;6.32,-27.88,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 125I-cyanopindolol from human adrenergic beta2 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50002346

(CHEMBL384584 | [D-pro10]Dynorphin A(1-11))Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCCN)C(O)=O Show InChI InChI=1S/C63H103N21O13/c1-5-37(4)51(58(94)80-44(20-13-29-74-63(70)71)59(95)84-30-14-21-48(84)57(93)81-45(60(96)97)17-9-10-26-64)83-54(90)43(19-12-28-73-62(68)69)78-53(89)42(18-11-27-72-61(66)67)79-55(91)46(31-36(2)3)82-56(92)47(33-38-15-7-6-8-16-38)77-50(87)35-75-49(86)34-76-52(88)41(65)32-39-22-24-40(85)25-23-39/h6-8,15-16,22-25,36-37,41-48,51,85H,5,9-14,17-21,26-35,64-65H2,1-4H3,(H,75,86)(H,76,88)(H,77,87)(H,78,89)(H,79,91)(H,80,94)(H,81,93)(H,82,92)(H,83,90)(H,96,97)(H4,66,67,72)(H4,68,69,73)(H4,70,71,74)/t37-,41-,42-,43-,44-,45-,46-,47-,48+,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Affinity against Opioid receptor mu 1 was determined by measuring the inhibition of [3H]-DAMGO binding to rat forebrain membranes |
J Med Chem 40: 2733-9 (1997)
Article DOI: 10.1021/jm960747t
BindingDB Entry DOI: 10.7270/Q2D50M2D |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50037135
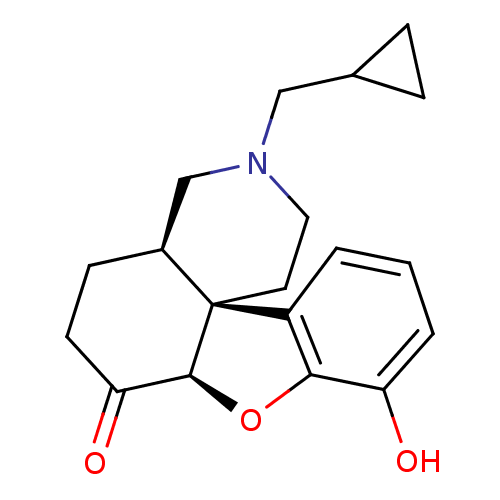
((4aR,7aR,12bS)-3-Cyclopropylmethyl-9-hydroxy-2,3,4...)Show SMILES Oc1cccc2c1O[C@H]1C(=O)CC[C@H]3CN(CC4CC4)CC[C@]213 Show InChI InChI=1S/C19H23NO3/c21-15-3-1-2-14-17(15)23-18-16(22)7-6-13-11-20(10-12-4-5-12)9-8-19(13,14)18/h1-3,12-13,18,21H,4-11H2/t13-,18-,19-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
In vivo binding affinity against mu opioid receptor was measured by using labeled ligand [3H]-Naloxone (0.5 nM) |
J Med Chem 37: 3121-7 (1994)
BindingDB Entry DOI: 10.7270/Q2PK0F6R |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569301

