

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50418800 (CHEMBL1797395) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.33 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Nav1.7 by cell based whole-cell voltage clamp electrophysiology assay | Bioorg Med Chem Lett 21: 3871-6 (2011) Article DOI: 10.1016/j.bmcl.2011.05.041 BindingDB Entry DOI: 10.7270/Q21837RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50418784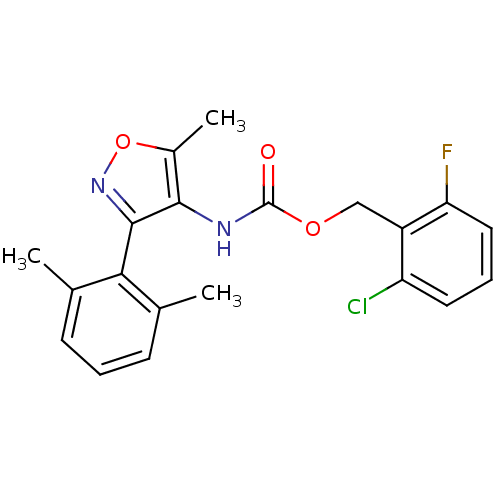 (CHEMBL1797399) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Nav1.7 by cell based whole-cell voltage clamp electrophysiology assay | Bioorg Med Chem Lett 21: 3871-6 (2011) Article DOI: 10.1016/j.bmcl.2011.05.041 BindingDB Entry DOI: 10.7270/Q21837RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398768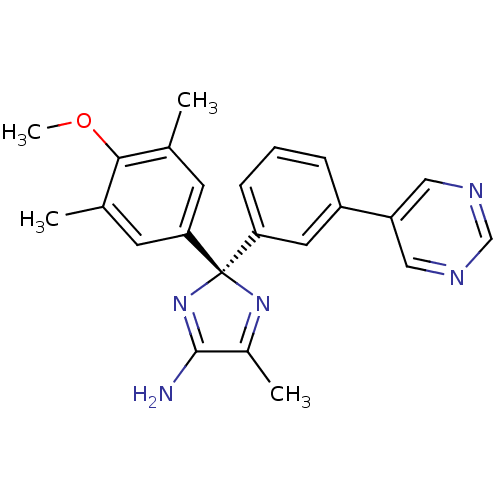 (CHEMBL2180030) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE1 using (europium)CEVNLDAEFK(Qsy7) as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by T... | J Med Chem 55: 9297-311 (2012) Article DOI: 10.1021/jm300991n BindingDB Entry DOI: 10.7270/Q2HH6M79 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398774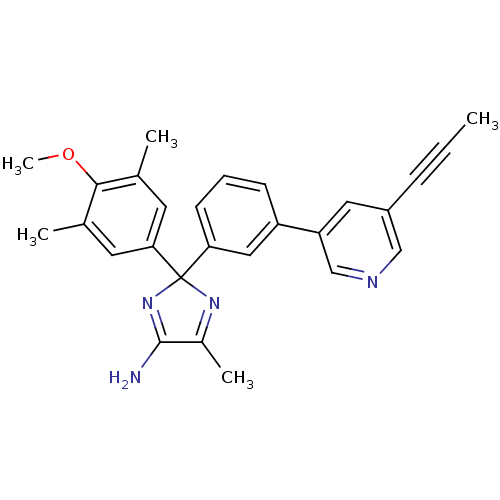 (CHEMBL2180031) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE1-mediated sAPPbeta release in human SH-SY5Y cells after 16 hrs by ELISA | J Med Chem 55: 9297-311 (2012) Article DOI: 10.1021/jm300991n BindingDB Entry DOI: 10.7270/Q2HH6M79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM181966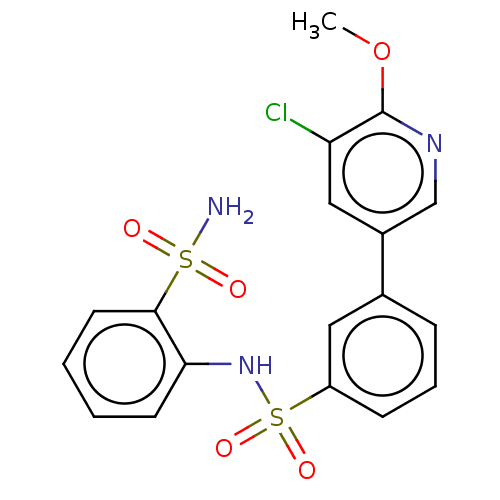 (US9145380, 169) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50418789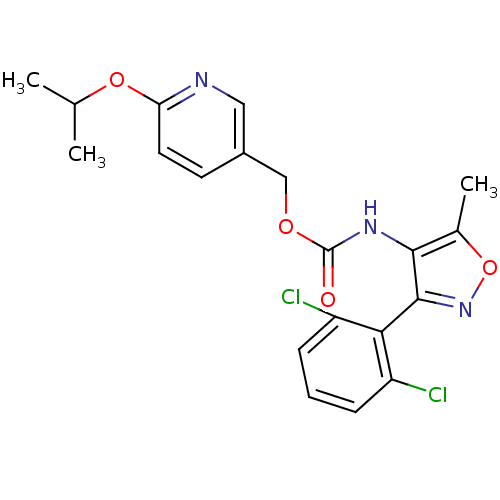 (CHEMBL1797391) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22.9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Nav1.7 by cell based whole-cell voltage clamp electrophysiology assay | Bioorg Med Chem Lett 21: 3871-6 (2011) Article DOI: 10.1016/j.bmcl.2011.05.041 BindingDB Entry DOI: 10.7270/Q21837RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50418791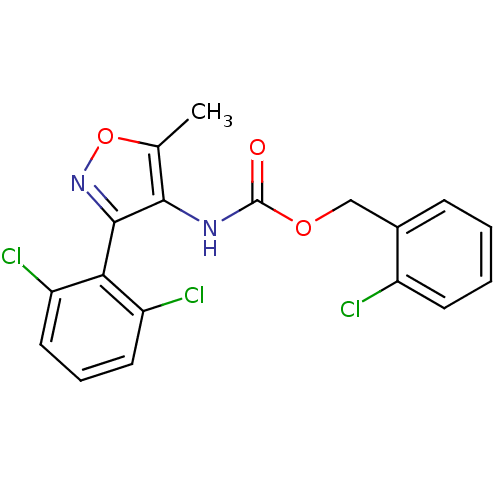 (CHEMBL1797388) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24.0 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Nav1.7 by cell based whole-cell voltage clamp electrophysiology assay | Bioorg Med Chem Lett 21: 3871-6 (2011) Article DOI: 10.1016/j.bmcl.2011.05.041 BindingDB Entry DOI: 10.7270/Q21837RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398768 (CHEMBL2180030) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE1-mediated sAPPbeta release in human SH-SY5Y cells after 16 hrs by ELISA | J Med Chem 55: 9297-311 (2012) Article DOI: 10.1021/jm300991n BindingDB Entry DOI: 10.7270/Q2HH6M79 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398774 (CHEMBL2180031) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE1 using (europium)CEVNLDAEFK(Qsy7) as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by T... | J Med Chem 55: 9297-311 (2012) Article DOI: 10.1021/jm300991n BindingDB Entry DOI: 10.7270/Q2HH6M79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50418774 (CHEMBL1797387) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28.2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Nav1.7 by cell