

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM50080514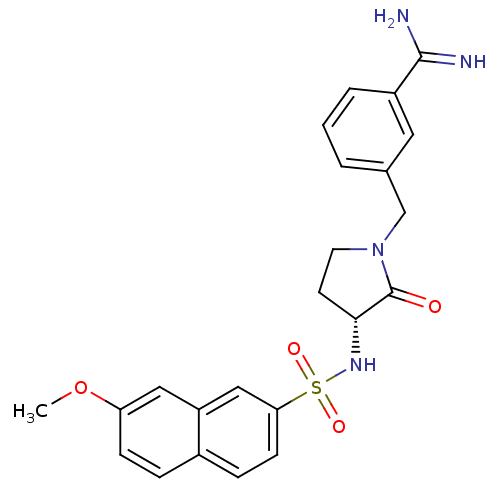 (3-[(R)-3-(7-Methoxy-naphthalene-2-sulfonylamino)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of human Coagulation factor Xa | J Med Chem 42: 3557-71 (1999) Article DOI: 10.1021/jm990040h BindingDB Entry DOI: 10.7270/Q2M04640 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50195684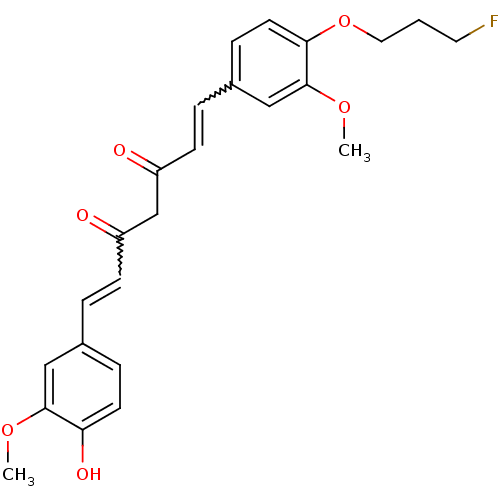 (1-[4-(3-fluoropropoxy)-3-methoxyphenyl]-5-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University School of Medicine Curated by ChEMBL | Assay Description Displacement of [125I]IMSB from beta amyloid protein 40 | J Med Chem 49: 6111-9 (2006) Article DOI: 10.1021/jm0607193 BindingDB Entry DOI: 10.7270/Q2J38TB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50100131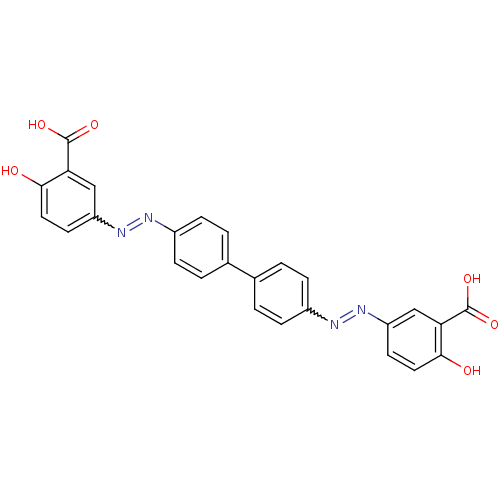 (5-(Biphenyl-4-ylazo)-bis (2-hydroxy-benzoic acid)(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University School of Medicine Curated by ChEMBL | Assay Description Displacement of [125I]IMSB from beta amyloid protein 40 | J Med Chem 49: 6111-9 (2006) Article DOI: 10.1021/jm0607193 BindingDB Entry DOI: 10.7270/Q2J38TB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50067040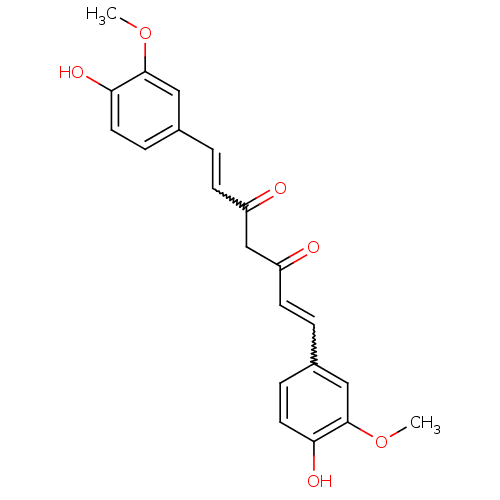 (((E,E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-hept...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.208 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University School of Medicine Curated by ChEMBL | Assay Description Displacement of [125I]IMSB from beta amyloid protein 40 | J Med Chem 49: 6111-9 (2006) Article DOI: 10.1021/jm0607193 BindingDB Entry DOI: 10.7270/Q2J38TB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50063266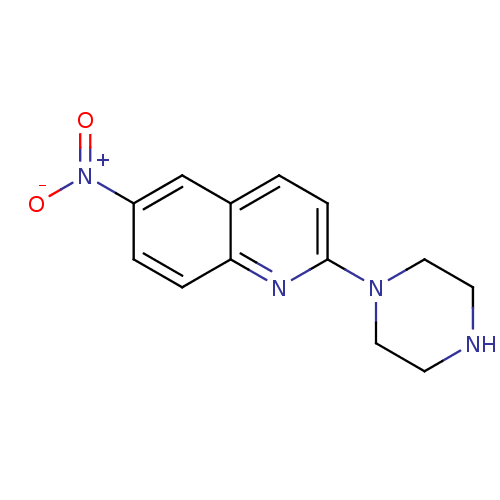 (6-Nitro-2-piperazin-1-yl-quinoline | 6-nitroquipaz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from Sprague-Dawley rat SERT | Bioorg Med Chem 15: 3499-504 (2007) Article DOI: 10.1016/j.bmc.2007.03.001 BindingDB Entry DOI: 10.7270/Q2SN08N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Mus musculus) | BDBM50057512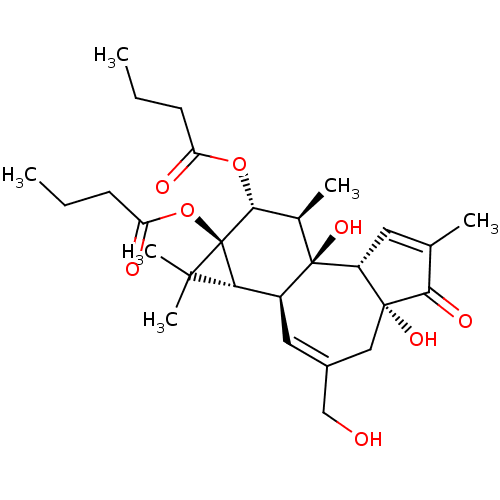 ((1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-4a,7b-dihydroxy-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Curated by ChEMBL | Assay Description Displacement of [3H]PDBu from mouse wild type C1b domain of PKCdelta expressed in Escherichia coli | J Med Chem 50: 3465-81 (2007) Article DOI: 10.1021/jm0702579 BindingDB Entry DOI: 10.7270/Q2RF5TQ5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50208770 (2-[2-(ethoxymethyl)piperazin-1-yl]-6-nitroquinolin...