(CHEMBL4854091)Show SMILES O[C@@H](CNCCc1ccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,18.19,wD:23.23,(1.74,-37.97,;3.08,-38.73,;4.41,-37.96,;5.75,-38.72,;7.08,-37.95,;8.41,-38.72,;9.75,-37.94,;11.08,-38.71,;12.41,-37.94,;12.4,-36.4,;13.74,-35.62,;15.07,-36.39,;16.4,-35.61,;16.39,-34.08,;17.71,-33.3,;19.06,-34.06,;19.06,-35.6,;17.73,-36.38,;20.4,-36.37,;21.73,-35.59,;23.07,-36.36,;23.07,-37.9,;24.4,-35.58,;25.74,-36.35,;25.73,-37.9,;27.06,-38.66,;28.39,-37.89,;28.39,-36.35,;27.05,-35.58,;27.81,-36.91,;26.28,-37.32,;20.41,-37.91,;19.08,-38.68,;19.08,-40.22,;20.42,-40.98,;21.76,-40.2,;21.74,-38.66,;11.06,-35.63,;9.73,-36.41,;3.08,-40.27,;1.76,-41.04,;1.75,-42.59,;3.09,-43.36,;3.09,-44.9,;4.42,-42.58,;5.75,-43.35,;7.09,-42.59,;8.42,-43.36,;7.09,-41.04,;5.75,-40.26,;4.42,-41.04,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 125I-cyanopindolol from human adrenergic beta2 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569298
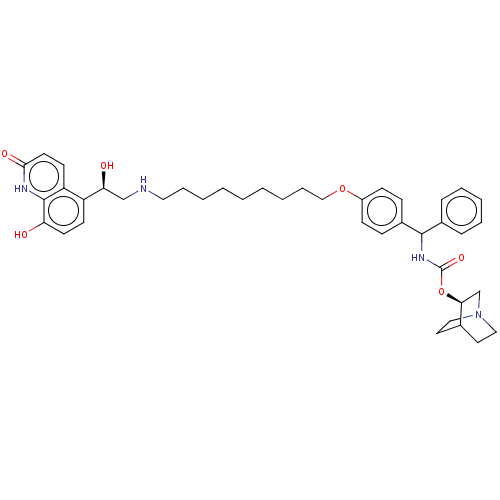
(CHEMBL4878395)Show SMILES O[C@@H](CNCCCCCCCCCOc1ccc(cc1)C(NC(=O)O[C@H]1CN2CCC1CC2)c1ccccc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,25.25,(3.18,-8.47,;4.52,-9.24,;5.85,-8.47,;7.19,-9.23,;8.52,-8.46,;9.85,-9.22,;11.18,-8.45,;12.52,-9.22,;13.85,-8.44,;15.19,-9.21,;16.52,-8.43,;17.85,-9.2,;19.19,-8.43,;20.52,-9.19,;21.85,-8.42,;23.19,-9.19,;24.52,-8.41,;24.51,-6.87,;23.17,-6.11,;21.84,-6.88,;25.84,-6.1,;27.18,-6.86,;28.51,-6.08,;28.5,-4.54,;29.85,-6.84,;31.18,-6.06,;31.17,-4.53,;32.5,-3.76,;33.84,-4.53,;33.84,-6.07,;32.52,-6.84,;33.21,-5.48,;31.73,-5.09,;25.84,-4.56,;27.17,-3.79,;27.16,-2.25,;25.82,-1.48,;24.49,-2.27,;24.5,-3.8,;4.52,-10.78,;3.19,-11.55,;3.19,-13.09,;4.53,-13.86,;4.53,-15.4,;5.86,-13.09,;7.19,-13.86,;8.53,-13.09,;9.86,-13.87,;8.53,-11.55,;7.19,-10.77,;5.86,-11.54,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 125I-cyanopindolol from human adrenergic beta2 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569301

(CHEMBL4854091)Show SMILES O[C@@H](CNCCc1ccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,18.19,wD:23.23,(1.74,-37.97,;3.08,-38.73,;4.41,-37.96,;5.75,-38.72,;7.08,-37.95,;8.41,-38.72,;9.75,-37.94,;11.08,-38.71,;12.41,-37.94,;12.4,-36.4,;13.74,-35.62,;15.07,-36.39,;16.4,-35.61,;16.39,-34.08,;17.71,-33.3,;19.06,-34.06,;19.06,-35.6,;17.73,-36.38,;20.4,-36.37,;21.73,-35.59,;23.07,-36.36,;23.07,-37.9,;24.4,-35.58,;25.74,-36.35,;25.73,-37.9,;27.06,-38.66,;28.39,-37.89,;28.39,-36.35,;27.05,-35.58,;27.81,-36.91,;26.28,-37.32,;20.41,-37.91,;19.08,-38.68,;19.08,-40.22,;20.42,-40.98,;21.76,-40.2,;21.74,-38.66,;11.06,-35.63,;9.73,-36.41,;3.08,-40.27,;1.76,-41.04,;1.75,-42.59,;3.09,-43.36,;3.09,-44.9,;4.42,-42.58,;5.75,-43.35,;7.09,-42.59,;8.42,-43.36,;7.09,-41.04,;5.75,-40.26,;4.42,-41.04,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50569299

(CHEMBL4866806)Show SMILES CCN(CCCNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12)C(=O)c1ccc(COc2cccc(c2)-c2ccc(cc2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1 |r,wU:47.50,8.8,wD:42.46,(11.54,-25.97,;12.87,-25.19,;12.87,-23.65,;11.53,-22.89,;10.2,-23.66,;8.87,-22.89,;7.54,-23.67,;6.2,-22.9,;4.87,-23.68,;3.53,-22.91,;4.87,-25.22,;3.54,-25.99,;3.54,-27.53,;4.88,-28.3,;4.88,-29.84,;6.21,-27.53,;7.54,-28.3,;8.88,-27.53,;10.21,-28.3,;8.88,-25.98,;7.54,-25.2,;6.21,-25.98,;14.2,-22.88,;14.2,-21.34,;15.54,-23.64,;15.54,-25.18,;16.87,-25.94,;18.21,-25.17,;19.54,-25.94,;20.87,-25.16,;22.21,-25.92,;22.22,-27.46,;23.55,-28.22,;24.88,-27.45,;24.87,-25.9,;23.53,-25.14,;26.2,-25.12,;27.54,-25.88,;28.87,-25.11,;28.86,-23.56,;27.53,-22.8,;26.21,-23.58,;30.19,-22.78,;31.53,-23.54,;32.86,-22.76,;32.85,-21.22,;34.2,-23.52,;35.53,-22.75,;35.52,-21.22,;36.85,-20.44,;38.19,-21.21,;38.19,-22.75,;36.87,-23.52,;37.56,-22.17,;36.07,-21.77,;30.19,-21.24,;31.52,-20.47,;31.51,-18.93,;30.17,-18.17,;28.84,-18.95,;28.85,-20.49,;18.2,-23.62,;16.86,-22.86,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methylscopolamine from human muscarinic M3 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50569297