based whole-cell voltage clamp electrophysiology assay | Bioorg Med Chem Lett 21: 3871-6 (2011) Article DOI: 10.1016/j.bmcl.2011.05.041 BindingDB Entry DOI: 10.7270/Q21837RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50418788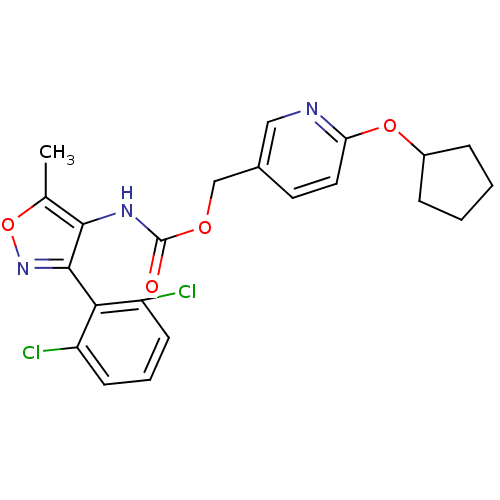 (CHEMBL1797392) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28.8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Nav1.7 by cell based whole-cell voltage clamp electrophysiology assay | Bioorg Med Chem Lett 21: 3871-6 (2011) Article DOI: 10.1016/j.bmcl.2011.05.041 BindingDB Entry DOI: 10.7270/Q21837RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50398767 (CHEMBL2180037) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE2 using (europium)CEVNLDAEFK(Qsy7) as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by T... | J Med Chem 55: 9297-311 (2012) Article DOI: 10.1021/jm300991n BindingDB Entry DOI: 10.7270/Q2HH6M79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM182037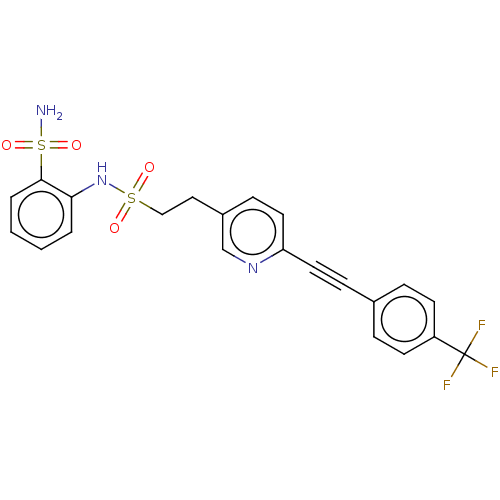 (US9145380, 240) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 31 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50491233 (CHEMBL2380450) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE1-mediated soluble APPbeta release in human SH-SY5Y cells after 16 hrs | J Med Chem 56: 4181-205 (2013) Article DOI: 10.1021/jm3011349 BindingDB Entry DOI: 10.7270/Q2MS3WP0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398768 (CHEMBL2180030) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE1 using (europium)CEVNLDAEFK(Qsy7) as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by