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from Sprague-Dawley rat SERT | Bioorg Med Chem 15: 3499-504 (2007) Article DOI: 10.1016/j.bmc.2007.03.001 BindingDB Entry DOI: 10.7270/Q2SN08N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50208769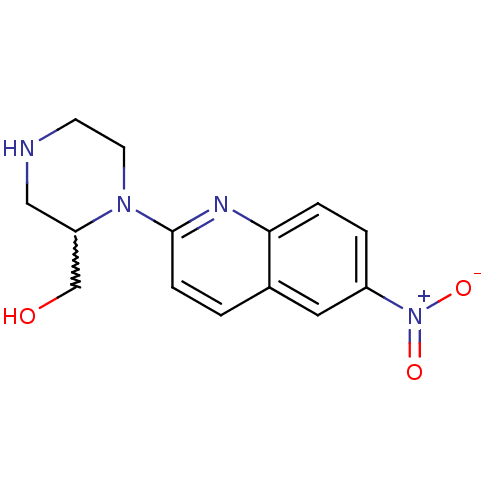 (2-[2-(hydroxymethyl)piperazin-1-yl]-6-nitroquinoli...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from Sprague-Dawley rat SERT | Bioorg Med Chem 15: 3499-504 (2007) Article DOI: 10.1016/j.bmc.2007.03.001 BindingDB Entry DOI: 10.7270/Q2SN08N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50208771 (2-[(2-methoxymethyl)piperazin-1-yl]-6-nitroquinoli...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from Sprague-Dawley rat SERT | Bioorg Med Chem 15: 3499-504 (2007) Article DOI: 10.1016/j.bmc.2007.03.001 BindingDB Entry DOI: 10.7270/Q2SN08N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM14059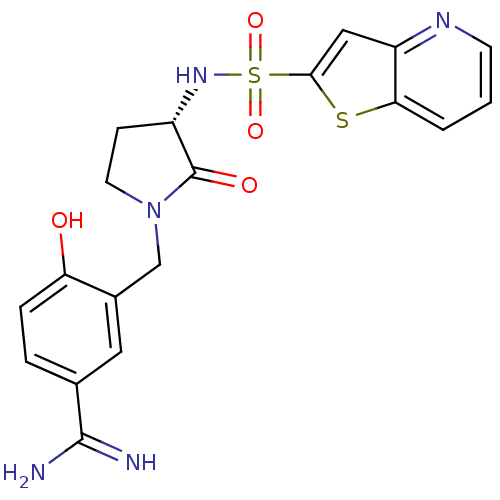 (4-hydroxy-3-[[(3S)-2-oxo-3-(9-thia-5-azabicyclo[4....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.700 | -51.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Aventis Pharma | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 43: 3226-32 (2000) Article DOI: 10.1021/jm000940u BindingDB Entry DOI: 10.7270/Q25H7DHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM14059 (4-hydroxy-3-[[(3S)-2-oxo-3-(9-thia-5-azabicyclo[4....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity against serine protease factor Xa (fXa) | Bioorg Med Chem Lett 9: 2753-8 (1999) BindingDB Entry DOI: 10.7270/Q2W66K02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50373326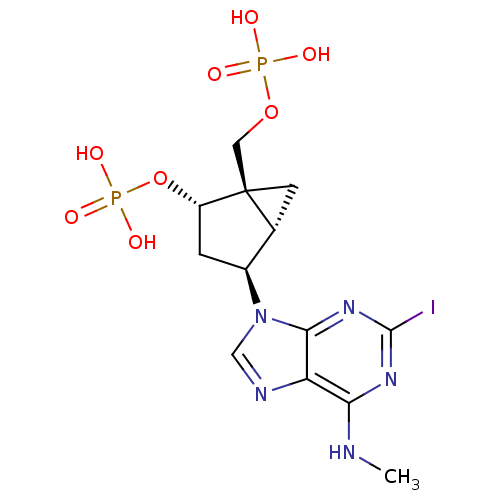 (CHEMBL444278) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK Curated by ChEMBL | Assay Description Inhibition of [3H]-5 binding to P2Y purinoceptor 1 expressed in Sf9 cells | J Med Chem 46: 4974-87 (2003) Article DOI: 10.1021/jm030127+ BindingDB Entry DOI: 10.7270/Q2NS0T9D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50123781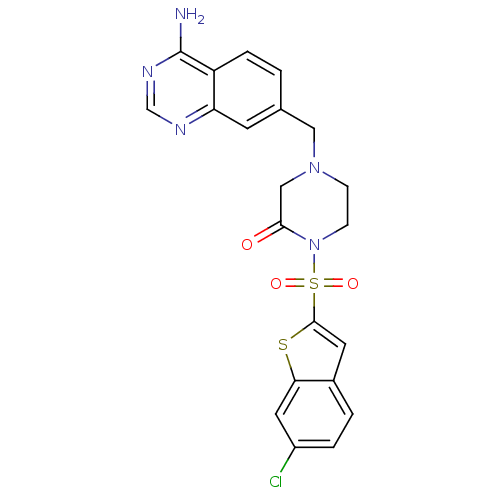 (4-(4-Amino-quinazolin-7-ylmethyl)-1-(6-chloro-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity (in vitro) of the compound towards human