(CHEMBL4846536)Show SMILES O[C@@H](CNCCCCCCCCCOc1cccc(c1)C(NC(=O)O[C@H]1CN2CCC1CC2)c1ccccc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:25.25,(48.77,-6.75,;50.1,-7.51,;51.43,-6.74,;52.77,-7.5,;54.1,-6.73,;55.44,-7.5,;56.77,-6.72,;58.11,-7.49,;59.44,-6.71,;60.77,-7.48,;62.1,-6.71,;63.44,-7.47,;64.77,-6.7,;66.11,-7.46,;67.44,-6.69,;67.43,-5.16,;68.75,-4.38,;70.1,-5.15,;70.1,-6.69,;68.77,-7.46,;71.44,-7.45,;72.77,-6.68,;74.11,-7.44,;74.11,-8.99,;75.44,-6.67,;76.77,-7.43,;76.77,-8.98,;78.1,-9.74,;79.43,-8.97,;79.43,-7.43,;78.09,-6.66,;78.85,-7.99,;77.32,-8.4,;71.44,-8.99,;70.11,-9.76,;70.11,-11.3,;71.45,-12.07,;72.78,-11.28,;72.77,-9.75,;50.11,-9.05,;48.78,-9.82,;48.78,-11.37,;50.11,-12.14,;50.11,-13.68,;51.44,-11.36,;52.77,-12.13,;54.11,-11.37,;55.44,-12.14,;54.12,-9.82,;52.78,-9.04,;51.44,-9.82,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methylscopolamine from human muscarinic M3 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM50474418
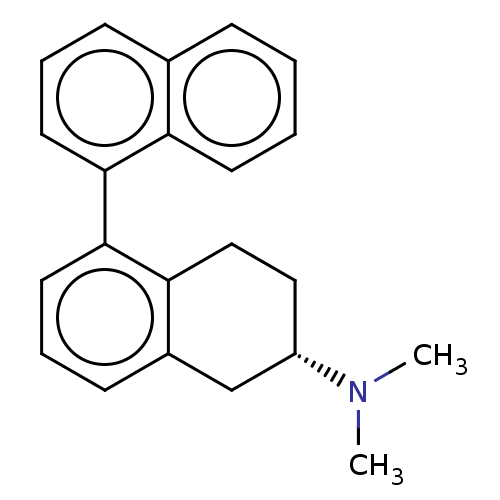
(CHEMBL611472)Show SMILES CN(C)[C@H]1CCc2c(C1)cccc2-c1cccc2ccccc12 |r| Show InChI InChI=1S/C22H23N/c1-23(2)18-13-14-20-17(15-18)9-6-12-22(20)21-11-5-8-16-7-3-4-10-19(16)21/h3-12,18H,13-15H2,1-2H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of Groningen
Curated by ChEMBL
| Assay Description
Binding affinity at rat 5-hydroxytryptamine 7 receptor. |
J Med Chem 46: 5365-74 (2003)
Article DOI: 10.1021/jm030826m
BindingDB Entry DOI: 10.7270/Q2K93B8V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50398597
(CHEMBL2179584)Show InChI InChI=1S/C21H30N2O3/c1-2-3-12-23-13-10-16(11-14-23)15-24-21-20-18(25-17-6-4-7-17)8-5-9-19(20)26-22-21/h5,8-9,16-17H,2-4,6-7,10-15H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting |
J Med Chem 55: 9240-54 (2012)
Article DOI: 10.1021/jm300953p
BindingDB Entry DOI: 10.7270/Q2FQ9XRB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50398593
(CHEMBL2179587)Show InChI InChI=1S/C21H32N2O3/c1-4-5-11-23-12-9-17(10-13-23)15-25-21-20-18(24-14-16(2)3)7-6-8-19(20)26-22-21/h6-8,16-17H,4-5,9-15H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from human 5HT4D receptor expressed in HEK293 cells after 30 mins by liquid scintillation counting |
J Med Chem 55: 9240-54 (2012)
Article DOI: 10.1021/jm300953p
BindingDB Entry DOI: 10.7270/Q2FQ9XRB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM50398598

(CHEMBL2152922)Show SMILES OC1(CN2CCC(COc3noc4cccc(O[C@@H]5CCOC5)c34)CC2)CCOCC1 |r| Show InChI InChI=1S/C23H32N2O6/c26-23(7-12-27-13-8-23)16-25-9-4-17(5-10-25)14-29-22-21-19(30-18-6-11-28-15-18)2-1-3-20(21)31-24-22/h1-3,17-18,26H,4-16H2/t18-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from 5HT4 receptor in rat striatal membrane after 30 mins by liquid scintillation counting |
J Med Chem 55: 9240-54 (2012)
Article DOI: 10.1021/jm300953p
BindingDB Entry DOI: 10.7270/Q2FQ9XRB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data