 Found 7277 hits with Last Name = 'du' and Initial = 'x'
Found 7277 hits with Last Name = 'du' and Initial = 'x' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM642537
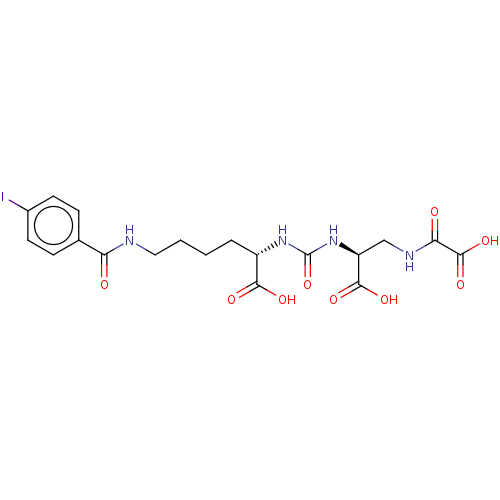
(US20230414794, Compound S2)Show SMILES OC(=O)[C@H](CCCCNC(=O)c1ccc(I)cc1)NC(=O)N[C@@H](CNC(=O)C(O)=O)C(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50481609
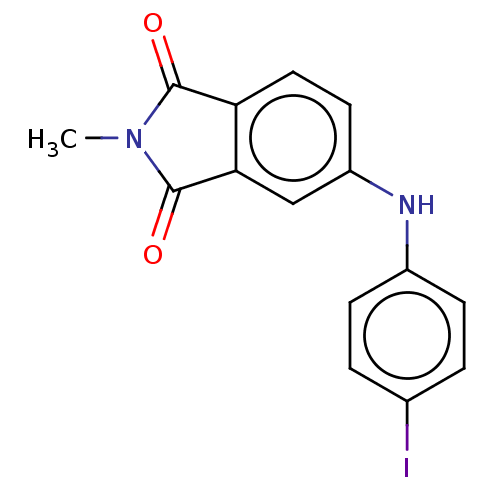
(CHEMBL610504)Show InChI InChI=1S/C15H11IN2O2/c1-18-14(19)12-7-6-11(8-13(12)15(18)20)17-10-4-2-9(16)3-5-10/h2-8,17H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I-N-Methyl-4-(4-bromoanilino)phthalimide from beta-amyloid plaques isolated from Alzheimer's disease patient brain |
Bioorg Med Chem 18: 1337-43 (2010)
Article DOI: 10.1016/j.bmc.2009.12.023
BindingDB Entry DOI: 10.7270/Q2445Q9V |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50229378
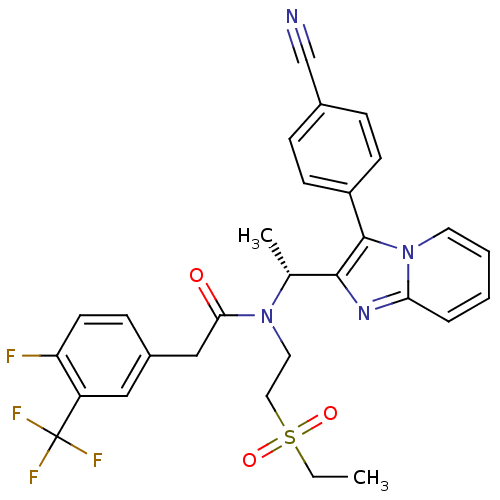
((R)-N-(1-(3-(4-cyanophenyl)H-imidazo[1,2-a]pyridin...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1nc2ccccn2c1-c1ccc(cc1)C#N)C(=O)Cc1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C29H26F4N4O3S/c1-3-41(39,40)15-14-36(26(38)17-21-9-12-24(30)23(16-21)29(31,32)33)19(2)27-28(22-10-7-20(18-34)8-11-22)37-13-5-4-6-25(37)35-27/h4-13,16,19H,3,14-15,17H2,1-2H3/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IP-10 from CXCR3 receptor expressed in human PBMC in RPMI-1640 buffer supplemented with 0.5% BSA |
Bioorg Med Chem Lett 18: 688-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.060
BindingDB Entry DOI: 10.7270/Q2WQ03HW |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50304738
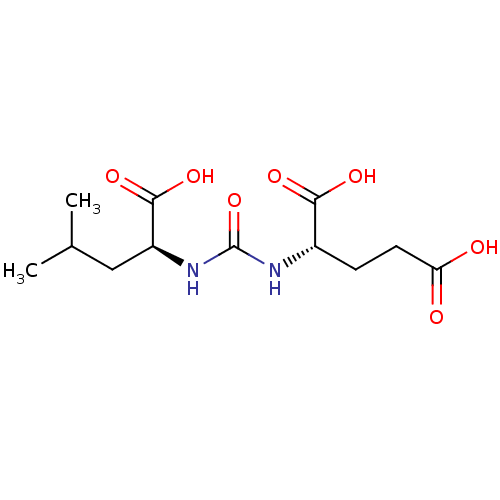
(2-(3-((S)-1-carboxy-3-methylbutyl)ureido)pentanedi...)Show SMILES CC(C)C[C@H](NC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C12H20N2O7/c1-6(2)5-8(11(19)20)14-12(21)13-7(10(17)18)3-4-9(15)16/h6-8H,3-5H2,1-2H3,(H,15,16)(H,17,18)(H,19,20)(H2,13,14,21)/t7-,8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| 3.53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50481608
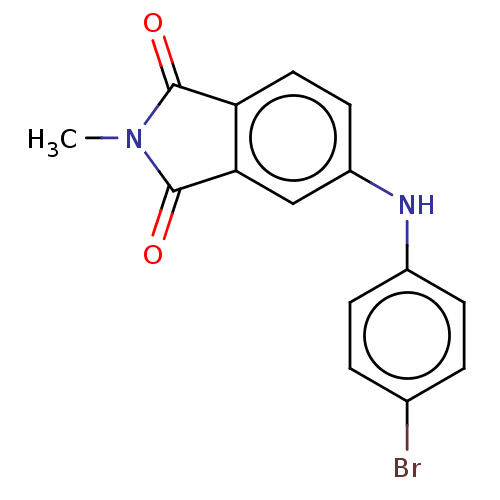
(CHEMBL598081)Show InChI InChI=1S/C15H11BrN2O2/c1-18-14(19)12-7-6-11(8-13(12)15(18)20)17-10-4-2-9(16)3-5-10/h2-8,17H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I-N-Methyl-4-(4-bromoanilino)phthalimide from beta-amyloid plaques isolated from Alzheimer's disease patient brain |
Bioorg Med Chem 18: 1337-43 (2010)
Article DOI: 10.1016/j.bmc.2009.12.023
BindingDB Entry DOI: 10.7270/Q2445Q9V |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50481605
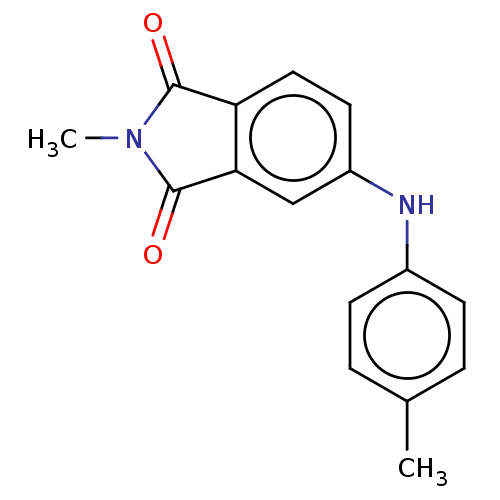
(CHEMBL598288)Show InChI InChI=1S/C16H14N2O2/c1-10-3-5-11(6-4-10)17-12-7-8-13-14(9-12)16(20)18(2)15(13)19/h3-9,17H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I-N-Methyl-4-(4-bromoanilino)phthalimide from beta-amyloid plaques isolated from Alzheimer's disease patient brain |
Bioorg Med Chem 18: 1337-43 (2010)
Article DOI: 10.1016/j.bmc.2009.12.023