Coagulation factor X was determined at 10 mg/kg peroral dose | J Med Chem 46: 681-4 (2003) Article DOI: 10.1021/jm020384z BindingDB Entry DOI: 10.7270/Q2KW5FDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Mus musculus) | BDBM50216859 (CHEMBL442232 | {2-(hydroxymethyl)-5-[5-methyl-3-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Curated by ChEMBL | Assay Description Displacement of [3H]PDBu from mouse wild type C1b domain of PKCdelta expressed in Escherichia coli | J Med Chem 50: 3465-81 (2007) Article DOI: 10.1021/jm0702579 BindingDB Entry DOI: 10.7270/Q2RF5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12597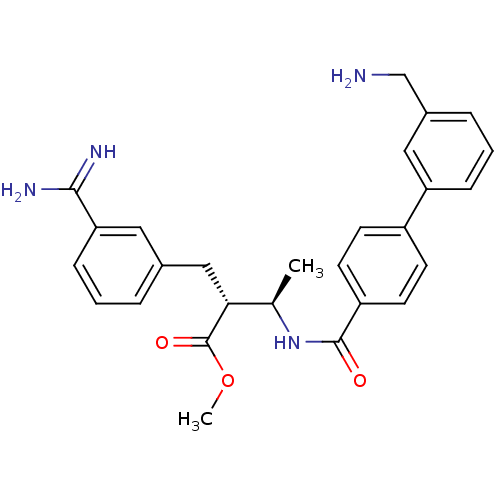 (CHEMBL48046 | RPR128515 | methyl (2R,3R)-2-{3-[ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.900 | -51.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Aventis Pharma | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 46: 685-90 (2003) Article DOI: 10.1021/jm0203837 BindingDB Entry DOI: 10.7270/Q2VH5M2S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12597 (CHEMBL48046 | RPR128515 | methyl (2R,3R)-2-{3-[ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.900 | -51.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Aventis Pharma | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 43: 3226-32 (2000) Article DOI: 10.1021/jm000940u BindingDB Entry DOI: 10.7270/Q25H7DHP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein kinase C delta type (Mus musculus) | BDBM50216857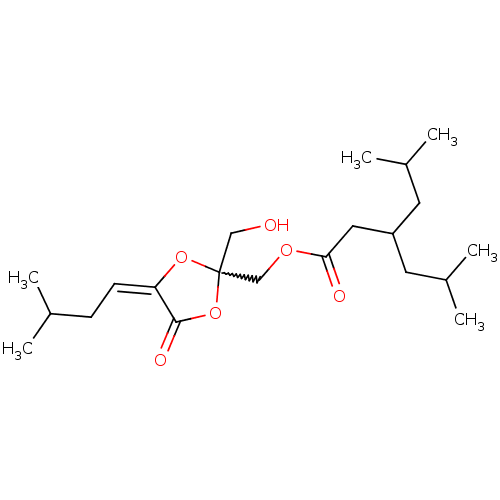 ((2-(hydroxymethyl)-4-(3-methylbutylidene)-5-oxo-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Curated by ChEMBL | Assay Description Displacement of [3H]PDBu from mouse wild type C1b domain of PKCdelta expressed in Escherichia coli | J Med Chem 50: 3465-81 (2007) Article DOI: 10.1021/jm0702579 BindingDB Entry DOI: 10.7270/Q2RF5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Mus musculus) | BDBM50216856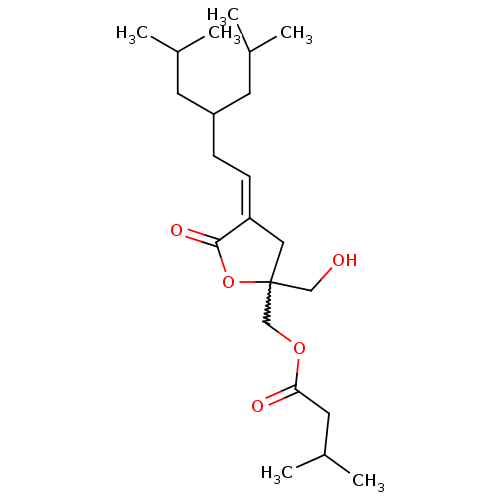 (CHEMBL231617 | Z-{2-(Hydroxymethyl)-4-[5-methyl-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Curated by ChEMBL | Assay Description Displacement of [3H]PDBu from mouse wild type C1b domain of PKCdelta expressed in Escherichia coli | J Med Chem 50: 3465-81 (2007) Article DOI: 10.1021/jm0702579 BindingDB Entry DOI: 10.7270/Q2RF5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12596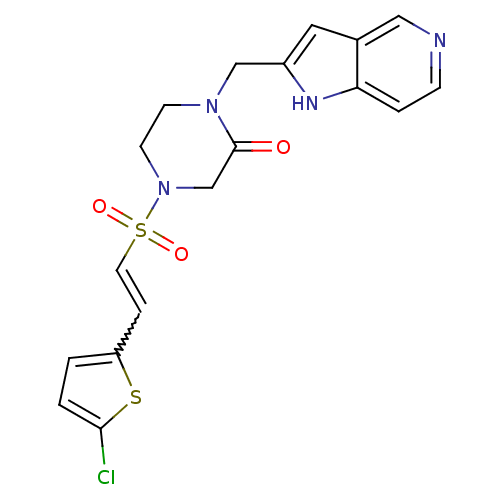 (4-{[(E)-2-(5-CHLOROTHIEN-2-YL)VINYL]SULFONYL}-1-(1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.10 | -50.