T... | J Med Chem 55: 9297-311 (2012) Article DOI: 10.1021/jm300991n BindingDB Entry DOI: 10.7270/Q2HH6M79 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM181846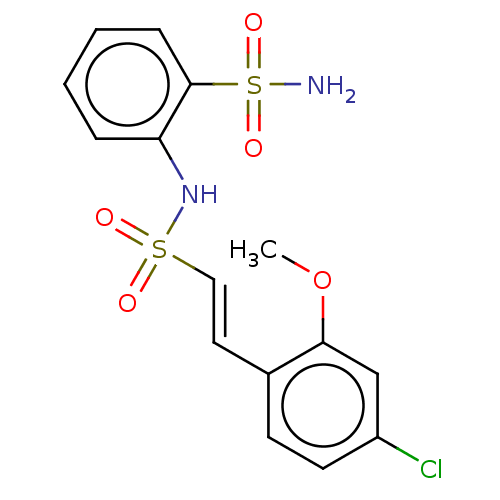 (US9145380, 49) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 36 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50418782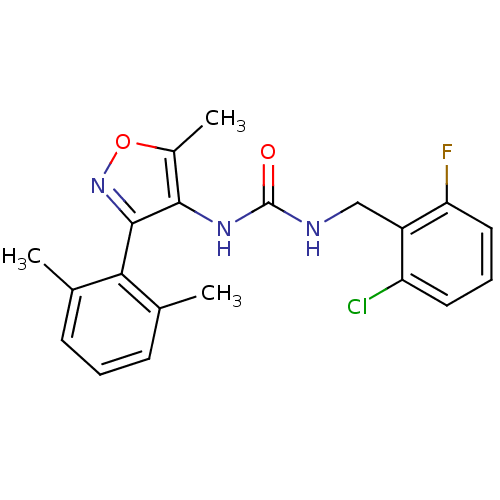 (CHEMBL1797401) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38.0 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Nav1.7 by cell based whole-cell voltage clamp electrophysiology assay | Bioorg Med Chem Lett 21: 3871-6 (2011) Article DOI: 10.1016/j.bmcl.2011.05.041 BindingDB Entry DOI: 10.7270/Q21837RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50491232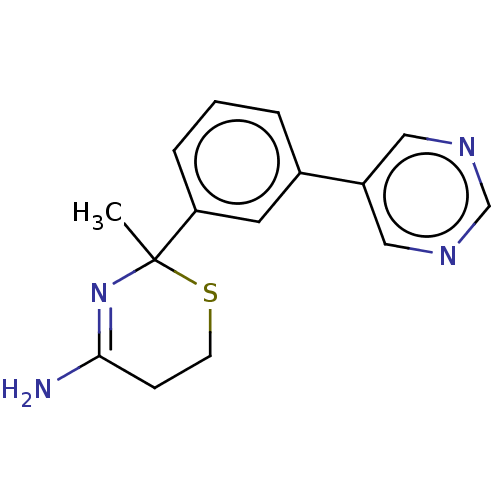 (CHEMBL2380445) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE1-mediated soluble APPbeta release in human SH-SY5Y cells after 16 hrs | J Med Chem 56: 4181-205 (2013) Article DOI: 10.1021/jm3011349 BindingDB Entry DOI: 10.7270/Q2MS3WP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM181802 (US9145380, 5) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM182009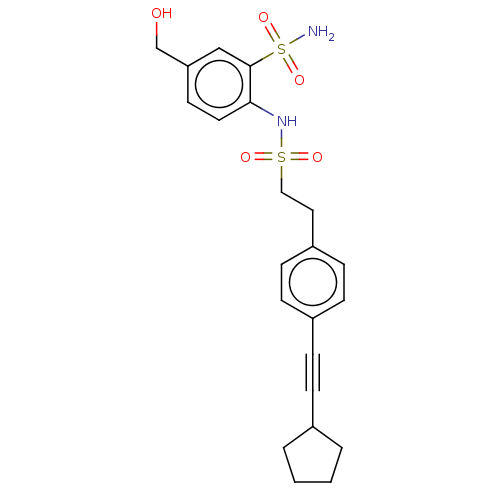 (US9145380, 212) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 41 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM181815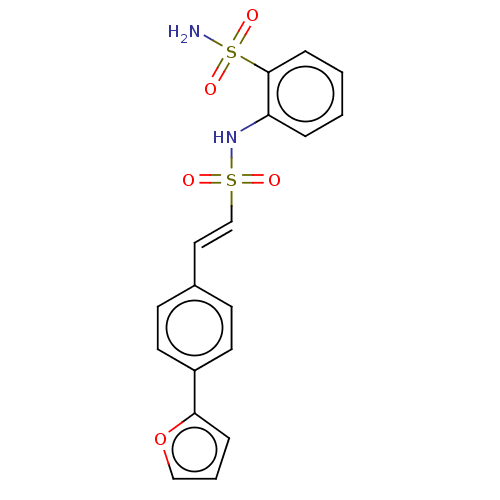 (US9145380, 18) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 44 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398768 (CHEMBL2180030) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE1-mediated sAPPbeta release