BindingDB Entry DOI: 10.7270/Q2445Q9V |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM642538
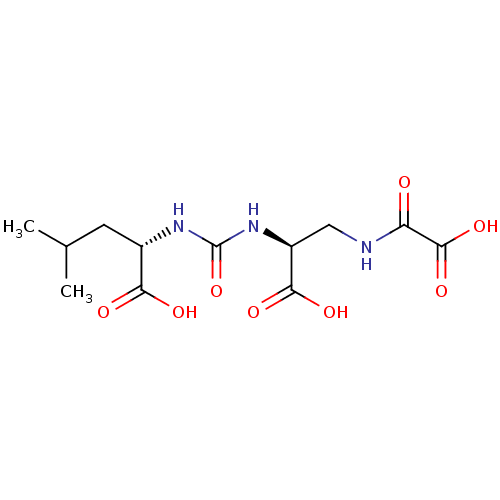
(US20230414794, Compound S3)Show SMILES CC(C)C[C@H](NC(=O)N[C@@H](CNC(=O)C(O)=O)C(O)=O)C(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 5.69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM642536
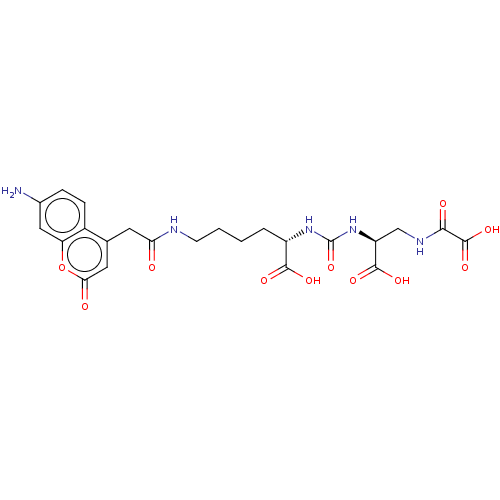
(US20230414794, Compound S1)Show SMILES Nc1ccc2c(CC(=O)NCCCC[C@H](NC(=O)N[C@@H](CNC(=O)C(O)=O)C(O)=O)C(O)=O)cc(=O)oc2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 5.96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50481604
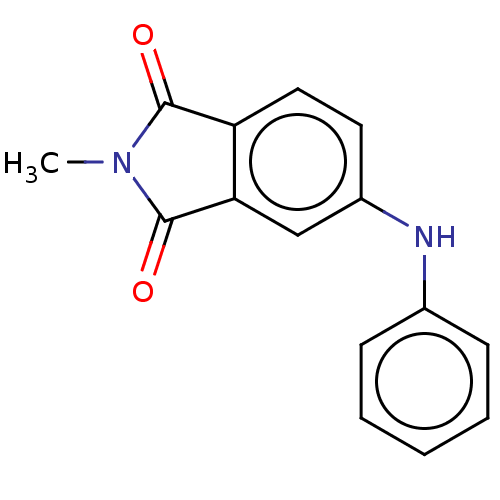
(CHEMBL610505)Show InChI InChI=1S/C15H12N2O2/c1-17-14(18)12-8-7-11(9-13(12)15(17)19)16-10-5-3-2-4-6-10/h2-9,16H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I-N-Methyl-4-(4-bromoanilino)phthalimide from beta-amyloid plaques isolated from Alzheimer's disease patient brain |
Bioorg Med Chem 18: 1337-43 (2010)
Article DOI: 10.1016/j.bmc.2009.12.023
BindingDB Entry DOI: 10.7270/Q2445Q9V |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50481606

(CHEMBL598082)Show InChI InChI=1S/C16H14N2O3/c1-18-15(19)13-8-5-11(9-14(13)16(18)20)17-10-3-6-12(21-2)7-4-10/h3-9,17H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I-N-Methyl-4-(4-bromoanilino)phthalimide from beta-amyloid plaques isolated from Alzheimer's disease patient brain |
Bioorg Med Chem 18: 1337-43 (2010)
Article DOI: 10.1016/j.bmc.2009.12.023
BindingDB Entry DOI: 10.7270/Q2445Q9V |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50301397

(CHEMBL571390 | [1-(4-Methyl-benzyl)-piperidin-4-yl...)Show SMILES Cc1ccc(CN2CCC(CC2)C(O)(c2ccccc2)c2ccccc2)cc1 Show InChI InChI=1S/C26H29NO/c1-21-12-14-22(15-13-21)20-27-18-16-25(17-19-27)26(28,23-8-4-2-5-9-23)24-10-6-3-7-11-24/h2-15,25,28H,16-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in CHO cell by betaplate scintillation counting |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50481607
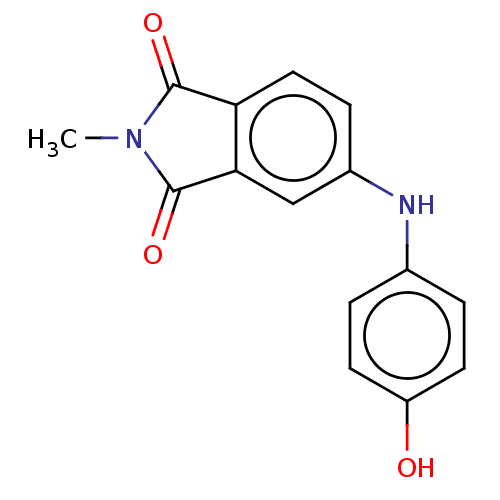
(CHEMBL598083)Show InChI InChI=1S/C15H12N2O3/c1-17-14(19)12-7-4-10(8-13(12)15(17)20)16-9-2-5-11(18)6-3-9/h2-8,16,18H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I-N-Methyl-4-(4-bromoanilino)phthalimide from beta-amyloid plaques isolated from Alzheimer's disease patient brain |
Bioorg Med Chem 18: 1337-43 (2010)
Article DOI: 10.1016/j.bmc.2009.12.023
BindingDB Entry DOI: 10.7270/Q2445Q9V |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50301394
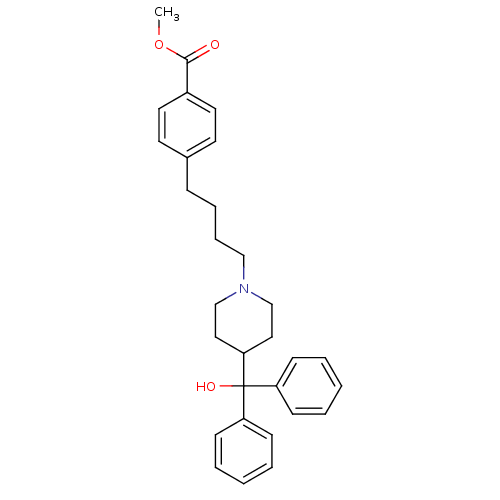
(CHEMBL568892 | methyl 4-(4-(4-(hydroxydiphenylmeth...)Show SMILES COC(=O)c1ccc(CCCCN2CCC(CC2)C(O)(c2ccccc2)c2ccccc2)cc1 Show InChI InChI=1S/C30H35NO3/c1-34-29(32)25-17-15-24(16-18-25)10-8-9-21-31-22-19-28(20-23-31)30(33,26-11-4-2-5-12-26)27-13-6-3-7-14-27/h2-7,11-18,28,33H,8-10,19-23H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in CHO cell by betaplate scintillation counting |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22874