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Aventis Pharma | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 46: 685-90 (2003) Article DOI: 10.1021/jm0203837 BindingDB Entry DOI: 10.7270/Q2VH5M2S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12596 (4-{[(E)-2-(5-CHLOROTHIEN-2-YL)VINYL]SULFONYL}-1-(1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity (in vitro) of the compound towards human Coagulation factor X was determined at 5 mg/kg peroral dose | J Med Chem 46: 681-4 (2003) Article DOI: 10.1021/jm020384z BindingDB Entry DOI: 10.7270/Q2KW5FDH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein kinase C delta type (Mus musculus) | BDBM50107112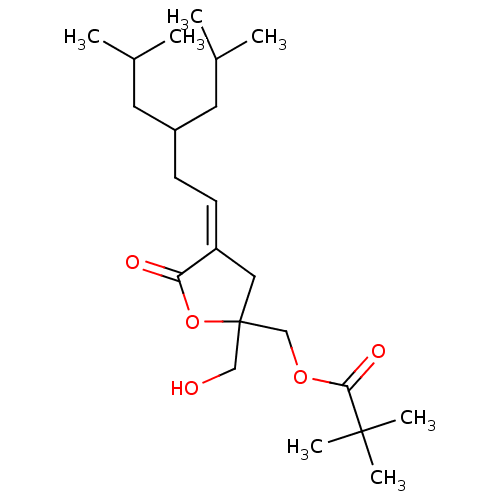 ((Z) 2,2-Dimethyl-propionic acid 2-hydroxymethyl-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Curated by ChEMBL | Assay Description Displacement of [3H]PDBu from mouse wild type C1b domain of PKCdelta expressed in Escherichia coli | J Med Chem 50: 3465-81 (2007) Article DOI: 10.1021/jm0702579 BindingDB Entry DOI: 10.7270/Q2RF5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50173647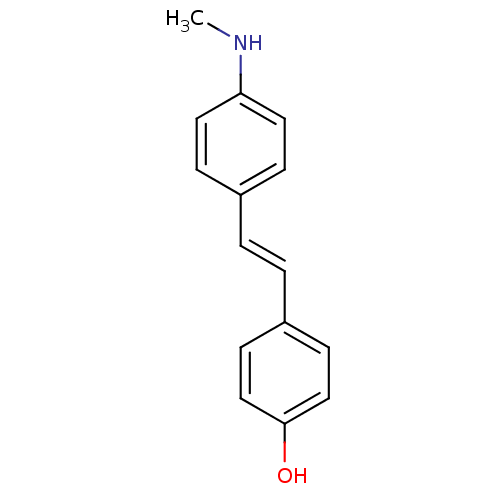 ((E)-4-(4-(methylamino)styryl)phenol | 4-(4-(methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University School of Medicine Curated by ChEMBL | Assay Description Binding affinity to amyloid beta (1-42) aggregates in human Alzheimer's disease brain sections | J Med Chem 55: 883-92 (2012) Article DOI: 10.1021/jm201400q BindingDB Entry DOI: 10.7270/Q2X92F5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Mus musculus) | BDBM50107112 ((Z) 2,2-Dimethyl-propionic acid 2-hydroxymethyl-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Curated by ChEMBL | Assay Description Binding affinity to mouse wildtype PKCdelta C1a expressed in Escherichia coli | J Med Chem 50: 3465-81 (2007) Article DOI: 10.1021/jm0702579 BindingDB Entry DOI: 10.7270/Q2RF5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12594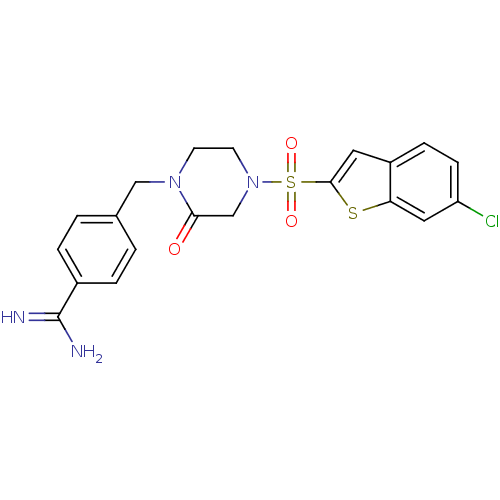 (4-({4-[(6-CHLORO-1-BENZOTHIEN-2-YL)SULFONYL]-2-OXO...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.30 | -50.