in human SH-SY5Y cells after 16 hrs by ELISA | J Med Chem 55: 9297-311 (2012) Article DOI: 10.1021/jm300991n BindingDB Entry DOI: 10.7270/Q2HH6M79 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM182005 (US9145380, 208) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM181833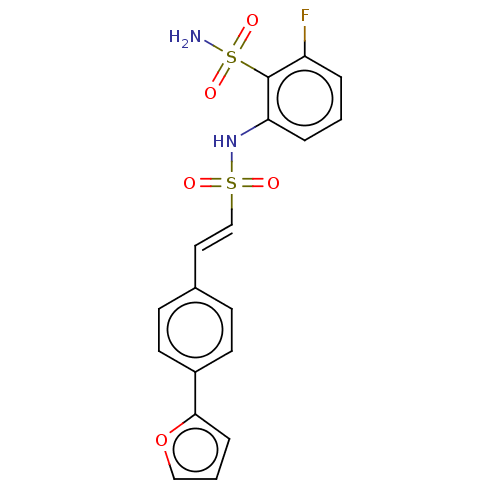 (US9145380, 36) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 57 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398769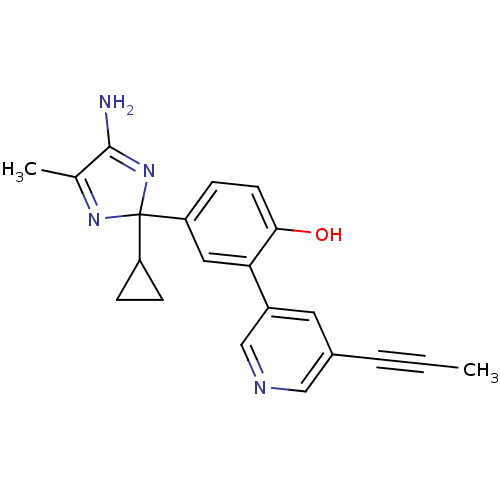 (CHEMBL2180014) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE1-mediated sAPPbeta release in human SH-SY5Y cells after 16 hrs by ELISA | J Med Chem 55: 9297-311 (2012) Article DOI: 10.1021/jm300991n BindingDB Entry DOI: 10.7270/Q2HH6M79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50418775 (CHEMBL1797413) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 58.9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Nav1.7 by cell based whole-cell voltage clamp electrophysiology assay | Bioorg Med Chem Lett 21: 3871-6 (2011) Article DOI: 10.1016/j.bmcl.2011.05.041 BindingDB Entry DOI: 10.7270/Q21837RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398769 (CHEMBL2180014) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE1 using (europium)CEVNLDAEFK(Qsy7) as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by T... | J Med Chem 55: 9297-311 (2012) Article DOI: 10.1021/jm300991n BindingDB Entry DOI: 10.7270/Q2HH6M79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50491225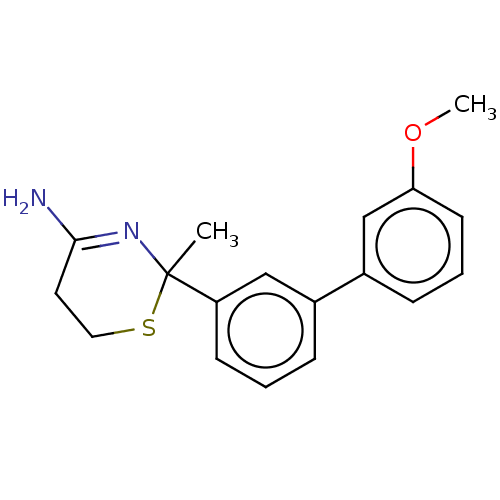 (CHEMBL2380446) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE1-mediated