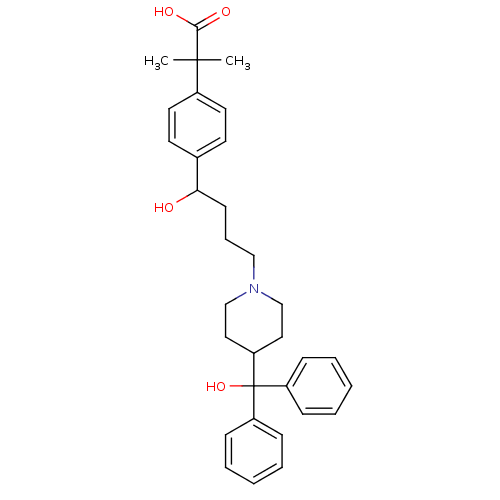
(2-(4-{1-hydroxy-4-[4-(hydroxydiphenylmethyl)piperi...)Show SMILES CC(C)(C(O)=O)c1ccc(cc1)C(O)CCCN1CCC(CC1)C(O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C32H39NO4/c1-31(2,30(35)36)25-17-15-24(16-18-25)29(34)14-9-21-33-22-19-28(20-23-33)32(37,26-10-5-3-6-11-26)27-12-7-4-8-13-27/h3-8,10-13,15-18,28-29,34,37H,9,14,19-23H2,1-2H3,(H,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in CHO cell by betaplate scintillation counting |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50095997
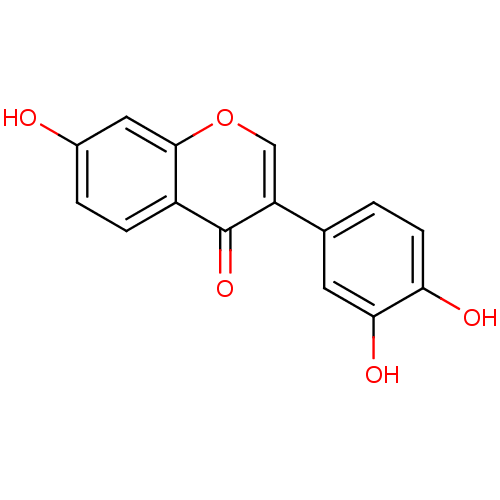
(3',4',7-trihydroxyisoflavone | CHEMBL13486)Show InChI InChI=1S/C15H10O5/c16-9-2-3-10-14(6-9)20-7-11(15(10)19)8-1-4-12(17)13(18)5-8/h1-7,16-18H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MIF tautomerase |
J Med Chem 52: 416-24 (2009)
Article DOI: 10.1021/jm801100v
BindingDB Entry DOI: 10.7270/Q208656D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50301391
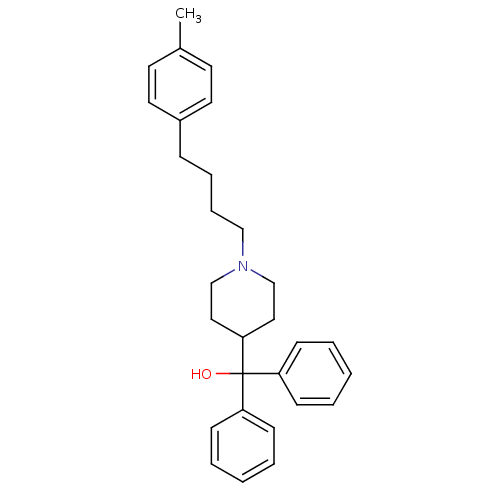
(1-[4-(4-methylphenyl)butyl]-alpha,alpha-diphenyl-4...)Show SMILES Cc1ccc(CCCCN2CCC(CC2)C(O)(c2ccccc2)c2ccccc2)cc1 Show InChI InChI=1S/C29H35NO/c1-24-15-17-25(18-16-24)10-8-9-21-30-22-19-28(20-23-30)29(31,26-11-4-2-5-12-26)27-13-6-3-7-14-27/h2-7,11-18,28,31H,8-10,19-23H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in CHO cell by betaplate scintillation counting |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50017376

((+/-)1-(4-tert-butylphenyl)-4-(4-(hydroxydiphenylm...)Show SMILES CC(C)(C)c1ccc(cc1)C(O)CCCN1CCC(CC1)C(O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C32H41NO2/c1-31(2,3)26-18-16-25(17-19-26)30(34)15-10-22-33-23-20-29(21-24-33)32(35,27-11-6-4-7-12-27)28-13-8-5-9-14-28/h4-9,11-14,16-19,29-30,34-35H,10,15,20-24H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in CHO cell by betaplate scintillation counting |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50301393
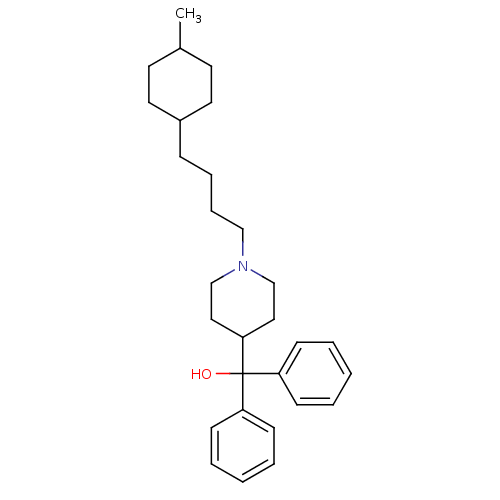
(1-[4-(4-methylcyclohexyl)butyl]alpha,alpha-dipheny...)Show SMILES CC1CCC(CCCCN2CCC(CC2)C(O)(c2ccccc2)c2ccccc2)CC1 |(10.08,-37.25,;8.75,-36.48,;7.41,-37.24,;6.08,-36.46,;6.09,-34.92,;4.76,-34.15,;3.42,-34.91,;2.09,-34.13,;.76,-34.9,;-.57,-34.12,;-1.91,-34.88,;-3.23,-34.11,;-3.23,-32.57,;-1.9,-31.8,;-.57,-32.58,;-4.56,-31.8,;-5.9,-31.03,;-4.56,-30.26,;-3.22,-29.5,;-3.21,-27.96,;-4.55,-27.19,;-5.89,-27.96,;-5.88,-29.5,;-5.89,-32.57,;-7.22,-31.79,;-8.55,-32.56,;-8.56,-34.1,;-7.21,-34.87,;-5.88,-34.1,;7.42,-34.16,;8.75,-34.93,)| Show InChI InChI=1S/C29H41NO/c1-24-15-17-25(18-16-24)10-8-9-21-30-22-19-28(20-23-30)29(31,26-11-4-2-5-12-26)27-13-6-3-7-14-27/h2-7,11-14,24-25,28,31H,8-10,15-23H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in CHO cell by betaplate scintillation counting |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50301398