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Aventis Pharma | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 46: 685-90 (2003) Article DOI: 10.1021/jm0203837 BindingDB Entry DOI: 10.7270/Q2VH5M2S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50081505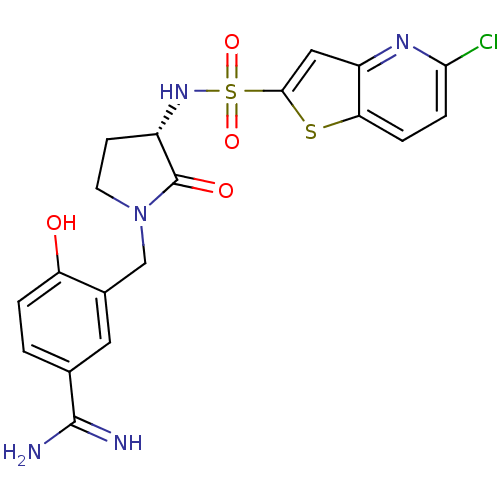 (3-[(S)-3-(5-Chloro-thieno[3,2-b]pyridine-2-sulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity against serine protease factor Xa (fXa) | Bioorg Med Chem Lett 9: 2753-8 (1999) BindingDB Entry DOI: 10.7270/Q2W66K02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50239956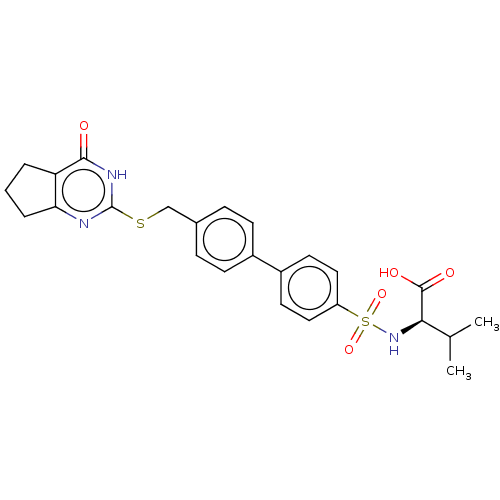 (CHEMBL4061792) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Scripps Florida , 130 Scripps Way, Jupiter, Florida 33458, United States. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human MMP13 expressed in mouse NSO cells using varying levels of fTHP-15 as substrate preincubated for 30 mins ... | J Med Chem 60: 5816-5825 (2017) Article DOI: 10.1021/acs.jmedchem.7b00514 BindingDB Entry DOI: 10.7270/Q23F4RTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50239954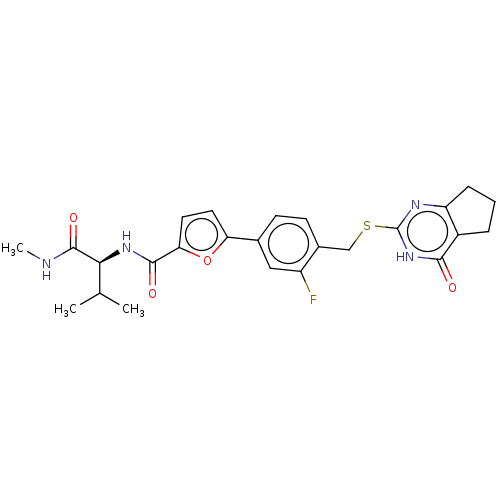 (CHEMBL4080520) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Scripps Florida , 130 Scripps Way, Jupiter, Florida 33458, United States. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human MMP13 expressed in mouse NSO cells using varying levels of fTHP-15 as substrate preincubated for 30 mins ... | J Med Chem 60: 5816-5825 (2017) Article DOI: 10.1021/acs.jmedchem.7b00514 BindingDB Entry DOI: 10.7270/Q23F4RTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50239957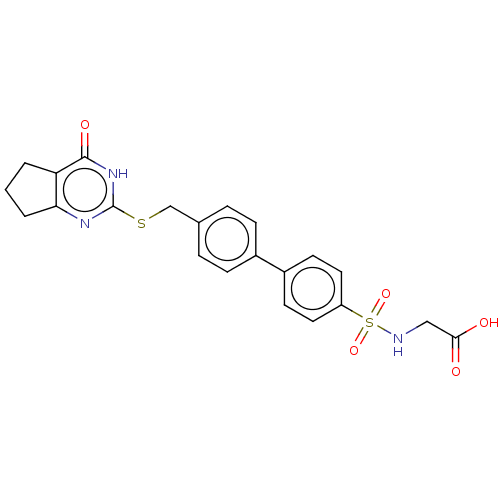 (CHEMBL4089861) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Scripps Florida , 130 Scripps Way, Jupiter, Florida 33458, United States. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human MMP13 expressed in mouse NSO cells using varying levels of fTHP-15 as substrate preincubated for 30 mins ... | J Med Chem 60: 5816-5825 (2017) Article DOI: 10.1021/acs.jmedchem.7b00514 BindingDB Entry DOI: 10.7270/Q23F4RTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13304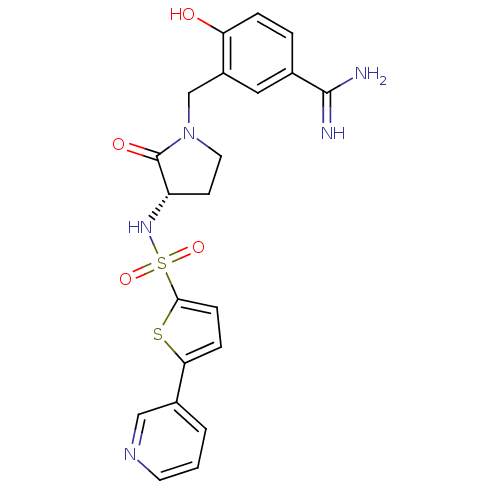 (4-hydroxy-3-[((3S)-2-oxo-3-{[(5-pyridin-3-ylthien-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50081512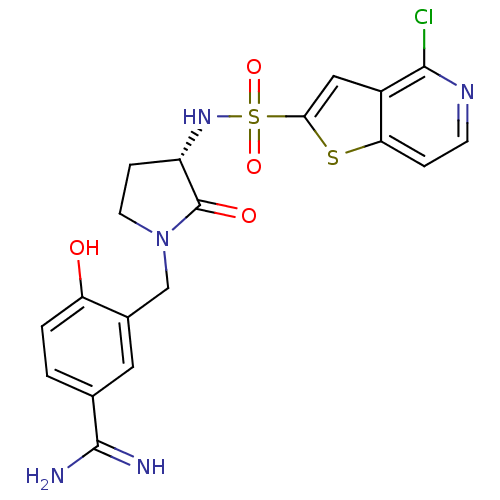 (3-[(S)-3-(4-Chloro-thieno[3,2-c]pyridine-2-sulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity against serine protease factor Xa (fXa) | Bioorg Med Chem Lett 9: 2753-8 (1999) BindingDB Entry DOI: 