soluble APPbeta release in human SH-SY5Y cells after 16 hrs | J Med Chem 56: 4181-205 (2013) Article DOI: 10.1021/jm3011349 BindingDB Entry DOI: 10.7270/Q2MS3WP0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50418795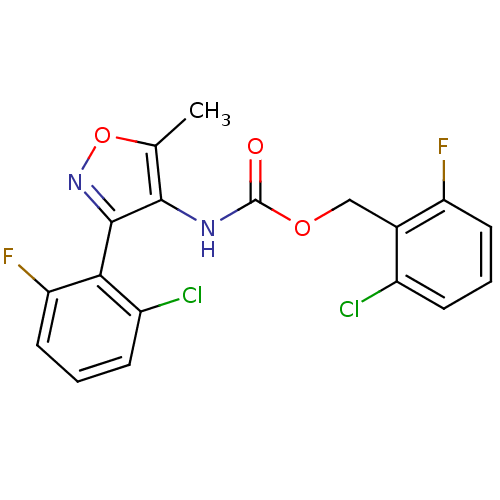 (CHEMBL1797398) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72.4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Nav1.7 by cell based whole-cell voltage clamp electrophysiology assay | Bioorg Med Chem Lett 21: 3871-6 (2011) Article DOI: 10.1016/j.bmcl.2011.05.041 BindingDB Entry DOI: 10.7270/Q21837RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM181803 (US9145380, 6) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 74 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM181903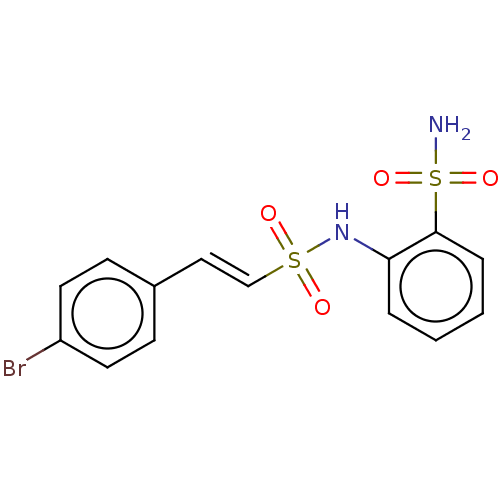 (US9145380, 106) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 74 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM182008 (US9145380, 211) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 75 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM181822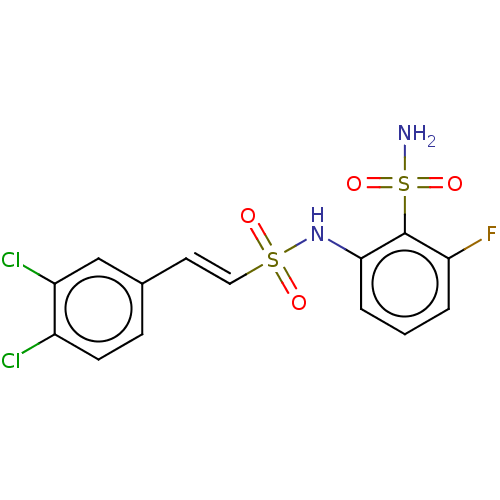 (US9145380, 25) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 77 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398767 (CHEMBL2180037) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE1 using (europium)CEVNLDAEFK(Qsy7) as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by T... | J Med Chem 55: 9297-311 (2012) Article DOI: 10.1021/jm300991n BindingDB Entry DOI: 10.7270/Q2HH6M79 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM181801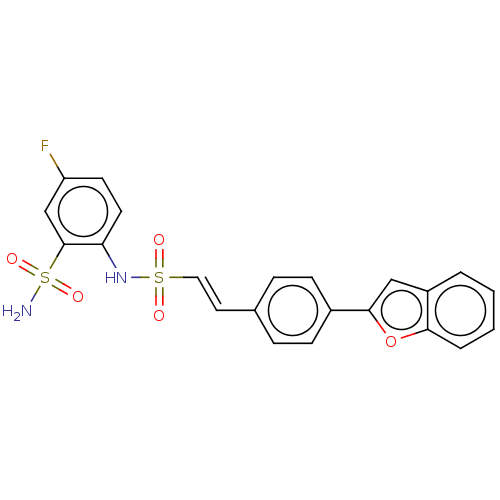 (US9145380, 4) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 79 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM181980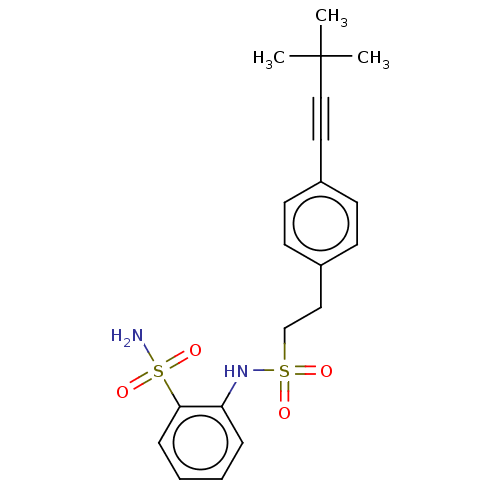 (US9145380, 183) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 81 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM181849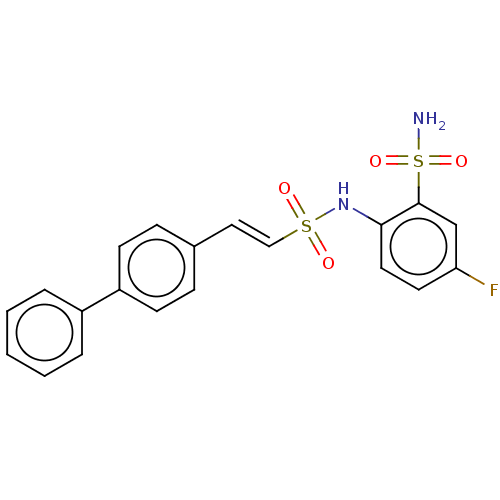 (US9145380, 52) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 84 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM181889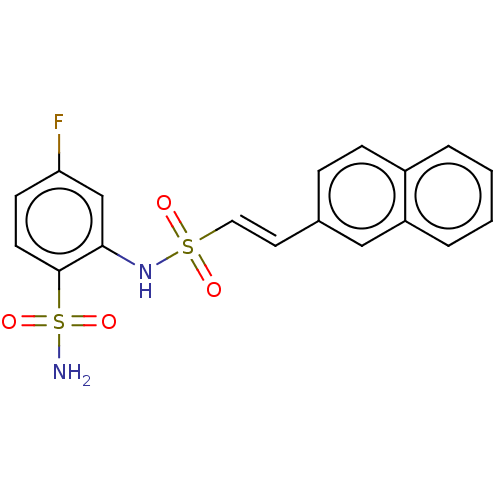 (US9145380, 92) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 91 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM182004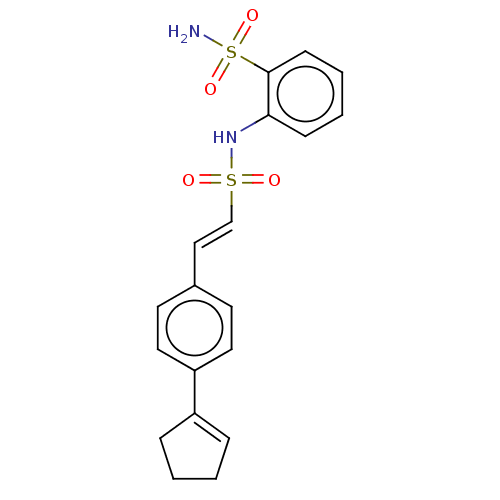 (US9145380, 207) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 97 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398776 (CHEMBL2180027) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE1 using (europium)CEVNLDAEFK(Qsy7) as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by T... | J Med Chem 55: 9297-311 (2012) Article DOI: 10.1021/jm300991n BindingDB Entry DOI: 10.7270/Q2HH6M79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50395945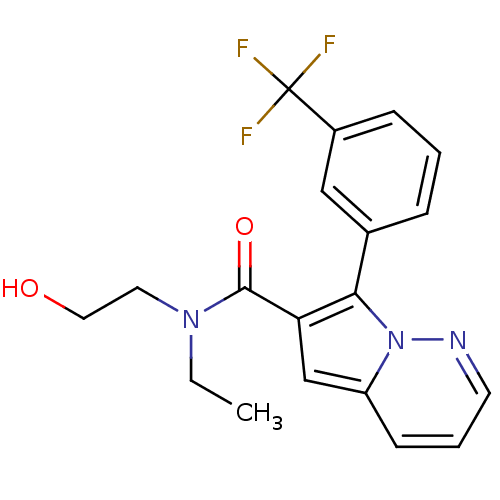 (CHEMBL2164565) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells by IonWorks assay | Bioorg Med Chem Lett 22: 6888-95 (2012) Article DOI: 10.1016/j.bmcl.2012.09.059 BindingDB Entry DOI: 10.7270/Q26W9C63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398786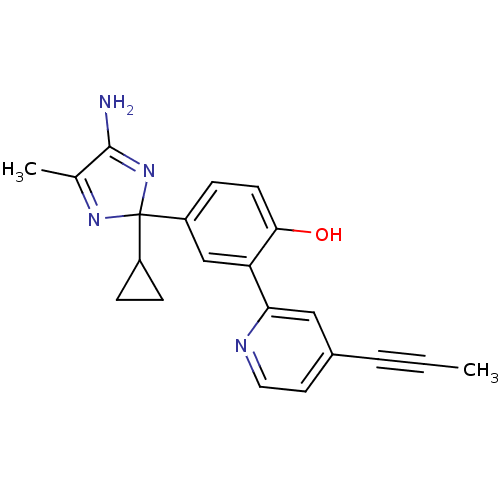 (CHEMBL2180015) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE1-mediated sAPPbeta release