(CHEMBL571391 | Diphenyl-[1-(2-p-tolyl-ethyl)-piper...)Show SMILES Cc1ccc(CCN2CCC(CC2)C(O)(c2ccccc2)c2ccccc2)cc1 Show InChI InChI=1S/C27H31NO/c1-22-12-14-23(15-13-22)16-19-28-20-17-26(18-21-28)27(29,24-8-4-2-5-9-24)25-10-6-3-7-11-25/h2-15,26,29H,16-21H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in CHO cell by betaplate scintillation counting |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50301396

(CHEMBL572034 | Phenyl-[1-(4-p-tolyl-butyl)-piperid...)Show InChI InChI=1S/C23H31NO/c1-19-10-12-20(13-11-19)7-5-6-16-24-17-14-22(15-18-24)23(25)21-8-3-2-4-9-21/h2-4,8-13,22-23,25H,5-7,14-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 121 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in CHO cell by betaplate scintillation counting |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50301399
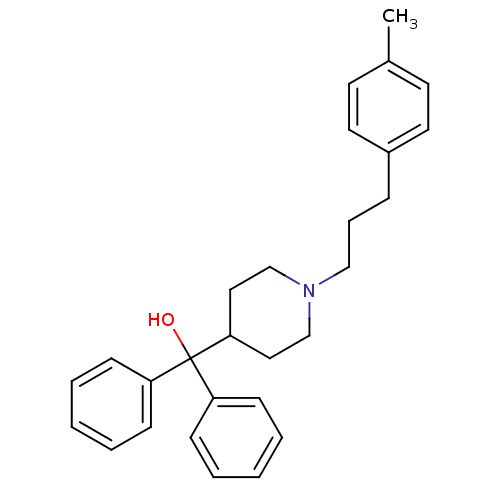
(CHEMBL570695 | Diphenyl-[1-(3-p-tolyl-propyl)-pipe...)Show SMILES Cc1ccc(CCCN2CCC(CC2)C(O)(c2ccccc2)c2ccccc2)cc1 Show InChI InChI=1S/C28H33NO/c1-23-14-16-24(17-15-23)9-8-20-29-21-18-27(19-22-29)28(30,25-10-4-2-5-11-25)26-12-6-3-7-13-26/h2-7,10-17,27,30H,8-9,18-22H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in CHO cell by betaplate scintillation counting |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50301392
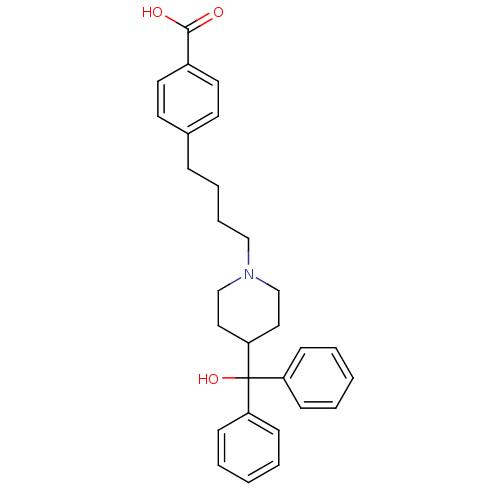
(4-[4-[4-[hydroxy(diphenyl)methyl]-1-piperidinyl]bu...)Show SMILES OC(=O)c1ccc(CCCCN2CCC(CC2)C(O)(c2ccccc2)c2ccccc2)cc1 Show InChI InChI=1S/C29H33NO3/c31-28(32)24-16-14-23(15-17-24)9-7-8-20-30-21-18-27(19-22-30)29(33,25-10-3-1-4-11-25)26-12-5-2-6-13-26/h1-6,10-17,27,33H,7-9,18-22H2,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 203 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in CHO cell by betaplate scintillation counting |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50096003
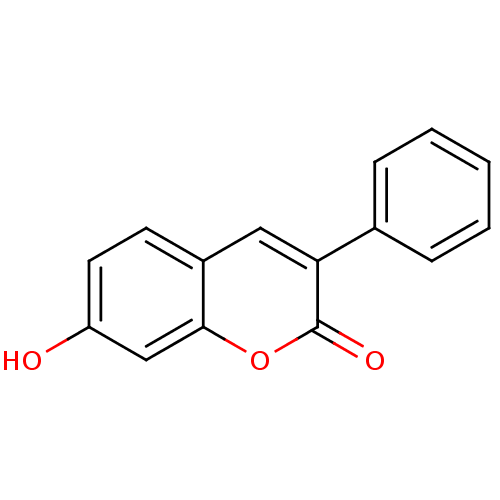
(7-Hydroxy-3-phenyl-chromen-2-one | 7-hydroxy-3-phe...)Show InChI InChI=1S/C15H10O3/c16-12-7-6-11-8-13(10-4-2-1-3-5-10)15(17)18-14(11)9-12/h1-9,16H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MIF tautomerase |
J Med Chem 52: 416-24 (2009)
Article DOI: 10.1021/jm801100v
BindingDB Entry DOI: 10.7270/Q208656D |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50096001
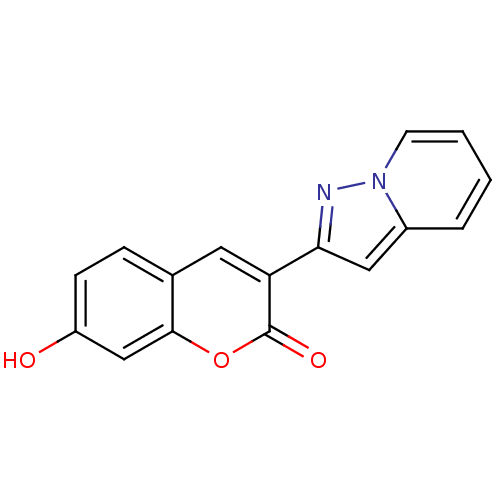
(7-Hydroxy-3-pyrazolo[1,5-a]pyridin-2-yl-chromen-2-...)Show InChI InChI=1S/C16H10N2O3/c19-12-5-4-10-7-13(16(20)21-15(10)9-12)14-8-11-3-1-2-6-18(11)17-14/h1-9,19H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MIF tautomerase |
J Med Chem 52: 416-24 (2009)
Article DOI: 10.1021/jm801100v
BindingDB Entry DOI: 10.7270/Q208656D |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50017724
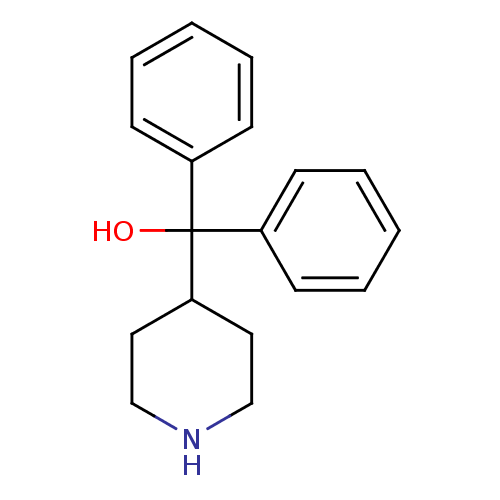
(CHEMBL127508 | Diphenyl-piperidin-4-yl-methanol | ...)Show InChI InChI=1S/C18H21NO/c20-18(15-7-3-1-4-8-15,16-9-5-2-6-10-16)17-11-13-19-14-12-17/h1-10,17,19-20H,11-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 659 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in CHO cell by betaplate scintillation counting |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50040929