10.7270/Q2W66K02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50377859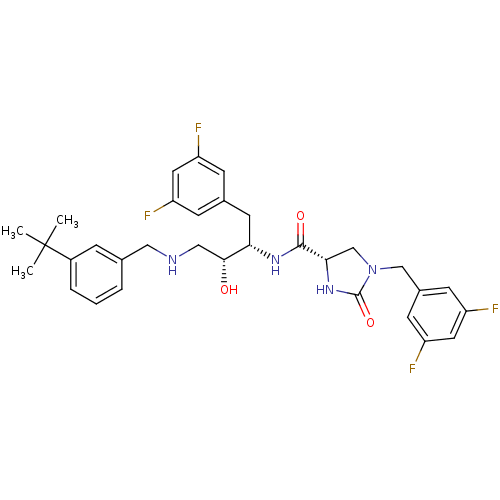 (CHEMBL260621) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Curated by ChEMBL | Assay Description Inhibition of human BACE1 | Bioorg Med Chem Lett 18: 2900-4 (2008) Article DOI: 10.1016/j.bmcl.2008.03.081 BindingDB Entry DOI: 10.7270/Q2RV0PKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50081499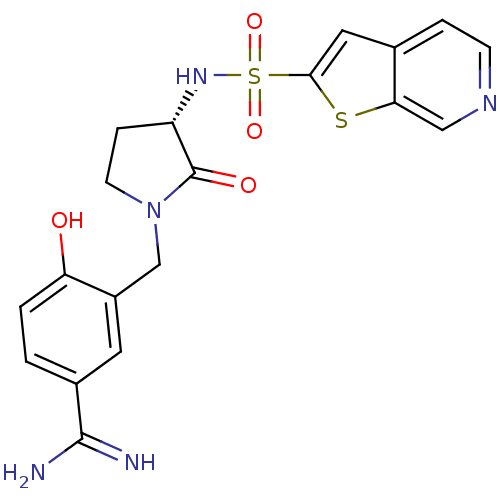 (4-Hydroxy-3-[(S)-2-oxo-3-(thieno[2,3-c]pyridine-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity against serine protease Coagulation factor X | Bioorg Med Chem Lett 9: 2753-8 (1999) BindingDB Entry DOI: 10.7270/Q2W66K02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Mus musculus) | BDBM50216860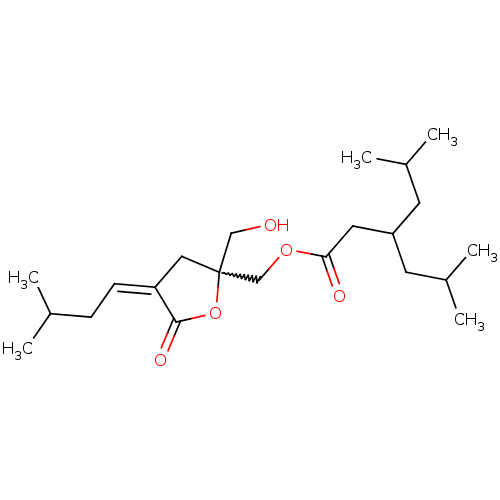 (CHEMBL230048 | Z-[2-(Hydroxymethyl)-4-(3-methylbut...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Curated by ChEMBL | Assay Description Displacement of [3H]PDBu from mouse wild type C1b domain of PKCdelta expressed in Escherichia coli | J Med Chem 50: 3465-81 (2007) Article DOI: 10.1021/jm0702579 BindingDB Entry DOI: 10.7270/Q2RF5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50485236 (BAY-949172 | CHEBI:79033 | FLORBETABEN F18 | Florb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University School of Medicine Curated by ChEMBL | Assay Description Binding affinity to amyloid beta (1-42) aggregates in human Alzheimer's disease brain sections | J Med Chem 55: 883-92 (2012) Article DOI: 10.1021/jm201400q BindingDB Entry DOI: 10.7270/Q2X92F5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50239961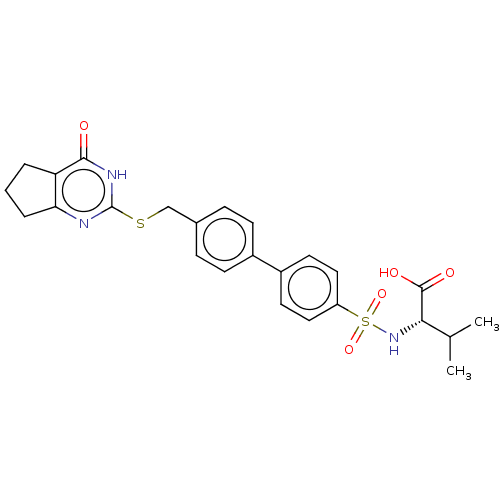 (CHEMBL4062836) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Scripps Florida , 130 Scripps Way, Jupiter, Florida 33458, United States. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human MMP13 expressed in mouse NSO cells using varying levels of fTHP-15 as substrate preincubated for 30 mins ... | J Med Chem 60: 5816-5825 (2017) Article DOI: 10.1021/acs.jmedchem.7b00514 BindingDB Entry DOI: 10.7270/Q23F4RTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50409757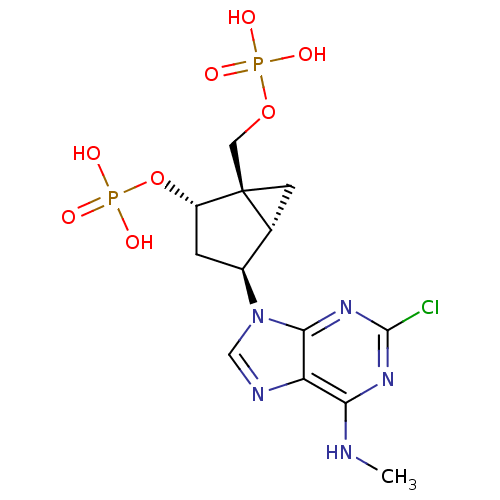 (CHEMBL2112864) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK Curated by ChEMBL | Assay Description Inhibition of [3H]-5 binding to P2Y purinoceptor 1 expressed in Sf9 cells | J Med Chem 46: 4974-87 (2003) Article DOI: 10.1021/jm030127+ BindingDB Entry DOI: 10.7270/Q2NS0T9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Mus musculus) | BDBM50216858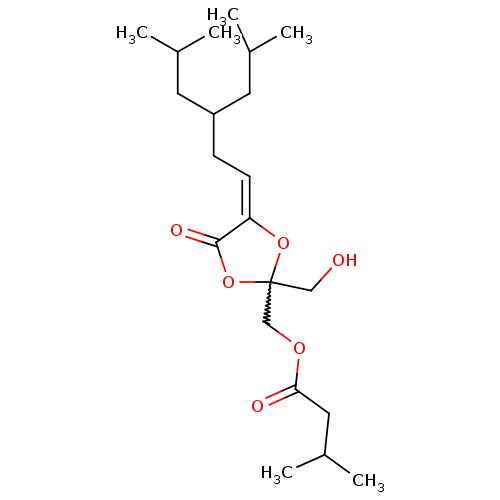 ((Z)-(2-(hydroxymethyl)-4-(3-isobutyl-5-methylhexyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Curated by ChEMBL | Assay Description Displacement of [3H]PDBu from mouse wild type C1b domain of PKCdelta expressed in Escherichia coli | J Med Chem 50: 3465-81 (2007) Article DOI: 10.1021/jm0702579 BindingDB Entry DOI: 10.7270/Q2RF5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50484946 ((18F)AV-45 | 18F-AV-45 | AV-45 F-18 | Amyvid | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University School of Medicine Curated by ChEMBL | Assay Description Binding affinity to amyloid beta (1-42) aggregates in human Alzheimer's disease brain sections | J Med Chem 55: 883-92 (2012) Article DOI: 10.1021/jm201400q BindingDB Entry DOI: 10.7270/Q2X92F5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50107112 ((Z) 2,2-Dimethyl-propionic acid 2-hydroxymethyl-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Binding affinity for Protein kinase C alpha C1a or C1b domain | J Med Chem 46: 2790-3 (2003) Article DOI: 10.1021/jm030082c BindingDB Entry DOI: 10.7270/Q2VH5PJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (MOUSE) | BDBM50107112 ((Z) 2,2-Dimethyl-propionic acid 2-hydroxymethyl-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Curated by ChEMBL | Assay Description Displacement of [3H]PDBu from mouse recombinant PKCalpha expressed in Escherichia coli | J Med Chem 50: 3465-81 (2007) Article DOI: 10.1021/jm0702579 BindingDB Entry DOI: 10.7270/Q2RF5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50085904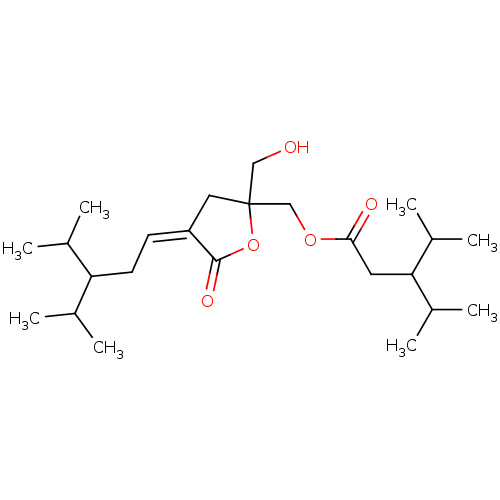 (3-Isopropyl-4-methyl-pentanoic acid 2-hydroxymethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Displacement of [20-3H]phorbol-12,13-dibutyrate (PDBu) from recombinant Protein kinase C alpha | J Med Chem 46: 1571-9 (2003) Article DOI: 10.1021/jm020476o BindingDB Entry DOI: 10.7270/Q2N58KQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13286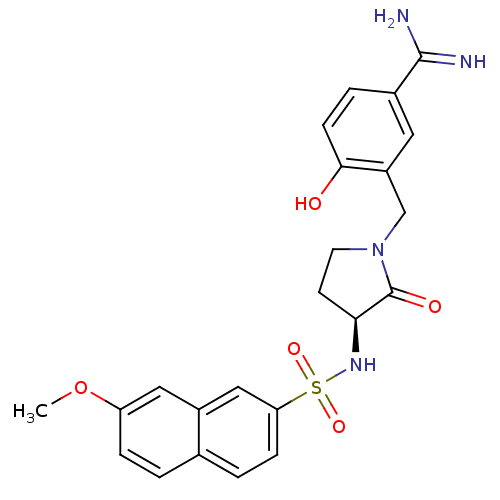 (4-Hydroxy-3-[3-(S)-(7-methoxynaphthalen-2-ylsulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13306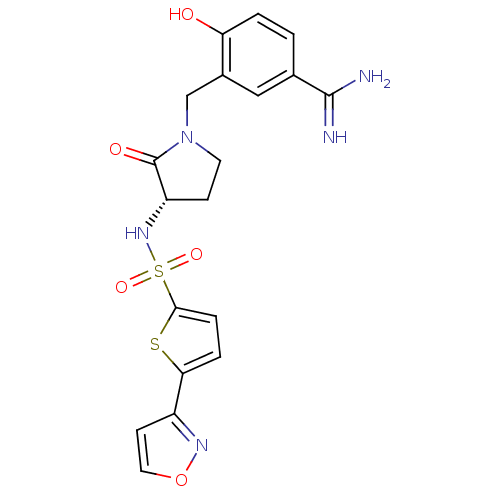 (4-Hydroxy-3-[3-(S)-(5-isoxazol-3-ylthiophene-2-yls...