in human SH-SY5Y cells after 16 hrs by ELISA | J Med Chem 55: 9297-311 (2012) Article DOI: 10.1021/jm300991n BindingDB Entry DOI: 10.7270/Q2HH6M79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM182021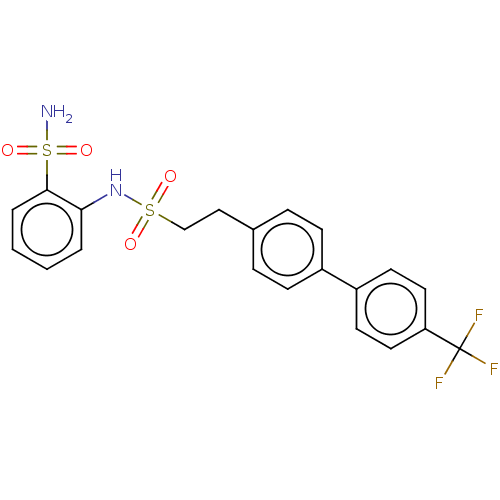 (US9145380, 224) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM182014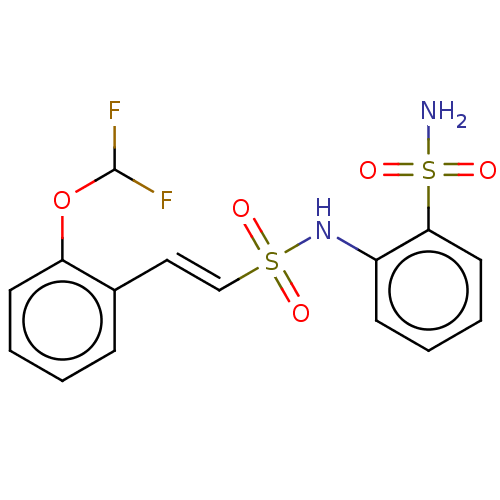 (US9145380, 217) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 110 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM181840 (US9145380, 43) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 110 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50398766 (CHEMBL2180033) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE2 using (europium)CEVNLDAEFK(Qsy7) as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by T... | J Med Chem 55: 9297-311 (2012) Article DOI: 10.1021/jm300991n BindingDB Entry DOI: 10.7270/Q2HH6M79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM181831 (US9145380, 34) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50418792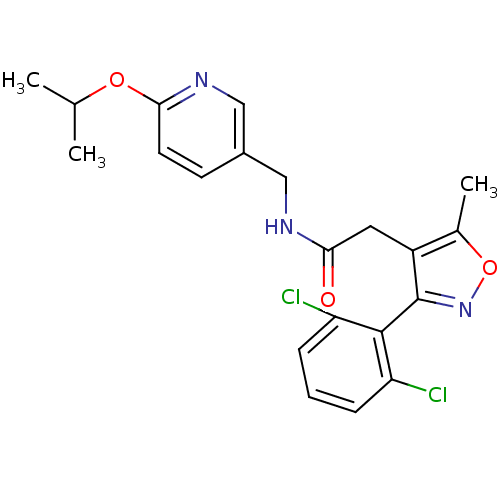 (CHEMBL1797409) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Nav1.7 by cell based whole-cell voltage clamp electrophysiology assay | Bioorg Med Chem Lett 21: 3871-6 (2011) Article DOI: 10.1016/j.bmcl.2011.05.041 BindingDB Entry DOI: 10.7270/Q21837RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM182013 (US9145380, 216) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM181798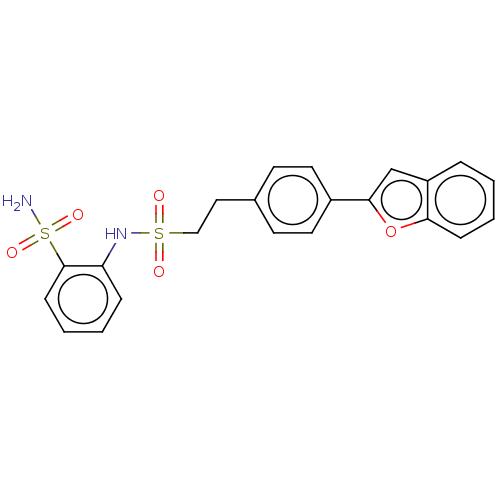 (US9145380, 1) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 467 total ) | Next | Last >> |