(4,5-dianilinophthalimide | 5,6-bis(phenylamino)-1H...)Show InChI InChI=1S/C20H15N3O2/c24-19-15-11-17(21-13-7-3-1-4-8-13)18(12-16(15)20(25)23-19)22-14-9-5-2-6-10-14/h1-12,21-22H,(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I-N-Methyl-4-(4-bromoanilino)phthalimide from beta-amyloid plaques isolated from Alzheimer's disease patient brain |
Bioorg Med Chem 18: 1337-43 (2010)
Article DOI: 10.1016/j.bmc.2009.12.023
BindingDB Entry DOI: 10.7270/Q2445Q9V |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM642541
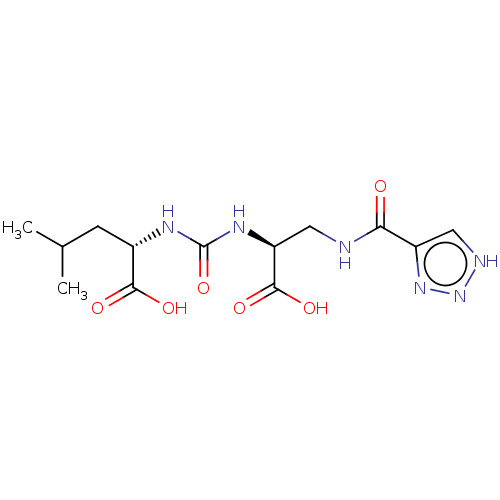
(US20230414794, Comparative Compound DS3 | US202304...)Show SMILES CC(C)C[C@H](NC(=O)N[C@@H](CNC(=O)c1c[nH]nn1)C(O)=O)C(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50122787
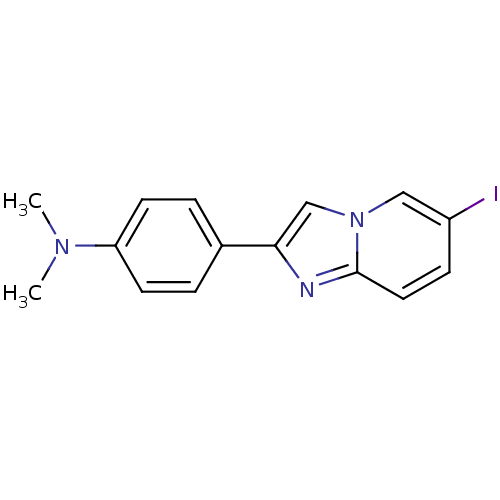
(2-(4'-dimethylaminophenyl)-6-iodoimidazo[1,2-a]pyr...)Show InChI InChI=1S/C15H14IN3/c1-18(2)13-6-3-11(4-7-13)14-10-19-9-12(16)5-8-15(19)17-14/h3-10H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I-N-Methyl-4-(4-bromoanilino)phthalimide from beta-amyloid plaques isolated from Alzheimer's disease patient brain |
Bioorg Med Chem 18: 1337-43 (2010)
Article DOI: 10.1016/j.bmc.2009.12.023
BindingDB Entry DOI: 10.7270/Q2445Q9V |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50040923
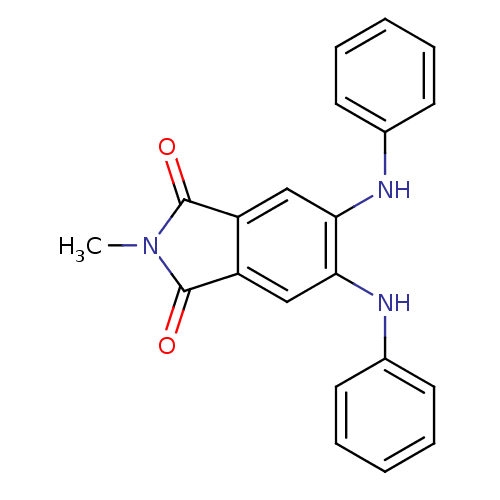
(2-Methyl-5,6-bis-phenylamino-isoindole-1,3-dione |...)Show InChI InChI=1S/C21H17N3O2/c1-24-20(25)16-12-18(22-14-8-4-2-5-9-14)19(13-17(16)21(24)26)23-15-10-6-3-7-11-15/h2-13,22-23H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I-N-Methyl-4-(4-bromoanilino)phthalimide from beta-amyloid plaques isolated from Alzheimer's disease patient brain |
Bioorg Med Chem 18: 1337-43 (2010)
Article DOI: 10.1016/j.bmc.2009.12.023
BindingDB Entry DOI: 10.7270/Q2445Q9V |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50079267
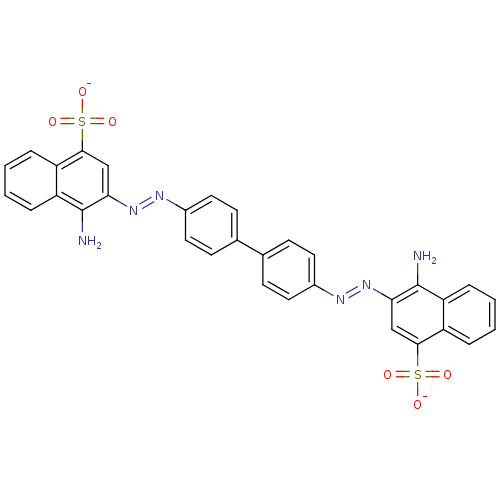
(Congo Red | Direct red 28 | Kongorot | Sodium diph...)Show SMILES Nc1c(cc(c2ccccc12)S([O-])(=O)=O)\N=N\c1ccc(cc1)-c1ccc(cc1)\N=N\c1cc(c2ccccc2c1N)S([O-])(=O)=O Show InChI InChI=1S/C32H24N6O6S2/c33-31-25-7-3-1-5-23(25)29(45(39,40)41)17-27(31)37-35-21-13-9-19(10-14-21)20-11-15-22(16-12-20)36-38-28-18-30(46(42,43)44)24-6-2-4-8-26(24)32(28)34/h1-18H,33-34H2,(H,39,40,41)(H,42,43,44)/p-2/b37-35+,38-36+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I-N-Methyl-4-(4-bromoanilino)phthalimide from beta-amyloid plaques isolated from Alzheimer's disease patient brain |
Bioorg Med Chem 18: 1337-43 (2010)
Article DOI: 10.1016/j.bmc.2009.12.023
BindingDB Entry DOI: 10.7270/Q2445Q9V |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50129793
(2-(4''-methylaminophenyl)-6-hydroxybenzothiazole |...)Show InChI InChI=1S/C14H12N2OS/c1-15-10-4-2-9(3-5-10)14-16-12-7-6-11(17)8-13(12)18-14/h2-8,15,17H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I-N-Methyl-4-(4-bromoanilino)phthalimide from beta-amyloid plaques isolated from Alzheimer's disease patient brain |