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 42: 3572-87 (1999) Article DOI: 10.1021/jm990041+ BindingDB Entry DOI: 10.7270/Q2KW5D9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12595 (4-[(6-CHLORO-1-BENZOTHIEN-2-YL)SULFONYL]-1-{[1-(2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Aventis Pharma | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 46: 685-90 (2003) Article DOI: 10.1021/jm0203837 BindingDB Entry DOI: 10.7270/Q2VH5M2S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50081510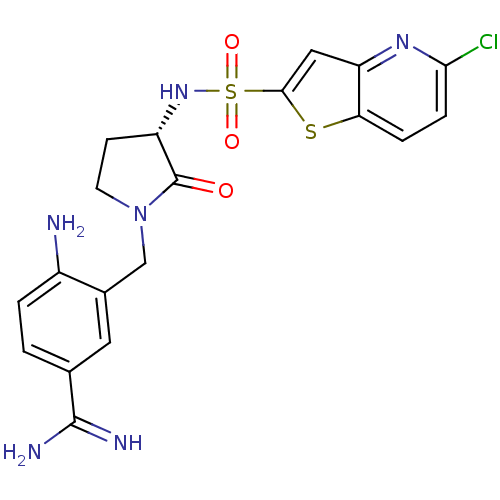 (4-Amino-3-[(S)-3-(5-chloro-thieno[3,2-b]pyridine-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity against serine protease factor Xa (fXa) | Bioorg Med Chem Lett 9: 2753-8 (1999) BindingDB Entry DOI: 10.7270/Q2W66K02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50081517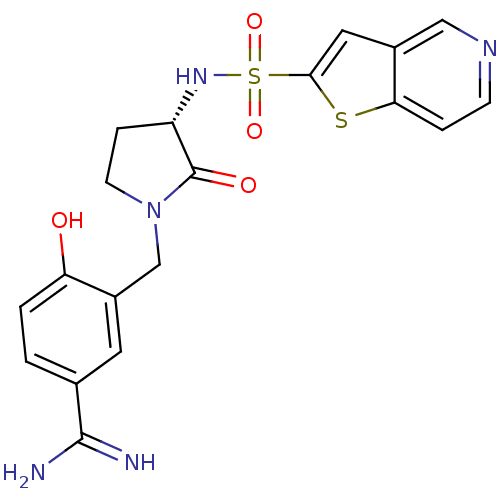 (4-Hydroxy-3-[(S)-2-oxo-3-(thieno[3,2-c]pyridine-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity against serine protease factor Xa (fXa) | Bioorg Med Chem Lett 9: 2753-8 (1999) BindingDB Entry DOI: 10.7270/Q2W66K02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13286 (4-Hydroxy-3-[3-(S)-(7-methoxynaphthalen-2-ylsulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity against serine protease Coagulation factor X | Bioorg Med Chem Lett 9: 2753-8 (1999) BindingDB Entry DOI: 10.7270/Q2W66K02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50123788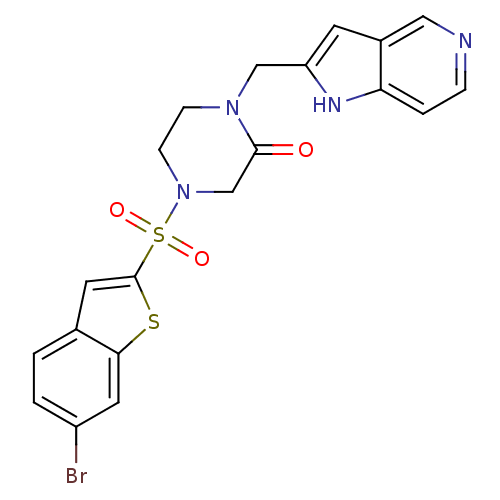 (4-(6-Bromo-benzo[b]thiophene-2-sulfonyl)-1-(1H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding affinity for human Coagulation factor X | J Med Chem 46: 681-4 (2003) Article DOI: 10.1021/jm020384z BindingDB Entry DOI: 10.7270/Q2KW5FDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50123766 (4-(6-Chloro-benzo[b]thiophene-2-sulfonyl)-1-(1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding affinity for human Coagulation factor X | J Med Chem 46: 681-4 (2003) Article DOI: 10.1021/jm020384z BindingDB Entry DOI: 10.7270/Q2KW5FDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12595 (4-[(6-CHLORO-1-BENZOTHIEN-2-YL)SULFONYL]-1-{[1-(2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity (in vitro) of the compound towards human Coagulation factor X was determined at 10 mg/kg peroral dose | J Med Chem 46: 681-4 (2003) Article DOI: 10.1021/jm020384z BindingDB Entry DOI: 10.7270/Q2KW5FDH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 2793 total ) | Next | Last >> |