Bioorg Med Chem 18: 1337-43 (2010)
Article DOI: 10.1016/j.bmc.2009.12.023
BindingDB Entry DOI: 10.7270/Q2445Q9V |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM642541

(US20230414794, Comparative Compound DS3 | US202304...)Show SMILES CC(C)C[C@H](NC(=O)N[C@@H](CNC(=O)c1c[nH]nn1)C(O)=O)C(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM642540
(US20230414794, Comparative Compound DS2)Show SMILES CC(C)C[C@H](NC(=O)N[C@@H](CNC(=O)c1c[nH]cn1)C(O)=O)C(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM642539
(US20230414794, Comparative Compound DS1)Show SMILES CC(C)C[C@H](NC(=O)N[C@@H](CNC(=O)c1ncc[nH]1)C(O)=O)C(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50100134

(2-(4-Dimethylamino-phenyl)-3,6-dimethyl-benzothiaz...)Show InChI InChI=1S/C17H19N2S/c1-12-5-10-15-16(11-12)20-17(19(15)4)13-6-8-14(9-7-13)18(2)3/h5-11H,1-4H3/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I-N-Methyl-4-(4-bromoanilino)phthalimide from beta-amyloid plaques isolated from Alzheimer's disease patient brain |
Bioorg Med Chem 18: 1337-43 (2010)
Article DOI: 10.1016/j.bmc.2009.12.023
BindingDB Entry DOI: 10.7270/Q2445Q9V |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50265450
(2-oxo-2-phenylethyl 7-hydroxy-2-oxo-2H-chromene-3-...)Show InChI InChI=1S/C18H12O6/c19-13-7-6-12-8-14(18(22)24-16(12)9-13)17(21)23-10-15(20)11-4-2-1-3-5-11/h1-9,19H,10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MIF tautomerase |
J Med Chem 52: 416-24 (2009)
Article DOI: 10.1021/jm801100v
BindingDB Entry DOI: 10.7270/Q208656D |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50096006
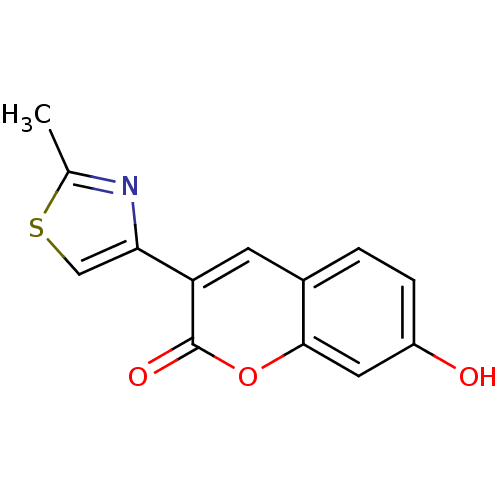
(7-Hydroxy-3-(2-methyl-thiazol-4-yl)-chromen-2-one ...)Show InChI InChI=1S/C13H9NO3S/c1-7-14-11(6-18-7)10-4-8-2-3-9(15)5-12(8)17-13(10)16/h2-6,15H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MIF tautomerase |
J Med Chem 52: 416-24 (2009)
Article DOI: 10.1021/jm801100v
BindingDB Entry DOI: 10.7270/Q208656D |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50301395

(1-(4-p-Tolyl-butyl)-piperidine | CHEMBL571073)Show InChI InChI=1S/C16H25N/c1-15-8-10-16(11-9-15)7-3-6-14-17-12-4-2-5-13-17/h8-11H,2-7,12-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in CHO cell by betaplate scintillation counting |
Bioorg Med Chem Lett 19: 5043-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.047
BindingDB Entry DOI: 10.7270/Q2NP24HJ |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50096008
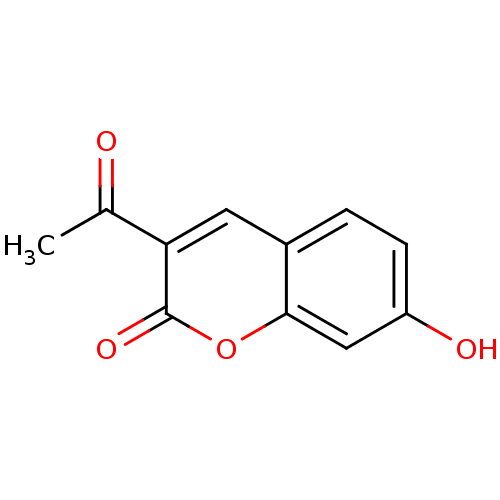
(3-Acetyl-7-hydroxy-chromen-2-one | 3-acetyl-7-hydr...)Show InChI InChI=1S/C11H8O4/c1-6(12)9-4-7-2-3-8(13)5-10(7)15-11(9)14/h2-5,13H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MIF tautomerase |
J Med Chem 52: 416-24 (2009)
Article DOI: 10.1021/jm801100v
BindingDB Entry DOI: 10.7270/Q208656D |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50095995
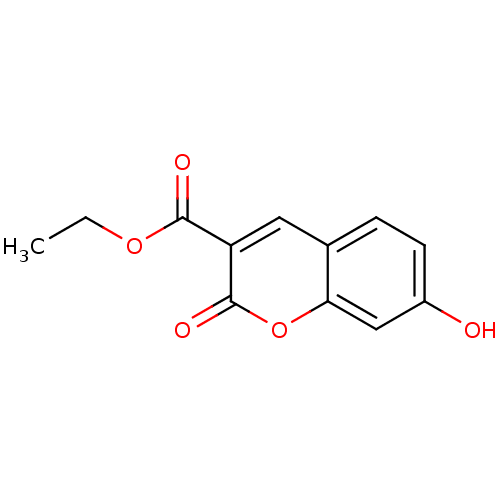
(7-HYDROXY-2-OXO-CHROMENE-3-CARBOXYLIC ACID ETHYL E...)Show InChI InChI=1S/C12H10O5/c1-2-16-11(14)9-5-7-3-4-8(13)6-10(7)17-12(9)15/h3-6,13H,2H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MIF tautomerase |
J Med Chem 52: 416-24 (2009)
Article DOI: 10.1021/jm801100v
BindingDB Entry DOI: 10.7270/Q208656D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1/3
(Homo sapiens (Human)) | BDBM28123

(3-cyanoquinoline, 8 | 4-({3-chloro-4-[(1-methyl-1H...)Show SMILES COc1cc2c(Nc3ccc(Sc4nccn4C)c(Cl)c3)c(cnc2cc1OCCCN1CCOCC1)C#N Show InChI InChI=1S/C28H29ClN6O3S/c1-34-8-6-31-28(34)39-26-5-4-20(14-22(26)29)33-27-19(17-30)18-32-23-16-25(24(36-2)15-21(23)27)38-11-3-7-35-9-12-37-13-10-35/h4-6,8,14-16,18H,3,7,9-13H2,1-2H3,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of MEK1 phosphorylation in LoVo cells |
Bioorg Med Chem Lett 13: 3031-4 (2003)
BindingDB Entry DOI: 10.7270/Q2P84B8T |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50611953

(CHEMBL5289716)Show SMILES CN(CC1(CC1)C(=O)NC12CC3CC(C1)CC(C3)(C2)C(N)=O)S(=O)(=O)c1ccccc1F |TLB:17:16:10.11.12:14,19:16:10:12.13.14,19:16:10.11.12:14,THB:15:13:10:16.17.18,15:16:10:12.13.14,17:11:14:15.16.18| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B alpha isoform
(Homo sapiens (Human)) | BDBM50061067

(15-(3-Guanidino-propyl)-8-isobutyl-18-((1E,3E)-6-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C49H74N10O12/c1-26(2)23-37-46(66)58-40(48(69)70)30(6)42(62)55-35(17-14-22-52-49(50)51)45(65)54-34(19-18-27(3)24-28(4)38(71-10)25-33-15-12-11-13-16-33)29(5)41(61)56-36(47(67)68)20-21-39(60)59(9)32(8)44(64)53-31(7)43(63)57-37/h11-13,15-16,18-19,24,26,28-31,34-38,40H,8,14,17,20-23,25H2,1-7,9-10H3,(H,53,64)(H,54,65)(H,55,62)(H,56,61)(H,57,63)(H,58,66)(H,67,68)(H,69,70)(H4,50,51,52)/b19-18+,27-24+/t28-,29-,30-,31+,34-,35-,36+,37-,38-,40+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Observed inhibition activity of the compounds against protein phosphatases 2A (PP2A) |
J Med Chem 40: 3199-206 (1997)
Article DOI: 10.1021/jm960873x
BindingDB Entry DOI: 10.7270/Q25Q4WS0 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor [G667C]
(Homo sapiens (Human)) | BDBM515356
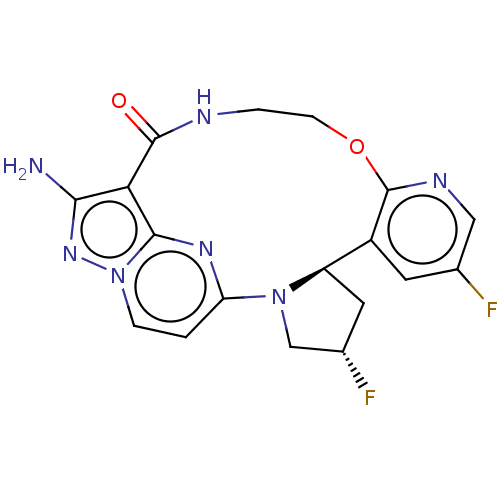
((13E,14E,22R,24S)-12-amino-24,35- difluoro-4-oxa-7...)Show SMILES Nc1nn2ccc3nc2c1C(=O)NCCOc1ncc(F)cc1[C@H]1C[C@H](F)CN31 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TrkAG667C (Kinase domain) kinase was expressed in Sf9 cells by using pIEX-Bac-4, and purified by using affinity chromatography on AKTA Purifier (GE c... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55FK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50061066

((5R,6S,9S,12S,13S,16R)-2-Eth-(Z)-ylidene-9-(3-guan...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)[C@@H](NC(=O)\C(=C\C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C41H60N8O10/c1-8-31-38(54)48-34(40(57)58)26(5)36(52)46-29(15-12-20-44-41(42)43)37(53)45-28(25(4)35(51)47-30(39(55)56)18-19-33(50)49(31)6)17-16-23(2)21-24(3)32(59-7)22-27-13-10-9-11-14-27/h8-11,13-14,16-17,21,24-26,28-30,32,34H,12,15,18-20,22H2,1-7H3,(H,45,53)(H,46,52)(H,47,51)(H,48,54)(H,55,56)(H,57,58)(H4,42,43,44)/b17-16+,23-21+,31-8-/t24-,25-,26-,28-,29-,30+,32-,34+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Observed inhibition activity against protein phosphatase 2A (PP2A) |
J Med Chem 40: 3199-206 (1997)
Article DOI: 10.1021/jm960873x
BindingDB Entry DOI: 10.7270/Q25Q4WS0 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM515368

((13E,14E,22R,24S)-12-amino- 24,35-difluoro-7-aza-1...)Show SMILES Nc1nn2ccc3nc2c1C(=O)NCCCc1ncc(F)cc1[C@H]1C[C@H](F)CN31 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A testing platform for TrkAWT kinase activity was established based on Homogeneous Time-Resolved Fluorescence (HTRF) assay, and the activities of the... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55FK |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor [G667C]
(Homo sapiens (Human)) | BDBM515377
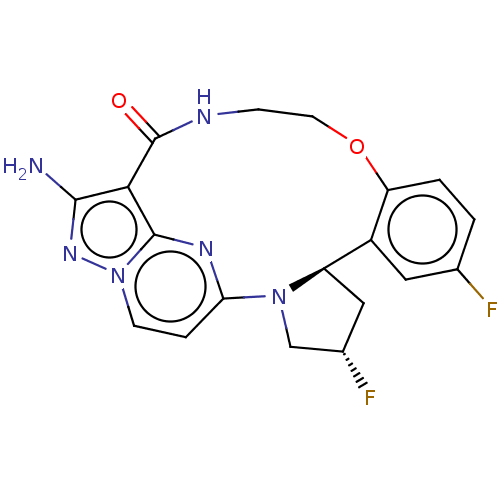
((13E,14E,22R,24S)-12-amino- 24,35-difluoro-4-oxa-7...)Show SMILES Nc1nn2ccc3nc2c1C(=O)NCCOc1ccc(F)cc1[C@H]1C[C@H](F)CN31 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TrkAG667C (Kinase domain) kinase was expressed in Sf9 cells by using pIEX-Bac-4, and purified by using affinity chromatography on AKTA Purifier (GE c... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55FK |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor [G667C]
(Homo sapiens (Human)) | BDBM515357
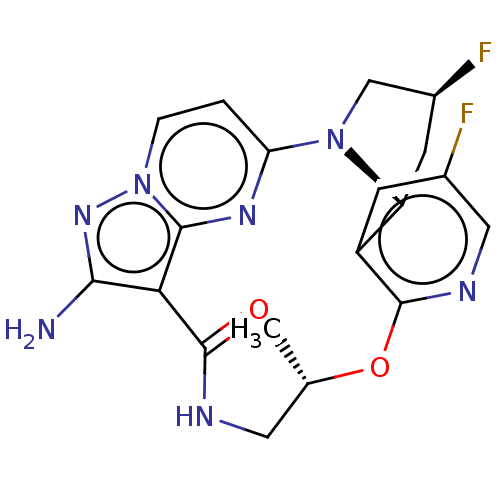
((13E,14E,22R,24S,5S)-12-amino-24,35-difluoro- 5-me...)Show SMILES C[C@H]1CNC(=O)c2c(N)nn3ccc(nc23)N2C[C@@H](F)C[C@@H]2c2cc(F)cnc2O1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TrkAG667C (Kinase domain) kinase was expressed in Sf9 cells by using pIEX-Bac-4, and purified by using affinity chromatography on AKTA Purifier (GE c... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55FK |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM515365
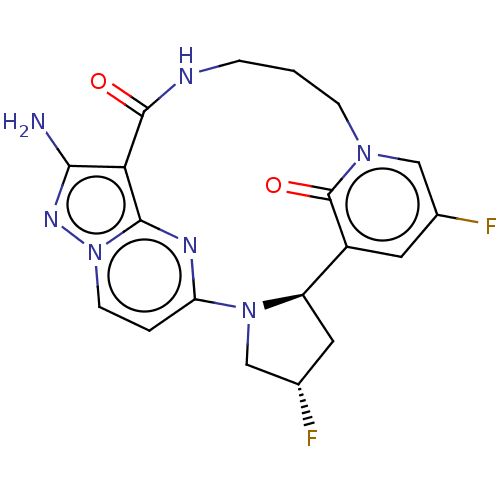
(US11098060, Example 23)Show SMILES Nc1nn2ccc3nc2c1C(=O)NCCCn1cc(F)cc([C@H]2C[C@H](F)CN32)c1=O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A testing platform for TrkAWT kinase activity was established based on Homogeneous Time-Resolved Fluorescence (HTRF) assay, and the activities of the... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55FK |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM515367
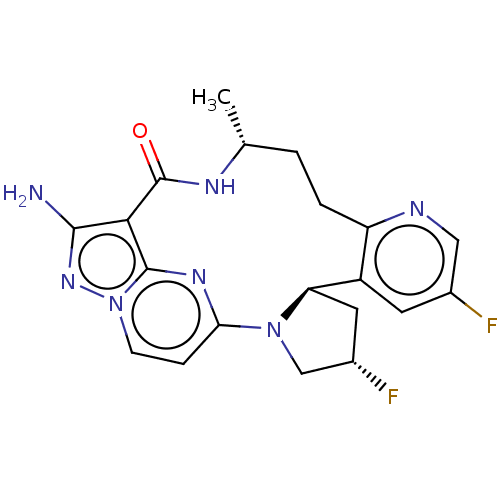
(US11098060, Example 26)Show SMILES C[C@@H]1CCc2ncc(F)cc2[C@H]2C[C@H](F)CN2c2ccn3nc(N)c(C(=O)N1)c3n2 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A testing platform for TrkAWT kinase activity was established based on Homogeneous Time-Resolved Fluorescence (HTRF) assay, and the activities of the... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55FK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data